Abstract
Germination is a crucial event in plant lifecycle mediated by a complex hormonal crosstalk. In this study, we revealed an antagonistic interaction between phytohormone abscisic acid (ABA) and gibberellins in epigallocatechin-3-gallate (EGCG)-retarded germination and early seedling growth in tomato. High concentrations of EGCG (0.5 mM and 1.0 mM) decelerated the seed germination and reduced the germination rate and biomass accumulation in seedlings. EGCG-induced inhibition in seedling growth was associated with oxidative stress and changes in antioxidant enzyme activity. Moreover, EGCG treatment increased the ABA content and decreased the GA3 content, leading to a significant reduction in the ratio of GA3/ABA in a dose-dependent manner. EGCG-induced changes in ABA and GA levels were attributed to the changes in expression levels of genes involved in their metabolism. Either exogenous GA application or endogenous ABA deficiency both alleviated EGCG-induced inhibition in early seedling growth. These results suggest that EGCG inhibits seed germination and early seedling growth by affecting the balance between ABA and GA in tomato. These findings are important to better understand the role of flavonoids in seed germination and their implications in controlling seed dormancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed germination is a crucial physiological process in plant life cycle, which initiates with the availability of specific environmental cues (Bewley et al. 2013). In nature, seeds remain dormant until the arrival of a favorable season. Although the success of seed germination depends on the availability of optimal environmental conditions, numerous endogenous signals actively participate in mediating the process of seed germination (Cao et al. 2019). While on-season dormancy release is essential for crop production, short seed dormancy can cause premature seed germination on the mother plants and reduce seed quality. On the other hand, long dormancy may result in asynchronous germination and seedling establishment (Tuan et al. 2018). Therefore, timely breaking of dormancy and germination of seeds are indispensable for sustaining a plant species as well as crop production.
Phytohormones play pivotal roles in dormancy release and seed germination. In particular, the dynamic balance between abscisic acid (ABA) and gibberellins (GAs) mediates the dormancy release, seed germination and post-germination seedling establishment (Cao et al. 2019; Tuan et al. 2018). Accordingly, changes occur in ABA–GA metabolism and signaling during seed germination and seedling establishment. ABA negatively regulates seed germination by inducing dormancy, while GAs positively regulate the germination by enhancing dormancy release (Vishal and Kumar 2018). Apart from this, the antagonistic interactions of ABA and GA regulate numerous plant developmental processes, including primary root growth and responses to abiotic stresses (Shu et al. 2018; Vishal and Kumar 2018). Although ABA, but not GA, is classically known as an abiotic stress hormone, recent studies highlight the importance of GAs in plant response to environmental stresses (Shu et al. 2018; Wang et al. 2017). A delay in soybean seed germination under salt stress or the inhibition of rice seed germination by aluminum stress is attributed to a reduction in GA/ABA ratio (Shu et al. 2017; Xu et al. 2017). However, manipulation of GA/ABA ratio by exogenous hydrogen that promotes the transcript abundances of GA biosynthetic genes (GA20ox1 and GA20ox2) and ABA catabolism genes (ABA8ox1 and ABA8ox2) could alleviate the deleterious effects of aluminum (Xu et al. 2017). Similarly, the elevation of GA/ABA ratio by exogenous salicylic acid accelerates the germination of maize seeds under low-temperature stress (Li et al. 2017b), further suggesting the vital role of GA/ABA ratio in mitigating environmental stresses during seed germination and early seedling growth.
