Abstract
Hydrogen sulfide (H2S) has emerged as a novel gaseous signal molecule with multifarious effects on seed germination, plant growth, development, and physiological processes. Due to its dominant role in plant stress tolerance and cross-adaptation, it is getting more attention nowadays, although it has been largely referred as toxic and environmental hazardous gas. In this review work, we are highlighting the importance of H2S as an essential gaseous molecule to help in signaling, metabolism, and stress tolerance in plants. Firstly, production of H2S from different natural and artificial sources were discussed with its transformation from sulfur (S) to sulfate (SO42−) and then to sulfite (SO32−). The importance of different kinds of transporters that helps to take SO42− from the soil solution was presented. Mainly, these transporters are SULTRs (H+/SO42− cotransporters) and multigene family encodes them. Furthermore, these SULTRs have LAST (Low affinity transport proteins), HAST (High affinity transport proteins), vacuole transporters, and plastid transporters. Since it is well known that there is strong relationship between SO42− and synthesis of hydrogen sulfide or dihydrogen sulfide or sulfane in plant cells. Thus, cysteine (Cys) metabolism through which H2S could be generated in plant cell with the role of different enzymes has been presented. Furthermore, H2S in interaction with other molecules could help to mitigate biotic and abiotic stress. Based on this review work, it can be concluded that H2S has potential to induce cross-adaptation to biotic and abiotic stress; thus, it is recommended that it should be considered in future studies to answer the questions like what are the receptors of H2S in plant cell, where in plants the physiological concentration of H2S is high in response to multiple stress and how it induces cross-adaptation by interaction with other signal molecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S) is a colorless, low molecular weight, and soluble gas which had been known for its bad odor and phytotoxic effects for centuries. It is present in atmosphere and mainly added through volcanos, salt marshes, wet land, geothermal vents, livestock, industry, combustion of biomass and fossil fuels, and bacterial anaerobic respiration. Hydrogen sulfide represents only 8.5% (i.e., 4.4 Tg) of the total annual natural sulfur emission (i.e., 52 Tg) (Watts 2000). The H2S is present in the atmosphere and plants may take it through foliage which negatively affects the normal sulfate metabolism in plants that is uptaken by plant roots (Ausma and Kok 2019). The H2S is absorbed through foliage via stomata and acts as substantial nutrient source of sulfur (S) in plants. For S homeostasis in plants, these must have to maintain the ability to emit it temporarily through foliage to atmosphere (Schröder 1993). The detoxification and removal of H2S from plants is more important when H2S is just a pollutant. In such circumstances, the role of H2S is not just a signaling molecule (Lisjak et al. 2013).
Most studies about H2S focused on animals, while studies on the effect of H2S on plants only started to be more frequent during the late part of the twentieth century (Wang 2002). Its endogenous generation in plants as signaling molecule and its direct and indirect role in stress tolerance and protection against diseases was realized after 1975, time until which it was only considered as a determinant of plant growth and development. At that time, results reported by Joshi et al. (1975) indicated that H2S inhibits the oxygen release from rice seedlings and nutrient uptake. A few years later, results about the impact of H2S fumigation on forest plant were published (Thompson and Kats 1978a, b).
Reported negative impacts of H2S include respiration inhibition in hemp (Martin and Maricle 2015), decrease in freezing tolerance of wheat (Stuiver et al. 1992), inhibition of photoreduction of NADP+ in spinach (De Kok et al. 1983), and inhibition of activity of cytochrome oxidase in the mitochondria (Dorman et al. 2002). However, the impact of H2S on plants are dose specific and low doses may rather be beneficial for crop growth and development (Dooley et al. 2013; Filipovic and Jovanović 2017). However, plants species vary greatly to atmospheric H2S phytotoxicity, revealing that various doses have differential effects on plants. These are mainly attributed due to differences in leave absorption capacity, leaf elongation rate, and other related physiological and morphological traits. Plants with tolerance to atmospheric H2S could be utilized in regions characterized as highly pollutant with H2S.
Most of the studies carried out on investigation of H2S on growth and physiological parameters were carried out with Sodium hydrosulfide (NaHS) as a donor of H2S. However, sodium sulfide (Na2S) has also been described as important H2S donor (Ziogas et al. 2018). The NaHS rapidly dissociated to generate H2S and hence used in most of studies to evaluate the H2S impacts on plants. The GYY4137 (morpholin4-ium 4 methoxyphenyl(morpholino) phosphinodithionate may be another donor of H2S (Lisjak et al. 2010). Nutritionally, it is important with respect to sulfur source, a major nutrient and component of s-containing amino acid such as cysteine and methionine.
Hydrogen sulfide acts as signaling molecules in stress along with interplay with other plant hormones, signaling molecules, and reactive oxygen species. Globally, now it is being applied for protection against stresses including drought (Ma et al. 2016a, b), waterlogging (Xiao et al. 2020), heavy metals (Thapa et al. 2012), salinity (Christou et al. 2011), inhibition of autophagy (Filipovic and Jovanović 2017), and fruit ripening (Ziogas et al. 2018). Some of the benefits associated with the use of H2S include enhancement in processes like seed germination, root proliferation, stomatal closure, leaf senescence, maintenance of K+/Na+ balance, and improvement in fruit shelf life and quality. Table 1 described the dose of NaHS application which is most important for exogenous application. Due its lipophilic and gaseous nature, H2S can be easily transported through cell membrane and within plant bodies. The waterlogged conditions create hypoxia stress and root respiration is primarily suffered oxygen deficiency in such conditions. The H2S application played a protective role against oxidative damages imposed by waterlogged conditions through reducing accumulation of reactive oxygen species in roots and leaves of peach (Xiao et al. 2020). It triggers the gene expression in grapevine related to biosynthesis of metabolites which are used to improve production of defensive compounds (Ma and Yang 2018). Moreover, evidence from tomato concluded that H2S application from NaHS regulated the expression of more five thousand genes (Guo et al. 2018). The H2S as stress tolerance molecules sustain crop growth and development through regulating the production of reactive oxygen species. Application of H2S may also increase the level of various antioxidant components, resulting in an improved tolerance (Corpas and Palma 2020). Keeping in view the important regulatory role in management of abiotic and biotic stress, it is now being applied exogenously for additional protection.
The post-harvest deterioration of fruit quality is an important issue of producers, marketing agents, and consumers. Exogenously applied H2S may impact the fruit ripening process and post-harvest quality, by reducing the activity of enzymes like superoxide dismutase, catalase, peroxidase, and other related enzymes for reducing the levels of reactive oxygen species. It is helpful to preserve vitamin C, soluble protein, and total phenols along with other fruit quality traits in apple and grapes (Zheng et al. 2016; Ni et al. 2016). The anti-ripening role of H2S results from the interplay with ethylene in fruits (Ge et al. 2017). The regulation of H2S concentration during transition of ripening stages was also reported in sweet pepper (Muñoz-Vargas et al. 2018). The current review mainly focused on H2S application in the field of agriculture in term of stress tolerance molecule, growth promotion, and preservation of post-harvest quality. The main objective of this review is to highlight the biosynthesis, sources, absorption, translocation, and regulatory role of H2S in stress tolerance for exogenous applications in field conditions for dealing various issues of crop production.
Artificial and Natural Sources of H2S for Plant Cells
To be effective in plant cells, H2S gas must be present in high concentration. There are two sources of H2S emission in environment, i.e., anthropogenic and natural source. Among anthropogenic activities, the main source of H2S emission is from combustion of fuels. Other manmade sources include geothermal industries, wastewater treatment, and agriculture activities. Areas located near geothermal site faced 50 ppb H2S. Modern cars that have catalytic inverters also emit H2S gas (Kourtidis et al. 2008). Drilling and refining, coke oven, paper making process and waste treatment processes emit H2S gas in sufficient amount. In mining process, during the decomposition of xanthates in presence of water also produce H2S (Bhomick and Rao 2014).
Natural gas is considered a more environment friendly fuel as it emits about 50% less carbon dioxide in atmosphere as compared to coal. Increasing demand of natural gas leads to the depletion of natural reservoirs, and trend is shifting toward the use of gas extracted from undesirable lands, such as shale gas reservoirs (Goodwin et al. 2015; Brace and Engelberth 2020). Natural gas obtained from such reservoirs is highly acidic and concentrated with H2S gas and considered as sour gas. In 2004, sour gas contributed 21% among total natural gas and predicted to be 27% in 2030, which will be a major health concern in future (Goodwin et al. 2015).
Volcanoes are considered to be major natural source of H2S gas emission (Aiuppa et al. 2005). Plants grown in such regions would be resistant from H2S. It is also released from marshlands, anoxic soils, ground water, and coastal sediments (Hansen et al. 1978). Areas near coastal regions face 5–30 µg/m3 H2S concentration in atmosphere (Kourtidis et al. 2008).
Sulfur Dynamics and Synthesis of H2S in Soil
Besides volcanoes, other natural sources of H2S include coastal marine sediments and anoxic soils of marshland. Archaea (e.g., Archaeoglobus) and Bacteria (e.g., Desulfovibrio, Desulfobacter) present in waterlogged soils and marshy places produce sulfur (S)-based compounds such as sulfate (SO42−) which is then transformed into sulfite (SO32−). According to the genesis point of view, H2S mainly comes from the four sources i.e., volcanic inorganic sources, SO42− reduction by bacteria (SRB), decomposition of organic S compounds by heat, and SO42− reduction by the thermochemical reactions (Ma et al. 2019).
Production of H2S in waterlogged soils is mainly due to the reduction of SO42−. The reduction that occurs only in these soils is due to the presence of anaerobic bacteria. This is also a major reaction of S cycling that prevails in waterlogged soils due to the readily decomposition of plant residues e.g., alfalfa (Medicago sativa). The SO42− reduction due to bacteria involves assimilation (SO42− is reduced to the thiol) or dissimilation (SO42− reduction leads to the production of H2S) processes. Since soil is main source of H2S production, it is essential to report about availability of S in the earth crust, which is the main reagent for the synthesis of H2S. The average S content of the earth crust is in the range of 0.06 to 0.10% and it is ranked as 13th most abundant element in nature (Tabatabai 2005). Minerals of S are available in rocks and soils as SO42− (e.g., Epsomaite (MgSO4.7H2O), Mirabilite (Na2SO4.10H2O), Gypsum (CaSO4.2H2O), Gypsum anhydorous (CaSO4)), and as S−2 (e.g., Arseno pyrite (FeS2.FeAs2), chalcopyrite (CuFeS2), cobaltite (CoAsS), galena (PbS), marcasite (FeS2), pentlandite (Fe, Ni)9S8, pyrrhotite (Fe11S12) sphalerite (ZnS)). There is continuous flow of S going on between terrestrial and marine masses (Fuentes-Lara et al. 2019). The simple biogeochemical cycle that shows transformation of S to different forms is shown in Fig. 1.
Generally, two types of S occur in soil i.e., Organic and inorganic. Organic S in soil accounts for 95% of the total S in the humid and semi humid regions. The S availability to the plants in agricultural ecosystems follows different mechanisms as shown in Fig. 2. From atmosphere, it comes from aerosols of S and S gaseous forms as well as dissolved S (SO42−) in snow and rain. Similarly, oxidation of S from soil organic matter and So also generates SO42−. These SO42− can be leached to the subsoil or fixed in the soil exchange matrix (Chao et al. 1962). Large quantities of SO42− in the subsoil of arid regions are also available as gypsum (CaSO4), while in the regions where rainfall is high this SO42− leached down to the lower horizons (Johnson and Cole 1980).
The SO42− in the soil is subjected to two kind of reactions i.e., dissimilatory (SO42− acts as final acceptor of electrons in the anaerobic metabolisms of microbes producing H2S that on re-oxidation goes to the atmosphere) and assimilatory reduction (Biosynthesis of organic compounds through algae, fungi, plants and prokaryotes e.g., amino acids). Assimilatory reduction of SO42− is not possible by animals and protists; thus, they depend on other organism that synthesizes the organic S compounds (Andreae 1990). The dissimilatory reduction of SO42− results in the production of S0 mostly under anoxic conditions. This S0 could be assimilated into S2− that will be part of the biomolecules or volatilized in the case of excess S (Fig. 2). Plant can be a source or sink of volatile forms of S such as H2S, DMS (dimethyl sulfide; \({CH}_{3}-S-{CH}_{3}\)), carbon disulfide (CS2) etc.
Availability of S for many crop species is very beneficial as it improves the nutritional quality as well as tolerance to the biotic and abiotic stresses (Tea et al. 2004; González-Morales et al. 2017; Corpas et al. 2020; Fuentes-Lara et al. 2019; Corpas and Palma 2020; Nawaz et al. 2020; Zhang and Liao 2020). Tabatabai (2005) reported significant relationship of Sulfur with Carbon (C), Nitrogen (N), and Phosphorus (P) in soils around the globe. However, S availability as inorganic form like N is very small as compared to the organic forms, but P is abundantly available in both forms. Thus, relationship between total N, organic P, organic C, and total S has been reported mostly. The mean C: N: P: S ratios for the agricultural soil is 130:10:1.3:1.3, while for the peat and organic soils it is 160:10:1.2:1.2 and the soil under native grass has ratio of 200:10:1:1.
Absorption and Translocation
The absorption of S in plant cell is mostly in the form of H2S, DMS, COS, and SO2. However, most of the S is taken up by the plants from the soil solution as SO42− (Wainwright 1984; Rennenberg 1989). Different kinds of transporters help to take SO42− from the soil solution. Mainly, these transporters are SULTRs (H+/ SO42− cotransporters) and multigene family encodes them. Furthermore, these SULTRs have LAST (Low affinity transport proteins), HAST (High affinity transport proteins), vacuole transporters and plastid transporters (Fig. 3). The concentration of SO42− that induces HAST is less than 10 mg dm−3 (Fuentes-Lara et al. 2019). Absorption of SO42− in the root is facilitated by HAST, SULTR 1:1, SULTR 1:2, and SULTR 1:3 in the presence of ATPase enzyme as shown in Fig. 3. In the epidermis and cortex of root HAST are present abundantly, while in the parenchyma cell adjacent to the vascular bundles (Xylem and phloem) LAST dominates.
The cotransporters SULTR 4:1 and 4:2 help the vacuoles to absorb SO42− which is then stored there. This can be further redistributed to different plant parts depending upon the demand. Xylem and phloem helps to transport and translocate SO42− from the roots to stems and afterward to the leaves and seeds through different kinds of SULTRs (Yoshimoto et al. 2003; Kataoka et al. 2004; Takahashi et al. 2011; Cao et al. 2013; Maruyama-Nakashita 2017; Kirschner et al. 2018; Singh and Schwan 2019). The absorbed SO42− is largely assimilated into proteins and other biomolecules, while in some plants e.g., Brassica spp., it might be present in the plant tissues. The SO42− absorbed by the xylem is discharged in the mesophyll cell of the leaf with the help of HAST and LAST. Some of this SO42− is stored in the vacuoles, while other moves to the chloroplast through SULTR3:1, SULTR3:2, SULTR 3:3, and SULTR 3:4, where it is reduced to S2− and assimilated to the biological molecules. The stored sulfate can be remobilized through the SULTR 4:1 and SULTR 4:2 (Fig. 3). Excessive accumulation of SO42− is possible if excess of S is available which further leads to the formation of volatile compounds e.g., H2S (Rennenberg 1989). Since H2S is a weak acid, it can be changed to different form as shown in the following equation:
Detrimental Effects of Hydrogen Sulfide
Hydrogen sulfide has been considered as a phytotoxin since long time, for its deleterious effects on plant growth (Lisjak et al. 2013). It is lipophilic in nature and can easily cross the cell membrane and its effects on the two important PTMs processes (persulfidation and S-nitrosation) by reaction with Cys-protein thiol group. These proteins regulate the productivity of redox species and signaling (Spadaro et al. 2010). Oxidation state of S varies from − 2 (thiol) to + 4 (sulfonic acid) and its variation further depends upon ROS contents.