Flavonoids are an important class of plant secondary metabolites, playing crucial roles in cell growth, differentiation, organ development, and response to stresses in plants (Agati et al. 2012; Brunetti et al. 2013). Although the role of (±)-catechin in plant disease resistance remains controversial depending on the plants and pathogens (Bais et al. 2014), exogenous catechin application has been shown to alleviate oxidative stress induced by waterlogging in tomato plants (Yiu et al. 2011). Oxidative stress is often associated with the accumulation of excessive reactive oxygen species (ROS) and stress relief entails enhancement of antioxidative potential (Li et al. 2018; Shi et al. 2016; Wang et al. 2010). Flavonoids promote the antioxidant potential to keep the ROS levels below a sub-lethal range (Du et al. 2018; Fan et al. 2012; Fei et al. 2018; Zhao et al. 2016). Epigallocatechin-3-gallate (EGCG), a flavan-3-ol class flavonoid, is generally considered as a positive regulator of plant stress response (Li et al. 2019). Low-to-moderate concentrations of exogenous EGCG could increase tomato seed germination and early seedling growth under salt stress (Ahammed et al. 2018). EGCG also induces tolerance to heat, cold, salt, and drought in tomato plants by stimulating the RESPIRATORY BURST OXIDASE HOMOLOG1 (RBOH1)-dependent H2O2 production (Li et al. 2019). However, studies relating to the antibacterial effects of EGCG on Escherichia coli showed that not H2O2 synthesis but endogenous oxidative stress caused by EGCG eventually inhibits the growth of E. coli, suggesting a role of prooxidant for EGCG (Xiong et al. 2017). In our previous study, we found that high concentrations of EGCG could inhibit seed germination and primary root growth in tomato; however, the underlying mechanisms remain unclear (Ahammed et al. 2018).
In the present study, we used tomato (Solanum lycopersicum L.), a widely cultivated vegetable with worldwide demand, as plant materials to unveil the mechanism of EGCG-induced seed germination and early seedling growth. The results showed that high concentrations of EGCG (0.5 mM and 1.0 mM) significantly reduced the seed germination rate and biomass accumulation in seedlings, which was attributed to increased biosynthesis of ABA and decreased biosynthesis of GA as well. Furthermore, pharmacological (GA treatment) and genetic (ABA biosynthetic mutants) experiments revealed that either exogenous GA application or endogenous ABA deficiency could alleviate EGCG-induced growth inhibition in tomato seedlings. This study helps to understand the role of flavonoids in seed germination and may have implications on controlling seed dormancy.
Materials and Methods
Plant Materials and Treatments
Firstly, warm water treatment (55 °C for 10 min, distilled water) was used to sterilize tomato seeds (S. lycopersicum L. cv. Zhongza No.9). One hundred seeds were then placed per Petri dish on sterile Whatman No. 1 filter papers (two layers) moisturized with 15 ml double-distilled water (control) or different concentrations of EGCG (0.5 mM and 1.0 mM) at 28 ± 3 °C. The concentrations of EGCG were chosen based on our previous experiments as these levels of EGCG could inhibit the seed germination rate in tomato (Ahammed et al. 2018). The seed germination rate (%) was calculated from 24 h after the initiation of treatment with 12-h intervals until 84 h, and the final count was recorded at 120 h. Respective treatment solutions were added into the Petri dishes daily to keep optimal moisture. Notably, prior to the addition of fresh solutions, prevailing liquid in the Petri dish was removed to minimalize metabolic effects due to root exudation. After 120 h, fresh weight was measured and the seedlings were harvested for different biochemical assays. Root samples were immediately frozen in liquid nitrogen and stored at − 80 °C until analysis. For the exogenous GA application experiment, tomato seeds were germinated in 5 μM GA solution with or without two levels of EGCG as described above. To confirm the role of ABA in EGCG-induced growth retardation, we used tomato seeds of wild-type Ailsa craig (Ailsa) and its ABA biosynthetic mutant notabilis (not). Ailsa and not tomato seeds were also germinated in two levels of EGCG (0.5 mM and 1.0 mM) following the same procedure as described above. Treatment with 0 µM EGCG, that is, ddH2O, served as control.
Lipid Peroxidation and Antioxidant Enzyme Activity Assay
For the malondialdehyde (MDA) content assay, fresh root samples were homogenized in freshly prepared ice cool extraction buffer (50 mM potassium phosphate buffer) and centrifuged at 12,000×g for 20 min. Afterward, 4 ml of 0.65% TBA in 20% trichloroacetic acid solution was added and the mixture was incubated at 95 °C for 30 min. The reaction was stopped by immediately placing the tubes in an ice bucket. Resulted homogenates were centrifuged at 10,000×g for 5 min and absorbance of the red adduct was recorded at 440, 532, and 600 nm. As an end product of lipid peroxidation, MDA equivalents per gram fresh weight were calculated as previously described (Hodges et al. 1999; Landi 2017). Antioxidant enzymes, such as catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) were extracted from roots and their activity was measured following the methods described previously (Ahammed et al. 2018; Zhang et al. 2019). All spectrophotometric analyses were performed on a Shimadzu spectrophotometer (UV-1800, Shimadzu, Japan). The activity of enzymes were expressed on the basis of protein content which was estimated using bovine serum albumin (BSA) as described elsewhere (Bradford 1976).