In 1975, it was first identified that at toxic level, H2S gas is detrimental for plant health. Joshi et al. (1975) reported inhibited O2 release from six rice cultivar seedlings, when exposed to H2S. In few cultivars, it also negatively affected on plant nutritional status especially phosphorous nutrition. Continuous fumigation of H2S at 3000 ppb on Medicago, sugar beet, pine, lettuce, and grapes caused leaf lesions leading to defoliation (Thompson and Kats 1978a, b). Defoliation is caused by mutation in two T-DNA, des1-1 and des 1–2 of Arabidopsis thaliana plants. Enzymes responsible for production of H2S in plants is l-cysteine desulfhydrase, and increased activity of this enzyme alters the gene expression and transcription factor which are associated with the leaf senescence due to increased cysteine concentration in plant leaf (Alvarez et al. 2010).
Furthermore, studies also reported the cross talk between H2S and NO, which is toxic at higher concentration (Wang 2003). H2S promotes the production of NO which controls the stomatal closure process by regulating the abscisic acid concentration (Jin et al. 2013). Increased H2S concentration in plant tissues also reduced the auxin translocation and synthesis followed by modulation in PIN proteins distribution resulting in restricted root growth (Jia et al. 2015). The cytochrome oxidase is an crucial enzyme in mitochondrial respiration which is involved in electron transport chain. The inhibition of cytochrome oxidase activity due to H2S had been recognized in experiments conducted for rice (Xiao et al. 2010).
Role of H2S Systems in Morpho-Physiological Processes
In recent years, H2S started to be considered as a signaling molecule in various physiological functions. It is now considered as third gaseous signaling molecule after CO2 and NO. From seed germination to maturity, it is thought to be involved in various physiological functions in aerobic and anaerobic organisms (Corpas and Palma 2020). In studies, a very narrow concentration of H2S application to plants showed positive response which showed that H2S acts as signaling molecule in plants (Joshi et al. 2020; Zhang et al. 2020). It actively takes part in various physiological processes like photosynthesis, defense and growth metabolism. Studies reported that it increases plant tolerance from heat, heavy metal stress, and nutrient stress by increasing photosynthesis rate via increasing ribulose-1, 5-bisphosphate carboxylase activity (Chen et al. 2011; Arnao and Hernandez-Ruiz 2015). It also improves stomatal density which further affects positively the photosynthesis rate. Seedlings treated with 0.01 mM H2S reduce photorespiration and increase stomatal density which leads to high photosynthesis activity. Photorespiration is reduced due to the downregulation of Glycolate oxidase, enzyme involved in C2 cycle in photorespiration (Duan et al. 2015).
Studies showed that H2S provides S to protein thiol group (-SH) and produces persulphide group (-SSH). This played a vital role in modulating the responses of selected proteins (Aroca et al. 2018). NaHS application, which is a donor of H2S improves seed germination rate by upregulating β-amylase activity in endosperm and downregulating the MDA and H2O2 contents. Increased starch hydrolysis by β-amylase increased the seed germination rate (Zhang et al. 2008, 2010). Exogenous H2S provoke some signaling molecules which increased cell division rate in roots and increased adventitious root growth.
Exogenous H2S application alleviates biotic and abiotic stress in plants. Earlier research only associated it with defense system in plants during pathogenic attacks (Bloem et al. 2004). Later in 2008, study revealed its protective effect against copper (Cu) toxicity (Zhang et al. 2008). After that, studies showed positive response of H2S application in alleviating the metal stress (e.g., aluminum, chromium, zinc, boron, and cadmium), drought, temperature, and nutrient stress in various plants as described in Table 2. H2S regulates homeostasis process in cells. During drought stress, it accumulates osmolytes proline and trehalose, which protect plants from drought effects. It is also involved in the stomata closing process, reducing the amount of water transpired by the plant under both normal and stressed conditions, thus increasing overall photosynthesis efficiency (Iqbal 2018). H2S also mediates stomatal opening by downregulating the ethylene-induced NO accumulation in stomatal cells (Liu et al. 2011; Iqbal 2018).
Several studies pointed to an inter-relationship between H2S and other plant hormones such as gibberellic acid, abscisic acid, and ethylene and modulate their signaling process (Hasanuzzaman et al. 2018). H2S controls the induction of abscisic acid (ABA) in plants. Studies highlighted the effect of increased ABA synthesis at high H2S concentration (Jin et al. 2011). High atmospheric or intracellular H2S concentration in plants influences ABA receptor expression and upstreams the ABA accumulation. This initiates signals for stomata closure, reduced water loss, and protection of plants from drought (Jin et al. 2013; Antoniou et al. 2020). Exposure of plant to ethylene initiates H2S synthesis in plants (Liu et al. 2011). Exposure of some plants with H2S increases indole acetic acid production (Zhang et al. 2009a, b). However, H2S alleviates the negative effect of jasmonic acid and gibberellic acid through signal transduction process (Xie et al. 2014; Hou et al. 2011). Jasmonic acid increases H2O2 contents in plants causing closure of stomata. H2O2 is another signaling molecule inter-related with H2S. There are plenty of evidences that proved these both signaling molecules have antagonistic effects (Hou et al. 2011), but few studies showed that seed priming with H2O2 improves H2S contents in endosperm and increases germination rate of Jatropha curcas (Li et al. 2012a, b). However, under plant stress conditions, H2S improves antioxidant system (Tang et al. 2020; Zhang et al. 2020).
Contrary effect of H2S is also reported with NO (signaling molecule). In Arabidopsis thaliana, application of NaSH reduced NO accumulation in epidermal cells due to increased H2S activity. Molecular analysis denoted that both NaSH and GYY4137 reduced the NO activity up to a large extent (Lisjak et al. 2010). H2S also inter-relates with Ca-signaling pathways. Calcium maintains the permeability and rigidity of cell wall. Pre-treatment of tobacco with NaSH improves Ca2+ uptake in plants through upstreaming of calmodulin (Li et al. 2013). Under metal toxicity, H2S ameliorates the plant tolerance by increasing proline contents. In short, H2S protects plant against biotic and abiotic stress and improves the growth of plants (Fu et al. 2019).
Hydrogen Sulfide and Abiotic Stress
Climate change is one of the significant factors that affect plant health and results in a decrease in production. Climate change imposes different environmental stresses on plants, i.e., salt stress, water deficit, extreme temperatures, and nutritional imbalance (Khalid et al. 2019). When the plants are exposed to these kinds of stresses, they generally increase the production and accumulation of reactive oxygen species (ROS), i.e., hydroxyl radicals, hydrogen peroxide, and superoxide ions in cells. To make the equilibrium in the cells, plant increases the activity of specific antioxidant enzymes, osmolytes, which decrease the accumulation of ROS (Suzuki et al. 2012).
In previous years, several reports have emphasized the impact of exogenous application of hydrogen sulfide on plants and their response to different environmental stresses, mainly its contribution in the regulation of cell signaling metabolism, upregulation, downregulation of gene expressions, and the activation of different antioxidant enzymes and osmolytes (Singh et al. 2019). Previous scientists also described a swift accumulation of endogenous hydrogen sulfide under different abiotic stress conditions to levels that trigger physiological responses (Fang et al. 2014; Lai et al. 2014). However, the molecular responses and signal transduction metabolism of hydrogen peroxide in plants are still elusive. The information on these metabolisms and the alterations that are carried out in different environmental conditions is essential to recognize how plants adequately respond to their environment.
Low temperature or cold stress significantly affects the plant's growth and development directly by constraining enzyme activities and metabolic reactions and, indirectly, through cold-induced osmotic stress (inhibition of water uptake and cellular dehydration) and oxidative stress (Chinnusamy et al. 2007). Hydrogen sulfide was stated to alleviate osmotic and oxidative stresses induced under cold or low-temperature stress by modifying the antioxidant enzymes activities, accelerates the accumulation of osmoprotectants (proline, total soluble sugars), and decreases cell membrane permeability (Shi et al. 2013). The mitogen-activated protein kinase (MAPK) pathway was also observed to be involved in the hydrogen sulfide-mediated response of Arabidopsis seedlings to low-temperature stress, with a specific effect on stomatal opening.
High temperature, water deficiency, and salt stress have reached an alarming concern in the context of environmental change (Hussain et al. 2018; Khalid et al. 2020). High temperature strictly affects plant growth and development, resulting in a substantial decline in yield (Akter and Islam 2017). The tolerance of plants against high temperatures is also improved by hydrogen sulfide. Numerous studies have delivered confirmation that the addition of sodium hydrogen sulfide, a hydrogen sulfide donor, improves plant germination and tissue viability (Li et al. 2013) and decreases the adverse phenotypic effects of high temperature such as wilting and curling of leaves (Christou et al. 2014). Different mechanisms of different plants against heat stress were reported: in corn, the hydrogen sulfide-induced tolerance, which was arbitrated by an increase in salicylic acid (SA) and proline (Li et al. 2015); in tobacco, it was suggested that hydrogen sulfide adjusts the entry of extracellular calcium ions across the plasma membrane by a direct effect on calmodulin (ubiquitous calcium-binding protein) (Li et al. 2012a, b). In strawberry, hydrogen sulfide alleviated oxidative stress. It helped to preserve root tissues against heat-induced damage by inducing gene expression of several antioxidants and heat shock proteins, including catalase, superoxide dismutase, HSP70, HSP80, and HSP90 (Christou et al. 2014).
The involvement of hydrogen sulfide in plant responses to water deficit has also been widely evaluated. It mainly depends on how much time and what concentration of heat was applied, hydrogen peroxide may encourage the closure or aperture of stomata. Both effects are mediated by a signaling molecule and involves the activity of calcium ion, cADP ribose, and slows anion channel 1 (Honda et al. 2015).
By knocking down the l-cysteine desulfhydrase in Arabidopsis plants, Jin et al. (2013) studied the function of hydrogen sulfide in stomatal movement and the relationship between hydrogen sulfide and abscisic acid metabolism in signaling transduction. They concluded that hydrogen sulfide is involved in the expression regulation of abscisic acid receptor candidates and potassium ions and calcium ion channels in guard cells. In addition to stomatal movement, numerous works have reported that hydrogen sulfide helps to provide tolerance to drought through the accumulation of osmolytes like proline and the association of calcium messenger system (Li et al. 2014).
Heavy metals such as cadmium, chromium, copper, and zinc are highly toxic for plants. Their accumulation in the intracellular compartments can cause DNA damage, enzyme inactivation, protein oxidation, and lipid peroxidation. The role of hydrogen sulfide in plant response to heavy metal stress has been recently reviewed by Hancock and Whiteman (2015) and Li et al. (2016b) (Table 3). In cucumber seedlings, pre-treatment with sodium hydrogen sulfide altered the expression of cell wall-associated proteins such as PME (CsPME) and expansin (CsExp), leading to the alleviation of boron-induced toxicity. Similarly, sodium hydrogen sulfide alleviated the inhibitory effects of copper and reduced the visible symptoms in germinated seeds and radical tips.
The roots are important plant organs for anchoring and nutrient and water uptake. Therefore, fast growth and development of root is essential for crop survival in stressful conditions. The knowledge about impact of H2S on roots formation and growth pattern is very important for stress tolerance. Several researchers reported positive impacts of application of this molecule on root of raddish (Carter et al. 2018), sweet potato, willow and soybean (Zhang et al. 2009a, b), and Chinese crab apple (Wei et al. 2017) and these are mainly as a result of H2S interaction with Indole Acetic Acid (IAA), Nitric Oxide (NO), Hydrogen per Oxide (H2O2), and Malondialdehyde (MDA) (Zhang et al. 2009a, b, Mei et al. 2017, Ma et al. 2016a, b). The pretreated seedlings with NaHS upregulated abscisic acid (ABA) biosynthesis in roots during drought. Moreover, the exogenous application of ABA increased the H2S contents in the roots in drought, and it showed cross talks between these two molecules (Ma et al. 2016a, b). The presence of excessive salt in soil resulted in salinity stress which disupts nutrients and water uptake along with oxidative damages and produces poor yield. The H2S mediates the NO production which is further utilized for maintaining ion (K+/Na+) homeostatis to improve salt tolerance (Chen et al. 2015).
Molecular Mechanism and Biosynthesis of H2S in Plants
Hydrogen sulfide gaseous signaling molecule is involved in the growth and development of plants. It regulates different physiological processes in plants, including germination, lateral and adventitious root formation, stomatal conductance and photosynthesis (Duan et al. 2015; Jia et al. 2015; Liu and Lal 2015; Jin and Pei 2016; Li et al. 2016a, b). Plant response to abiotic stress is also regulated by H2S and thus considered as versatile regulator (Guo et al. 2015). The emission of H2S in plant was first observed by DeCormis in 1968 (Rennenberg, 1989). Exposure of some plants e.g., pumpkin, cantaloupe, corn, cucumber, and soybean to light results in the synthesis of H2S as reported by Wilson et al. (1978). Similarly, emission of H2S in cucumber leaf tissue was reported earlier (Sekiya et al. 1982).
H2S is recognized as an important secondary messenger in various plant developmental processes, stress responses, and due to its small size it can easily navigate between cells. Thus, it behaves as a gasotransmitter at lower cellular concentrations. It does not require any transporter assistance; therefore, it can move through hydrophobic plasma and organelle membranes (Mathai et al. 2009; Shivaraj et al. 2020). Hydrogen sulfide accumulation occurs in chloroplasts via a photosynthetic sulfate assimilation pathway, and the reaction is triggered by sulfide reductase (SiR) (Garcia et al. 2015). The cytosolic hydrogen sulfide is accumulated mainly with the action of the enzyme l-cysteine desulhydrase 1 (DES1), and it is of interest that the sulfide concentration in chloroplasts is always higher than in cytosol (Kabil and Banerjee 2010).
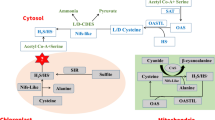
Multiple enzymatic systems are involved in the biosynthesis of H2S in plants. According to Aroca et al. (2015), H2S production mainly occurs in the chloroplast and partly in the mitochondria and cytosol. It includes sulfite reductase (SiR), l‐Cysteine desulfhydrase (LCD), d‐Cysteine desulfhydrase (DCD), cysteine synthase (CS), beta‐cyanoalanine synthase (CAS), carbonic anhydrase, Nitrogenase Fe-S cluster (NFS 1& 2), O-acetyl-l-serine (thiol) lyase (OASTL), and Desulfhydrase (DES1). The enzymatic role in various steps of synthesis of H2S is shown in Fig. 4. l‐Cysteine desulfhydrase is the major player involved in the production of H2S as it catalyzes the conversion of l-Cys to pyruvate, NH4+ , and H2S as shown in Fig. 4. The cofactor in this reaction is pyridoxal phosphate (Gotor et al. 2015). Similarly, d-Cys is converted to pyruvate, ammonia, and H2S in the cytoplasm through DCD (Riemenschneider et al. 2005).
Until today, there are six different pathways reported in the plants through which H2S has been generated. Firstly, SO42− is reduced to S2− and then it became a part of organic metabolites. Before this reduction reaction, SO42− is activated by adenylation to form adenosine 5ʹ phosphosulfate (APS), which is catalyzed by ATP sulfurylase (ATPS). APS reductase (APR) enzyme in plastid converts APS to sulfite that is further reduced to sulfide and H2S through ferredoxin-dependent sulfite reductase (SiR) enzyme (Fig. 4). At the end, sulfide is incorporated into amino acids skeleton of OAS (O-acetylserine) by OAS-TL (O-acetylserine thiol lyase) to form cysteine. The revers of this reaction generates H2S (Li 2015). In these all reactions, OAS is product of serine and catalyzed by SAT (serine acetyltransferase). H2S, pyruvate, and NH4+1 synthesis due to the degradation of l-Cysteine were first reported by Harrington and Smith (1980). Xie et al. (2013) found that H2S in higher plants was produced by enzymatic reaction of LCD on substrate l-cysteine. Furthermore, l-cysteine was converted to l-alanine and elemental S through the action of enzyme LCD. The elemental S could be further reduced to H2S if reductant is present (Papenbrock et al. 2007a, b).
Léon et al. (2002) reported two genes (AtNFS1 and AtNFS2) that encoded NifS-like cysteine desulfurase catalyzing cysteine to form H2S. d-cysteine could also be catalyzed by DCD to generate H2S as reported by Riemenschneider et al. (2005) in their study on Arabidopsis thaliana. The subcellular localization, enzymatic inhibitors, and substrates for both LCD and DCD seem different. Another enzyme, i.e., b-cyanoalanine synthase (CAS) can also act on l-Cysteine and cyanide to form H2S and b-cyanoalanine as reported by Hatzfeld et al. (2000). Similarly, it has been found that three genes, i.e., CYSC1,CYS-D1, and CYS-D2 encode enzyme CAS that confirm that l-Cysteine is involved in the synthesis of H2S (Jost et al. 2000). The enzyme cyanoalanine synthase cI (CAS-CI) also produces hydrogen sulfide in the mitochondria during the synthesis of β-cyanoalanine (Yamaguchi et al. 2000).