Plant Hormone Analysis
To measure the concentrations of ABA in roots, 0.1 g fresh root samples were ground in liquid nitrogen. After grinding, 1 ml of ethyl acetate was added to the samples with 10 µl of internal standard (1 µg/ml) of abscisic acid (ABA) purchased from OlChemIm (Olomouc, Czech Republic). Afterward, the samples were vortexed thoroughly and shaked overnight in dark at 4 °C. Then, the samples were centrifuged for 10 min at 12,000 rpm and the obtained extracts were concentrated to dryness under nitrogen gas. The obtained residues were dissolved in 500 µl of chromatographic pure methanol and quantified by HPLC–MS according to the previously described method (Wang et al. 2016). The concentrations of GAs were measured following the method described previously (Liu et al. 2018). Root samples were spiked with isotope standard during the extraction, and samples were determined using an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, USA) equipped with an electrospray ionization source, operated in the negative ion or positive multiple‐reaction monitoring mode.
Extraction of Total RNA and Quantitative Real-Time PCR (QRT-PCR) Assay
Total RNA was extracted from ~ 100 mg root tissues using total RNA extraction kit (Tiangen, Shanghai, China). A 1 µg aliquot of total RNA was reverse-transcribed to generate cDNA using a ReverTra Ace qPCR RT Kit (Toyobo, Japan). The SYBR Green PCR Master Mix (Takara, Tokyo, Japan) was used for qRT-PCR assay and the analysis was performed with LightCycler@ 480 II Real-Time PCR Detection System (Roche, Basel, Swiss) under the default thermal cycling conditions with an added melting curve. Gene-specific primers for qRT-PCR were designed for the genes NCED1, NCED2, GA20ox1 and GA2ox2; and the marker gene actin was used as an internal control (Supplementary Table S1). The PCR was run at 95 °C for 3 min, followed by 40 cycles at 95 °C for 30 s, then 30 s at 58 °C, and 30 s at 72 °C. Relative transcript levels were calculated as previously described (Livak and Schmittgen, 2001).
Statistical Analysis
Four replicates were performed for each treatment in Petri dishes. The results were averaged and expressed as means (± SD). Tukey’s least significant difference (LSD) test was used to determine statistical significance at P < 0.05. Significant differences are indicated by different lower-case letters.
Results
High Concentrations of EGCG Inhibit Seed Germination and Seedling Growth in Tomato
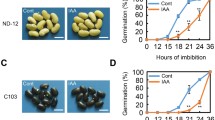
Although EGCG is generally considered as a positive regulator for plant growth, our previous study suggests that high concentrations of EGCG might negatively affect plant growth (Ahammed et al. 2018). To verify this assumption, we performed germination test under two apparently high concentrations of EGCG (0.5 mM and 1.0 mM). As expected, high levels of EGCG significantly retarded tomato seed germination as evidenced by the phenotypes after 48 h of treatment (Fig. 1a). In addition, the inhibitory effect of EGCG was dose-dependent, i.e., 1.0 mM EGCG inhibited the radicle growth more profoundly compared with the 0.5 mM EGCG. Time course of germination rate (%) revealed that the high concentrations of EGCG not only decreased the germination rate but also decelerated the germination of tomato seeds (Fig. 1b). For instance, the germination rates at 24, 36, and 48 h after the EGCG treatment were 49.8, 55.9, and 41.1% lower in 1.0 mM EGCG treatment compared with that in control. However, at later stages the differences became narrow, and at 120 h after the EGCG treatment, the germination rates decreased by 5.7 and 10.6% in 0.5 mM and 1.0 mM EGCG treatment compared with that in control. Fresh weight analysis of young seedlings at 120 h after the EGCG treatment showed that biomass accumulation decreased by 20.1% and 38.3% in 0.5 mM and 1.0 mM EGCG, respectively, compared with the control (Fig. 1c). These results suggest that EGCG at higher concentrations could significantly reduce seed germination as well as post-germination seedling growth in tomato.