Hydrogen Sulfide and its Interaction with Other Signaling Molecules During Plant Abiotic Stress
Hydrogen sulfide has been considered as a toxic gas for living organisms; however, it has emerged as a new molecule in living cells for signaling, considering equally crucial as hydrogen peroxide (H2O2), carbon monoxide (CO), and nitric oxide (NO) (Kimura 2014). Hydrogen sulfide is a major component in plant responses against stress. Many stresses may result in an increase in hydrogen sulfide in plants, and this may result in the mediation of cell stress by different cellular metabolisms (Fig. 5).
Numerous reports have been available on this gasotransmitter related to the growth of vegetative plants and affecting the disease resistance, protective roles against stresses such as oxidative stresses (Fang et al. 2016), drought and heat tolerance (Li et al. 2012a, b; Shen et al. 2013), osmotic and saline stresses, stomatal closure/aperture (Papanatsiou et al. 2015), modulating in photosynthetic machinery (Chen et al. 2011), and autophagy regulation (Laureano-Marin et al. 2016).
Moreover, hydrogen sulfide is a reactive molecule to alter the signals concerning the plant hormones (Liu et al. 2011; Lisjak et al. 2010a; Zhang et al. 2010). Concerning the primary mechanism of action, a post-translational modification (PTM) of proteins (persulfidation) is formed when the conversion of the thiol group (−SH) into the persulfide group (−SSH) occurred at the reactive cysteine residues on the target proteins. Persulfidation showed an increased nucleophilicity than the thiol group; thus, modified cysteines demonstrated a more significant challenge activity leading to a higher percentage of proteins compared to the reactive nitrogen and oxygen species. The modified targets of hydrogen sulfide is unclear; however, the sulfane-sulfur atom has a unique ability to bind reversibly to other sulfur atoms to generate hydrosulfides and polysulfides. Polysulfides seemed to be effective in the persulfidation since these are more nucleophilic than hydrogen sulfide (Toohey 1989). These newly emerged low molecular weight compound persulfides are the potential mediators in sulfide signaling. In conclusion, the extent of the interaction between signaling molecules needs more study on the biochemical cascade triggered in the plant cells toward different stress conditions. Hydrogen peroxide molecules are small in size and have ability to cross by cellular membranes and its movement also occurs through aquaporin channels in different compartments of cells. Hydrogen sulfide is also involved in feeding of electrons in complex II of mitochondria by enzyme quinone oxidoreductase. Hydrogen sulfide also involved in regulation of phosphorylation of the plasma membrane to maintain homeostasis of ions (Li et al. 2013).
The optimum concentration of hydrogen sulfide will increase growth and activate different metabolisms. However, variation in the concentration of hydrogen sulfide will ultimately restrict the development of primary roots (Zhang et al. 2017). Hydrogen sulfide toxicity repressed primary root growth by triggering a signal transduction pathway involving reactive oxygen species (ROS) accumulation, MITOGEN-ACTIVATED PROTEIN KINASE 6 (MPK6) activation,, and nitric oxide (NO) production.
Conclusion and Future Perspectives
The role of H2S is alleviating various stresses through endogenous production and exogenous application is quite clear. The literature highlighted that advent of biotic and abiotic stress stimulates the generation of H2S to provide protection against damages imposed by stress and is even helpful for protection against diseases. There is detailed description of alleviating the role of H2S against heavy metals, salinity, drought, and water logging and others. It is a potential tool for preservation of post-harvest fruit quality.
The phytotoxic and beneficial impacts of H2S largely depend upon the dose, the detailed research on dose standardization for various crops should be focused for its practical application in agriculture. It is still unknow that the actual site of H2S production in plants and its interaction with other molecules in plant metabolism (Corpas and Palma 2020). The H2S and NO displayed interplay with each other and biochemical basis of interplay still need to be worked out for deep understanding of the phenomenon. Suppose H2S is endogenously produced as signaling molecules, then it should be measured. Furthermore, considering H2S signaling molecules, its production should be stopped when not needed. Because plants remove H2S when it is just a pollutant, the process of removal of H2S still needs to be studied (Lisjak et al. 2013).
Although H2S is widely being used for improving post-harvest quality of climacteric and non-climacteric fruits, understanding the mechanism of signaling H2S with fruit ripening is required for future investigation (Ziogas et al. 2018). Although detail studies on role of H2S in fruits have been carried out and its practical application has been suggested regarding preservation of post-harvest fruit quality, the exact biochemical basis of interaction of H2S with NO should be exploited (Ziogas et al. 2018). The levels of biological sulfide measuring techniques differ greatly according to testing procedure. The future investigation is required for uniform readings. Keeping in view the phytotoxicity, the crops like brassica species may be grown in areas where H2S concentration is high in atmosphere (Ausma and Kok 2019).
Abbreviations
- ABA:
-
Abscisic acid
- CAS:
-
Cyano alanine synthase
- CS:
-
Cysteine synthase
- Cys:
-
Cysteine
- DES1:
-
l-Cysteine Desulhydrase
- DMS:
-
Dimethyl sulfide
- HAST:
-
High affinity transport proteins
- LAST:
-
Low affinity transport proteins
- LCDSH/DCDSH:
-
l- and d-cysteine desulfhydrase
- MAPK:
-
Mitogen-activated protein kinase
- PTMs:
-
Persulfidation and S-nitrosation
- SR:
-
Sulfite reductase
References
Aiuppa A, Inguaggiato S, McGonigle AJS, O’Dwyer M, Oppenheimer C, Padgett MJ, Rouwet D, Valenza M (2005) H2S fluxes from Mt. Etna, Stromboli, and Vulcano (Italy) and implications for the sulfur budget at volcanoes. Geochim Cosmochim Acta 69:1861–1871
Akter N, Islam MR (2017) Heat stress effects and management in wheat. A review. Agron Sustain Dev 37:37
Alvarez C, Calo L, Romero LC, Garcia I, Gotor C (2010) An O-acetylserine (thiol) lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol 152:656–669
Andreae MO (1990) Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar Chem 30:1–29
Antoniou C, Xenofontos R, Chatzimichail G, Christou A, Kashfi K, Fotopoulos V (2020) Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomoleclues 10(1):E120
Arnao MB, Hernandez-Ruiz J (2015) Functions of melatonin in plants: a review. J Pineal Res 59:133–150
Aroca Á, Serna A, Gotor C, Romero LC (2015) S-sulfhydration: a cysteine post-translational modification in plant systems. Plant Physiol 168:334–342
Aroca A, Gotor C, Romero LC (2018) Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front Plant Sci 9:1369
Ausma T, De Kok LJ (2019) Atmospheric H2S: impact on plant functioning. Front Plant Sci 10:743
Bharwana SA, Ali S, Farooq MA, Ali B, Iqbal N, Abbas F, Ahmad MSA (2014a) Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ Sci Pollut Res 21:717–731
Bharwana SA, Ali S, Farooq MA, Ali B, Iqbal N, Abbas F, Ahmad MSA (2014b) Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ Sci Pollut Res 21(1):717–731
Bhomick PC, Rao KS (2014) Sources and effects of hydrogen sulfide. J Appl Chem 3(3):914–918