High concentrations of EGCG decelerate seed germination and decrease biomass accumulation in tomato seedlings. a Phenotype of germinating seeds at 48 h after the EGCG treatment, b time course of germination rate, and c fresh weight of seedlings after 120 h of EGCG treatment. Seeds were germinated with either ddH2O or two concentrations of EGCG (0.5 M and 1.0 mM) in Petri dishes. Each Petri dish contains 100 seeds on filter papers moistened with the aforementioned solutions. Data are the averages of four replicates and the error bars indicate ± standard deviations. Mean denoted by different letters indicate statistically significant differences according to Tukey’s test at P < 0.05
EGCG-Induced Growth Inhibition is Attributed to Oxidative Stress
To understand the potential mechanism of EGCG-induced growth inhibition, we compared the levels of lipid peroxidation in roots by measuring the content of MDA. The MDA concentrations increased significantly and dose-dependently under EGCG treatment (Fig. 2a). We then analyzed the activity of some key antioxidant enzymes, such as CAT, POD, and APX. The activity of these enzymes was altered differentially by two levels of EGCG. The CAT activity was not significantly influenced by 0.5 mM EGCG; however, its activity was significantly increased by 1.0 mM EGCG treatment compared with the control. Meanwhile, the POD activity was suppressed by 0.5 mM EGCG and it was elevated by 1.0 mM EGCG compared with the control. Interestingly, the activity of APX decreased dose-dependently. More precisely, the APX activity decreased by 26.4% and 34.6% under 0.5 mM and 1.0 mM EGCG, respectively, compared with the control. These results suggest that EGCG-induced suppression in seed germination and early seedling growth is associated with the occurrence of oxidative stress.
High concentrations of EGCG induce oxidative stress and alter antioxidative enzyme activity in tomato roots. a Malondialdehyde (MDA) content, b catalase (CAT) activity, c peroxidase (POD) activity, and d ascorbate peroxidase (APX) activity. Root samples were collected at 120 h after placing the seeds in 0.5 mM and 1.0 mM EGCG solution for germination. Data are the averages of four replicates and the error bars indicate ± standard deviations. Mean denoted by different letters indicate statistically significant differences according to Tukey’s test at P < 0.05
EGCG Alters Hormone Balance in Tomato Seedlings
Phytohormones play a crucial role in modulating seed germination and early seedling growth (Shu et al. 2018). Therefore, we analyzed the concentrations of two important growth regulatory hormones (ABA and GAs) in tomato seedlings. The ABA content was significantly increased by 0.5 mM and 1.0 mM EGCG, although no significant difference was found in ABA content between 0.5 and 1.0 mM EGCG treatments (Fig. 3a). However, GA3 concentrations decreased with increasing concentration of EGCG. The GA3 concentrations in 0.5 mM and 1.0 mM were 24.2% and 34.0% lower than that in control (Fig. 3b). Unlike GA3, the 0.5 mM EGCG, but not 1.0 mM EGCG, significantly increased the GA4 content in roots of tomato seedlings (Fig. 3c). All these alterations resulted in more profound changes in the ratios of GA3 to ABA and GA4 to ABA. Briefly, the ratio of GA3 to ABA decreased by 34.4% and 45.9% in 0.5 mM and 1.0 mM EGCG treatment, respectively (Fig. 3d). Although the ratio of GA4/ABA was not altered by 0.5 mM EGCG, it was significantly decreased by 1.0 mM EGCG. These results suggest that high concentrations of EGCG increased ABA concentration and decreased GA3 concentrations, leading to significant decreases in the ratios of GA3/ABA.