Bloem E, Riemenschneider A, Volker J, Papenbrock J, Schmidt A, Salac I, Haneklaus S, Schnug E (2004) Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J Exp Bot 55(406):2305–2312
Brace EC, Engelberth AS (2020) Assessing viability of soybean oils to remove hydrogen sulfide from natural gas. ACS Sustain Chem Eng 8:9377
Cao M-J, Wang Z, Wirtz M, Hell R, Oliver DJ, Xiang C-B (2013) SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J 73:607–616
Carter JM, Brown EM, Grace JP, Salem AK, Irish EE, Bowden NB (2018) Improved growth of pea, lettuce, and radish plants using the slow release of hydrogen sulfide from GYY-4137. PLoS ONE 13(12):e0208732
Chao TT, Harward ME, Fang SC (1962) Adsorption and desorption phenomena of sulfate ions in soils. Soil Sci Soc Am J 26:234–237
Chen J, Wu FH, Wang WH, Zheng CJ, Lin GH, Dong XJ, He JX, Pei ZM, Zheng HL (2011) Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot 62:4481–4493
Chen J, Wang W-H, Wu F-H, He E-M, Liu X, Shangguan Z-P, Zheng H-L (2015) Hydrogen sulfide enhances salt tolerance through nitric oxide mediated maintenance of ion homeostasis in barley seedling roots. Sci Rep 5:12516
Chen X, Chen Q, Zhang X, Li R, Jia Y, Ef A, Jia A, Hu L, Hu H (2016) Hydrogen sulfide mediates nicotine biosynthesis in tobacco (Nicotiana tabacum) under high temperature conditions. Plant Physiol Biochem 104:174–179
Cheng W, Zhang L, Jiao C, Su M, Yang T, Zhou L, Peng R, Wang R, Wang C (2013) Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol Biochem 70:278–286
Christou A, Manganaris GA, Papadopoulos I, Fotopouls V (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64:1953–1966
Chinnusamy V, Zhu J, Zhu J-K (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12:444–451
Christou A, Manganaris G, Papadopoulos I, Fotopoulos V (2011) Hydrogen sulfide confers systemic tolerance to salt and polyethylene glycol stress in strawberry plants. In: Abstracts book of the 10th International Conference on Reactive Oxygen and Nitrogen Species in Plants, 5–8 July 2011, Budapest, Hungary, p 159
Christou A, Filippou P, Manganaris GA, Fotopoulos V (2014) Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol 14:42
Corpas FJ, Palma JM (2020) H2S signaling in plants and applications in agriculture. J Adv Res 24:131–137
De Kok LJ, Thompson CR, Kuiper PJC (1983) Sulfide-induced oxygen uptake by isolated spinach chloroplasts catalyzed by photosynthetic electron transport. Physiol Plant 59:19–22
Deng YQ, Bao J, Yuan F, Liang X, Feng ZT, Wang BS (2016) Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul 79:391–399
Dooley FD, Nair SP, Ward PD (2013) Increased growth and germination success in plants following hydrogen sulfide administration. PLoS ONE 8:e62048
Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF (2002) Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci 65:18–25
Duan B, Ma Y, Jiang M, Yang F, Ni L, Lu W (2015) Improvement of photosynthesis in rice (Oryza sativa L) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul 75(1):33–44
Fang H, Jing T, Liu Z, Zhang L, Jin Z, Pei Y (2014) Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 56:472–481
Fang H, Liu Z, Jin Z, Zhang L, Liu D, Pei Y (2016) An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ Pollut 213:870–877
Filipovic MR, Jovanović VM (2017) More than just an intermediate: hydrogen sulfide signalling in plants. J Exp Bot 68(17):4733–4736
Fu PN, Wang WJ, Hou LX, Liu X (2013) Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc Bot Pol 82:295–302
Fu MM, Dawood M, Wang NH, Wu F (2019) Exogenous hydrogen sulfide reduces cadmium uptake and alleviates cadmium toxicity in barley. Plant Growth Regul 89(2):227–237
Fuentes-Lara LO, Medrano-Macías J, Pérez-Labrada F, Rivas-Martínez EN, García-Enciso EL, González-Morales S, Juárez-Maldonado A, Rincón-Sánchez F, Benavides-Mendoza A (2019) From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 24:2282
Garcia I, Gotor C, Romero LC (2015) Cysteine homeostasis. In: D’mello JPF (ed) Amino acids in higher plants. CABI Publishing, Wallingford, pp 219–233
Ge Y, Hu KD, Wang SS, Hu LY, Chen XY, Li YH, Yang Y, Yang F, Zhang H (2017) Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE 12:e0180113
González-Morales S, Pérez-Labrada F, García-Enciso EL, Leija-Martínez P, Medrano-Macías J, Dávila-Rangel IE, Juárez-Maldonado A, Rivas-Martínez EN, Benavides-Mendoza A (2017) Selenium and sulfur to produce allium functional crops. Molecules 22(4):558
Goodwin MJ, Musa OM, Steed JW (2015) Problems associated with sour gas in the oilfield industry and their solutions. Energy Fuel 29(8):4667–4682
Gotor C, Laureano-Marín AM, Moreno I, Aroca Á, García I, Romero LC (2015) Signaling in the plant cytosol: cysteine or sulfide? Amino Acids 47:2155–2164
Guo H, Xiao T, Zhou H, Xie Y, Shen W (2015) Hydrogen sulfide: a versatile regulator of environmental stress in plants. Acta Physiol Plant 38:16
Guo Z, Liang Y, Yan J, Yang E, Li K, Xu H (2018) Physiological response and transcription profiling analysis reveals the role of H2S in alleviating excess nitrate stress tolerance in tomato roots. Plant Physiol Biochem 124:59–69
Hancock JT, Whiteman M (2015) Hydrogen sulfide and reactive friends: the interplay with reactive oxygen species and nitric oxide signalling pathways. In: Hawkesford M, Rennenberg H, Saito K, Schnug E (eds) Molecular physiology and ecophysiology of sulfur. Springer, Cham, pp 153–168
Hansen MH, Ingvorsen K, Jorgensen BB (1978) Mechanisms of hydrogen sulfide release from coastal marine sediments to the atmosphere. Limnol Oceanogr 23:68–76
Harrington HM, Smith IK (1980) Cysteine metabolism in cultured tobacco cells. Plant Physiol 65:151–155
Hasanuzzaman M, Bhuyan MHMB, Mahmud JA, Nahar K, Mohsin SM, Parvin K, Fujita M (2018) Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal Behav 13:1477905
Hatzfeld Y, Maruyama A, Schmidt A, Noji M, Ishizawa K, Saito K (2000) β-Cyanoalanine synthase is a mitochondrial cysteine synthase-like protein in spinach and arabidopsis. Plant Physiol 123:1163–1172
Honda K, Yamada N, Yoshida R, Ihara H, Sawa T, Akaike T, Iwai S (2015) 8-Mercapto-cyclic GMP mediates hydrogen sulfide-induced stomatal closure in Arabidopsis. Plant Cell Physiol 56:1481–1489
Hou Z, Liu J, Hou L, Li X, Liu X (2011) H2S may function downstream of H2O2 in jasmonic acid-induced stomatal closure in Vicia faba. Chin Bull Bot 46(4):396–406
Hu LY, Hu SL, Wu J, Li YH, Zheng JL, Wei ZJ, Liu J, Wang HL, Liu YS, Zhang H (2012) Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J Agric Food Chem 60(35):8684–8693
Hussain S, Khalid MF, Saqib M, Ahmad S, Zafar W, Rao MJ, Morillon R, Anjum MA (2018) Drought tolerance in citrus rootstocks is associated with better antioxidant defense mechanism. Acta Physiol Plant 40(8):135
Iqbal MJ (2018) Role of osmolytes and antioxidant enzymes for drought tolerance in wheat. Global Wheat Prod. https://doi.org/10.5772/intechopen.75926
Jia H, Hu Y, Fan T, Li J (2015) Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci Rep 5:8251
Jin Z, Pei Y (2016) Hydrogen sulfide: the shutter button of stomata in plants. Sci China Life Sci 59:1187–1188
Jin Z, Shen J, Qiao Z, Yang G, Wang R, Pei Y (2011) Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Comm 414(3):481–486
Jin Z, Xue S, Luo Y, Tian B, Fang H, Li H, Pei Y (2013) Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62:41–46
Johnson DW, Cole DW (1980) Anion mobility in soils: relevance to nutrient transport from forest ecosystems. Environ Int 3:79–90
Joshi MM, Ibrahium IKA, Hollis JP (1975) Hydrogen sulphide: effects on the physiology of rice plants and relation to straighthead disease. Phytophatology 65:1165–1170