EGCG alters the balance between abscisic acid (ABA) and gibberellins (GAs) in tomato roots. a ABA concentration, b GA3 concentration, c GA4 concentration, and d ratio of GA to ABA (GA/ABA). Root samples were collected at 120 h after placing the seeds in 0.5 mM and 1.0 mM EGCG solution for germination. Data are the averages of four replicates and the error bars indicate ± standard deviations. Mean denoted by different letters indicate statistically significant differences according to Tukey’s test at P < 0.05
EGCG-Induced Changes in Hormone Levels are Related to Their Metabolism
To unveil the mechanism of EGCG-induced changes in hormone levels, we analyzed the transcript levels of ABA and GA metabolism genes. Time course of NCED1, an important gene in ABA biosynthetic pathway, showed that EGCG treatment maintained an increased transcript level of NCED1 over the course of experiment, resulting in a 4.5- and 6.6-fold increase at 120 h after the 0.5 mM and 1.0 mM EGCG treatment, respectively, compared with the control (Fig. 4a). The transcripts of NCED2 in 0.5 mM and 1.0 mM EGCG were 1.6- and 1.5-fold greater than the control at 60 h after the treatment (Fig. 4b). At 72 h after the EGCG treatment, the transcript levels of NCED2 in EGCG treatments were 4.9- and 3.8-fold greater in 0.5 mM and 1.0 mM EGCG, respectively, which then gradually decreased over the time of treatment. On the contrary, the transcript levels of GA biosynthetic gene, GA20ox1, remarkably increased in control during germination; however, such changes were abolished by the EGCG treatment (Fig. 4c). At 120 h after the EGCG treatment, the transcript levels of GA20ox1 in control remained 2.7- and 2.2-fold increased compared with 0.5 mM and 1.0 mM EGCG treatment, respectively. The transcript levels of GA2ox2, a GA inactivation (catabolism) gene, in 0.5 mM and 1.0 mM EGCG treatment were higher than that of control at 60 h after the treatment, which were slightly increased at 72 h, but then its levels decreased to the levels of control at 120 h after the EGCG treatment (Fig. 4d). All these results suggest that the expression patterns of ABA and GA metabolism genes are more or less consistent with the changes in ABA and GA levels under EGCG treatments.
EGCG-induced changes in ABA and GA levels are associated with the expression of their metabolism-related genes. Time course of the transcript levels of ABA biosynthetic genes, a NCED1, b NCED2, GA biosynthetic gene, c GA20ox1, and GA inactivation (catabolism) gene, d GA2ox2 in tomato roots. Root samples for qRT-PCR were collected at different time points after placing the seeds in 0.5 mM and 1.0 mM EGCG solution for germination. Data are the averages of four replicates and the error bars indicate ± standard deviations. Mean denoted by different letters indicate statistically significant differences according to Tukey’s test at P < 0.05
GA Alleviates EGCG-Induced Growth Inhibition
Since we found a significant decrease in GA3 content upon EGCG treatment, we intended to confirm the role of GA in EGCG-induced growth inhibition. Therefore, we simultaneously applied GA and EGCG, and compared the root length and fresh weight of tomato seedlings. While EGCG treatments significantly reduced the root length in a dose-dependent manner (42.8% and 61.6% in 0.5 and 1.0 mM EGCG, respectively), exogenous GA application remarkably alleviated the EGCG-induced inhibition in root length. The GA treatment increased the root length by 67.0% and 116.9% under 0.5 and 1.0 mM EGCG treatment, respectively, compared with the sole treatment of EGCG. Similarly, the GA treatment significantly increased the fresh weight of tomato seedlings under EGCG treatment compared with the individual treatment of EGCG. These results revealed that exogenous GA can alleviate the EGCG-induced growth inhibition, suggesting that EGCG-induced reduction in GA content is one of the main reasons behind the EGCG-induced growth retardation.
ABA is a Prime Factor in EGCG-Induced Growth Inhibition
To further ascertain the role of ABA in EGCG-induced early seedling growth, we germinated seeds of wild-type Ailsa craig (Ailsa) and its ABA biosynthetic mutant notabilis (not) in high concentrations of EGCG. Although EGCG inhibited the root length in Ailsa, EGCG-induced root growth retardation was significantly attenuated in ABA-deficient not seedlings. Similarly, 0.5 mM EGCG and 1.0 mM EGCG decreased fresh weight by 49.8% and 58.7% in Ailsa seedlings; however, the fresh weight in ABA-deficient not seedlings was 62.1% and 55.2% higher than Ailsa under 0.5 mM and 1.0 mM EGCG treatments, respectively. These results suggest that ABA deficiency attenuates the inhibitory effect of EGCG on early seedling growth.