Joshi NC, Yadav D, Ratner K, Kamara I, Aviv-Sharon E, Irihimovitch V, Charuvi D (2020) Sodium hydrosulfide priming improves the response of photosynthesis to overnight frost and day high light in avocado (Persea americana Mill, cv. ‘Hass’). Physiol Plant 168(2):394–405
Jost R, Berkowitz O, Wirtz M, Hopkins L, Hawkesford MJ, Hell R (2000) Genomic and functional characterization of the oas gene family encoding O-acetylserine (thiol) lyases, enzymes catalyzing the final step in cysteine biosynthesis in Arabidopsis thaliana. Gene 253:237–247
Kabil O, Banerjee R (2010) Redox biochemistry of hydrogen sulfide. J Biol Chem 285:21903–21907
Kataoka T, Hayashi N, Yamaya T, Takahashi H (2004) Root-to-shoot transport of sulfate in arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol 136:4198–4204
Kaya C, Ashraf M (2019) The mechanism of hydrogen sulfide mitigation of iron deficiency-induced chlorosis in strawberry (Fragaria × ananassa) plants. Protoplasma 256:371–382. https://doi.org/10.1007/s00709-018-1298-x
Khalid MF, Hussain S, Ahmad S, Ejaz S, Zakir I, Ali MA, Ahmed N, Anjum MA (2019) Impacts of abiotic stresses on growth and development of plant. Plant tolerance to environmental stress. CRC Press, Boca Raton, pp 1–8
Khalid MF, Hussain S, Anjum MA, Ahmad S, Ali MA, Ejaz S, Morillon R (2020) Better salinity tolerance in tetraploid vs diploid volkamer lemon seedlings is associated with robust antioxidant and osmotic adjustment mechanisms. J Plant Physiol 244:153071
Kharbech O, Houmani H, Chaoui A, Corpas FJ (2017) Alleviation of Cr(VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPHrecycling metabolisms. J Plant Physiol 219:71–80
Kimura H (2014) The physiological role of hydrogen sulfide and beyond. Nitric Oxide 41:4–10
Kirschner S, Woodfield H, Prusko K, Koczor M, Gowik U, Hibberd JM, Westhoff P (2018) Expression of SULTR2;2, encoding a low-affinity sulphur transporter, in the Arabidopsis bundle sheath and vein cells is mediated by a positive regulator. J Exp Bot 69:4897–4906
Kourtidis K, Kelesis A, Petrakakis M (2008) Hydrogen sulfide (H2S) in urban ambient air. Atmos Environ 42(32):7476–7482
Lai D, Mao Y, Zhou H, Li F, Wu M, Zhang J, He Z, Cui W, Xie Y (2014) Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci 225:117–129
Laureano-Marin AM, Moreno I, Romero LC, Gotor C (2016) Negative regulation of autophagy by sulfide is independent of reactive oxygen species. Plant Physiol 171:1378–1391
Léon S, Touraine B, Briat J-F, Lobréaux S (2002) The AtNFS2 gene from Arabidopsis thaliana encodes a NifS-like plastidial cysteine desulphurase. Biochem J 366:557–564
Li ZG (2015) Analysis of some enzymes activities of hydrogen sulfide metabolism in plants. In: Cadenas E, Packer L (eds) Methods in enzymology. Academic Press, Cambridge, pp 253–269
Li ZG, Gong M, Liu P (2012a) Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha Curcas”. Acta Physiol Plantarum 34(6):2207–2213
Li ZG, Gong M, Xie H, Yang L, Li J (2012b) Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci 185:185–189
Li ZG, Ding XJ, Du PF (2013) Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747
Li SP, Hu KD, Hu LY, Li YH, Jiang AM, Xiao F, Han Y, Liu YS, Zhang H (2014) Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J Agric Food Chem 62(5):1119–1129
Li ZG, Xie LR, Li XJ (2015) Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J Plant Physiol 177:121–127
Li Q, Wang Z, Zhao Y, Zhang X, Zhang S, Bo L, Wang Y, Ding Y, An L (2016a) Putrescine protect shul less barley from damage due to UV-B stress via H2S-and H2O2-mediated signaling pathways. Plant Cell Rep 35:1155–1168
Li ZG, Min X, Zhou ZH (2016b) Hydrogen sulfide: a signal molecule in plant cross-adaptation. Front Plant Sci 7:1621
Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, Wilson I, Wood ME, Whiteman M, Hancock JT (2010a) A novel hydrogen sulphide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem 48:931–935
Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT (2013) Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ 36:1607–1616
Liu R, Lal R (2015) Effects of low-level aqueous hydrogen sulfide and other sulfur species on lettuce (Lactuca sativa) seed germination. Commun Soil Sci Plant Anal 46:576–587
Liu J, Hou L, Liu G, Liu X, Wang X (2011) Hydrogen sulfide induced by nitric oxide mediates ethylene-induced stomatal closure of Arabidopsis thaliana. Chin Sci Bull 56(33):3547–3553
Ma Q, Yang J (2018) Transcriptome profiling and identification of functional genes involved in H2S response in grapevine tissue cultured plantlets. Genes Genomics 40(12):1287–1300
Ma L, Yang L, Zhao J, Wei J, Kong X, Wang C, Zhang X, Yang Y, Hu X (2015) Comparative proteomic analysis reveals the role of hydrogen sulfide in the adaptation of the alpine plant Lamiophlomis rotate to altitude gradient in the Northern Tibetan Plateau. Planta 241:887–906
Ma D, Ding H, Wang C, Qin H, Han Q, Hou J, Lu H, Xie Y, Guo T (2016a) Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS ONE 11(9):e0163082
Ma D, Ding H, Wang C, Qin H, Han Q, Hou J et al (2016b) Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS ONE 2016(11):e0163082
Ma X, Zheng G, Liang M, Xie D, Martinelli G, Sajjad W, Xu W, Fan Q, Li L, Du L, Zhao Y (2019) Occurrence and origin of H2S from volcanic reservoirs in Niudong area of the Santanghu Basin, NW China. Geofluids 2019:1279658
Mostofa MG, Rahman A, Ansary MMU, Watanabe A, Fujita M, Tran LP (2015) Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep 5:14078
Martin NM, Maricle BR (2015) Species-specific enzymatic tolerance of sulfide toxicity in plant roots. Plant Physiol Biochem 88:36–41
Maruyama-Nakashita A (2017) Metabolic changes sustain the plant life in low-sulfur environments. Curr Opin Plant Biol 39:144–151
Mathai JC, Missner A, Kügler P, Saparov SM, Zeidel ML, Lee JK, Pohl P (2009) No facilitator required for membrane transport of hydrogen sulfide. PNAS 106:16633–16638
Muñoz-Vargas MA, González-Gordo S, Cañas A, López-Jaramillo J, Palma JM, Corpas FJ (2018) Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide 81:36–45
Nawaz F, Majeed S, Aqib M, Ahmad KS, Ghaffar A, Usmani MM, Shabbir RN, Shafiq BA (2020) Sulfur-mediated physiological and biochemical alterations to improve abiotic stress tolerance in food crops. In: Hasanuzzaman M (ed) Plant ecophysiology and adaptation under climate change: mechanisms and perspectives ii: mechanisms of adaptation and stress amelioration. Springer, Singapore, pp 415–441
Mei YD, Chen HT, Shen WB, Shen W, Huang LQ (2017) Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol 17:1–12
Ni ZJ, Hu KD, Song CB, Ma RH, Li ZR, Zheng JL, Fu LH, Wei ZJ, Zhang H (2016) Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid Med Cell Longev 2016:4715651
Papanatsiou M, Scuffi D, Blatt MR, García-Mata C (2015) Hydrogen sulfide regulates inward-rectifying KC channels in conjunction with stomatal closure. Plant Physiol 168:29–35
Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A (2007a) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants—from the field to the test tube and back. Plant Biol 9(5):582–588
Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A (2007b) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants-from the field to the test tube and back. Plant Biol 9:582–588
Rennenberg H (1989) Synthesis and emission of hydrogen sulfide by higher plants. In: Saltzman ES, Cooper WJ (eds) Biogenic sulfur in the environment. ACS Publications, Washington, DC, pp 44–57
Riemenschneider A, Nikiforova V, Hoefgen R, De Kok LJ, Papenbrock J (2005) Impact of elevated H2S on metabolite levels, activity of enzymes and expression of genes involved in cysteine metabolism. Plant Physiol Biochem 43:473–483
Schröder P (1993) Plants as sources of atmospheric sulfur. In: Deok LJ, Stulen I, Rennenberg H, Brunold C, Rauser WE (eds) Sulfur nutrition and assimilation in higher plants: Regulatory, agricultural and environmental aspects. SPB Academic Publishing, The Hague, pp 253–270
Sekiya J, Schmidt A, Wilson LG, Filner P (1982) Emission of hydrogen sulfide by leaf tissue in response to L-Cysteine. Plant Physiol 70:430–436
Shen J, Xing T, Yuan H, Liu Z, Jin Z, Zhang L et al (2013) Hydrogen sulfide improves drought tolerance in Arabidopsis thaliana by microRNA expressions. PLoS ONE 8:e77047
Shi H, Ye T, Chan Z (2013) Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 71:226–234
Shi H, Ye T, Han N, Bian H, Liu X, Chan Z (2015) Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Integr Plant Biol 57:628–640
Shivaraj SM, Vats S, Bhat JA, Dhakte P, Goyal V, Khatri P, Kumawat S, Singh A, Prasad M, Sonah H, Sharma TR, Deshmukh R (2020) Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol Plant 168:437–455
Singh SP, Schwan AL (2019) 4.18—sulfur metabolism in plants and related biotechnologies. In: Moo-Young M (ed) Comprehensive biotechnology, 3rd edn. Pergamon, Oxford, pp 221–236
Singh S, Kumar V, Kapoor D, Kumar S, Singh S, Dhanjal DS, Datta S, Samuel J, Dey P, Wang S (2019) Revealing on hydrogen sulfide and nitric oxide signals co-ordination for plant growth under stress conditions. Physiol Plant 168:301
Spadaro D, Yun BW, Spoel SH, Chu C, Wang YQ, Loake GJ (2010) The redox switch: dynamic regulation of protein function by cysteine modifications. Physiol Plantarum 138:360–371
Stuiver CEE, De Kok LJ, Kuiper PJC (1992) Freezing tolerance and biochemical changes in wheat shoots as affected by H2S fumigation. Plant Physiol Biochem 30:47–55
Suzuki N, Koussevitzky S, Mittler RON, Miller GAD (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Tabatabai MA (2005) Sulfur in soils | overview. In: Hillel D (ed) Encyclopedia of soils in the environment. Elsevier, Oxford, pp 76–85
Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62:157–184
Tang X, An B, Cao D, Xu R, Wang S, Zhang Z, Liu X, Sun X (2020) Improving photosynthetic capacity, alleviating photosynthetic inhibition and oxidative stress under low temperature stress with exogenous hydrogen sulfide in blueberry seedlings. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00108
Tea I, Genter T, Naulet N, Boyer V, Lummerzheim M, Kleiber D (2004) Effect of foliar sulfur and nitrogen fertilization on wheat storage protein composition and dough mixing properties. Cereal Chem 81:759–766
Thapa G, Sadhukhan A, Panda SK, Sahoo L (2012) Molecular mechanistic model of plant heavy metal tolerance. Biometals 25:489–505
Thompson CR, Kats G (1978a) Effects of continuous hydrogen sulphide fumigation on crop and forest plants. Environ Sci Technol 12:550–553
Thompson CR, Kats G (1978b) Effects of continuous hydrogen sulfide fumigation on crop and forest plants. Environ Sci Technol 12(5):550–553
Toohey JI (1989) Sulphane sulphur in biological systems: a possible regulatory role. Biochem J 264:625–632
Wainwright M (1984) Sulfur oxidation in soils. In: Brady NC (ed) Advances in agronomy. Academic Press, Cambridge, pp 349–396
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798
Wang R (2003) The gasotransmitter role of hydrogen sulfide. Antioxid Redox Sign. https://doi.org/10.1089/152308603768295249
Watts SF (2000) The mass budgets of carbonyl sulfide, dimethyl sulfide, carbon disulfide and hydrogen sulfide. Atmos Environ 34:761–779
Wei GQ, Cao H, Sun YG, Deng B, Zhang WW, Yang HQ (2017) Effects of hydrogen sulfide on root architecture, leaf reactive oxygen and photosynthetic characteristics of Malus hupehensis under waterlogging. J Appl Ecol 28(10):3267–3273
Wilson LG, Bressan RA, Filner P (1978) Light-dependent emission of hydrogen sulfide from plants. Plant Physiol 61:184–189
Xiao M, Ma J, Li H, Jin H, Feng H (2010) Effects of hydrogen sulfide on alternative pathway respiration and induction of alternative oxidase gene expression in rice Suspension Cells. Zeitschrift für Naturforschung C 65:463–471
Xiao Y, Wu X, Sun M, Peng F (2020) Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front Plant Sci 11:696
Xie Y, Lai D, Mao Y, Zhang W, Shen W, Guan R (2013) Molecular cloning, characterization, and expression analysis of a novel gene encoding l-Cysteine desulfhydrase from brassica napus. Mol Biotechnol 54:737–746
Xie Y, Zhang C, Lai D, Sun Y, Samma MK, Zhang J, Shen W (2014) Hydrogen sulfide delays GA triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J Plant Physiol 171(2):53–62
Yamaguchi Y, Nakamura T, Kusano T, Sano H (2000) Three Arabidopsis genes encoding proteins with differential activities for cysteine synthase and beta-cyanoalanine synthase. Plant Cell Physiol 41:465–476
Yao GF, Wei ZZ, Li TT, Tang J, Huang ZQ, Yang F, Li YH, Han Z, Hu F, Hu LY, Hu KD (2018) Modulation of enhanced antioxidant activity by hydrogen sulfide antagonization of ethylene in tomato fruit ripening. J Agric Food Chem 66(40):10380–10387
Yoshimoto N, Inoue E, Saito K, Yamaya T, Takahashi H (2003) Phloem-localizing sulfate transporter, Sultr1;3, mediates re-distribution of sulfur from source to sink organs in arabidopsis. Plant Physiol 131:1511–1517
Zhang J, Liao W (2020) Protein S-nitrosylation in plant abiotic stresses. Funct Plant Biol 47:1–10
Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529
Zhang H, Tang J, Liu XP, Wang Y, Yu W, Peng WY, Fang F, Ma DF, Wei ZJ, Hu LY (2009a) Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol 51(12):1086–1094
Zhang H, Tang J, Liu XP, Wang Y, Yu W, Peng WY, Fang F, Ma DF, Wei ZJ, Hu LY (2009b) Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol 51:1086–1094
Zhang H, Dou W, Jiang CX, Wei ZJ, Liu J, Jones RL (2010) Hydrogen sulfide stimulates β-amylase activity during early stages of wheat grain germination. Plant Signal Behav 5(8):1031–1033
Zhang L, Pei Y, Wang H, Jin Z, Liu Z, Qiao Z, Fang H, Zhang Y (2015) Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. pekinensis. Oxid Med Cell Longev 2015:1–11
Zhang P, Luo Q, Wang R, Xu J (2017) Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway. Sci Rep 7:868
Zhang Q, Cai W, Ji TT, Ye L, Lu YT, Yuan TT (2020) WRKY13 enhances cadmium tolerance by promoting D-Cysteine Desulfhydrase and hydrogen sulfide production. Plant Physiol 183(1):345–357
Zheng JL, Hu LY, Hu KD, Wu J, Yang F, Zhang H (2016) Hydrogen sulfide alleviates senescence of fresh-cut apple by regulating antioxidant defense system and senescence-related gene expression. Hort Sci 51:152–158
Zhu CQ, Zhang JH, Sun LM, Zhu LF, Abliz B, Hu WJ, Zhong C, Bai ZG, Sajid H, Cao XC, Jin QY (2018) Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast alcontents in rice. Front Plant Sci 9:294
Ziogas V, Molassiotis A, Fotopoulos V, Tanou G (2018) Hydrogen sulfide: a potent tool in postharvest fruit biology and possible mechanism of action. Front Plant Sci 9:1375
Author information
Authors and Affiliations
Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used: Conceptualization MA, SF, and SA; methodology MA, SH, MT, FI, and SU; software SA; validation HMH and WN; formal analysis CW; resources HL; writing—original draft preparation MA, SF, and SA; writing—review and editing, SF and MA; visualization SF, and supervision SF.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Tariq Aftab.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, M., Fahad, S., Ali, M.A. et al. Hydrogen Sulfide: A Novel Gaseous Molecule for Plant Adaptation to Stress. J Plant Growth Regul 40, 2485–2501 (2021). https://doi.org/10.1007/s00344-020-10284-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10284-0