Discussion
Seed is the basis for sustaining plant species generation after generation (Bewley et al. 2013). It is the ultimate product of agricultural production in the case of food crops. Thus, optimal germination and seedling establishment are crucial for harvesting the final yield (Shu et al. 2018). Phytohormone ABA and GA function antagonistically in the process of seed germination and their balance largely determines the seedling growth (Vishal and Kumar 2018). In this study, we unveiled the critical roles of ABA and GA in EGCG-retarded seed germination in tomato. While high concentrations of EGCG retarded seed germination and seedling growth by impairing the balance between ABA and GA, exogenous GA or endogenous ABA deficiency alleviated the EGCG-retarded growth inhibition in tomato seedling. This study clarified the well-known antagonistic interaction between ABA and GA in mediating the EGCG-induced seed germination and early seedling establishment.
Seed germination is a multifarious physiological process that occurs through the integration of multiple environmental cues and endogenous signals (Bewley et al. 2013). After imbibition, the production of ROS increases along with the progress of seed germination (Bailly, 2019). ROS can interact with the hormones and affect seed germination; nonetheless, the antioxidant system actively participates to keep the levels of ROS below the toxic levels (Bicalho et al. 2018). Under stressful conditions, ROS production exceeds the non-toxic levels and impair redox homeostasis, leading to oxidative stress (Hasan et al. 2019; Zhang et al. 2020). Oxidative stress is closely associated with high levels of membrane lipid peroxidation (Ahammed et al. 2020a,b). In the current study, EGCG-induced reduction in seed germination and seedling growth was associated with an increased level of lipid peroxidation in roots (Fig. 2a), suggesting that high concentrations of EGCG-induced oxidative stress during seed germination. Although the activity of some antioxidant enzymes was slightly elevated by EGCG, APX activity was reduced dose-dependently, suggesting that enhanced MDA content was partly attributed to the reduction of APX activity (Fig. 2d). Moreover, higher activity of CAT and POD under 1.0 mM might be a signature of oxidative stress, but these increases were potentially incapable of maintaining ROS at non-toxic levels and thus failed to mitigate oxidative stress. EGCG-induced reduction in seed germination in the current study was consistent with the effect of EGCG on seed germination in Arabidopsis and tomato (Ahammed et al. 2018; Hong et al. 2015).
Phytohormone ABA and GA play a prime role in seed germination and post-germination seedling establishment (Shu et al. 2017; Tuan et al. 2018; Vishal and Kumar, 2018). Their endogenous levels are maintained by both anabolism and catabolism at the cellular level through some rate-limiting enzymes. In the current study, EGCG enhanced the ABA content (Fig. 3a), which was attributed to the upregulation of the ABA biosynthetic gene, NCED1 and NCED2 (Fig. 4), encoding for the rate-limiting enzyme nine-cis-epoxycarotenoid dioxygenase (NCED). On the other hand, EGCG reduced the concentrations of GA3 (Fig. 3b), which were related to the downregulation of GA biosynthetic gene, GA20ox1, and upregulation of GA inactivation gene, GA2ox2 (Fig. 4c, d). These changes in ABA and GA levels under EGCG treatment eventually reduced the ratio of GA/ABA (Fig. 3d), which potentially reduced seed germination and early seedling growth (Fig. 1). The ratio of GA/ABA is crucial for seed germination under stressful conditions (Li et al. 2017b; Vishal and Kumar, 2018). Moreover, root development is controlled by both ABA and GA. While ABA inhibits primary root growth by delaying the cell expansion and proliferation (Shu et al. 2018), GA positively regulates root growth, by promoting the cell expansion in root tips through maintenance of GA levels via suppression of GA inactivation gene, GA2ox2 (Li et al. 2017a). In the present study, EGCG-induced root growth retardation was alleviated by exogenous GA application (Fig. 5), confirming the positive role of GA in promoting root growth and biomass accumulation under stressful conditions. On the other hand, the negative effect of EGCG on root growth and biomass accumulation was attenuated in ABA biosynthesis mutant not (Fig. 6), suggesting that ABA is required for EGCG-induced growth retardation in tomato. Thus the antagonism between ABA and GA controls the seed germination and early seedling growth in tomato (Shu et al. 2018).
Exogenous gibberellin (GA) alleviates EGCG-induced growth inhibition in tomato seedlings. aRoot length, and b fresh weight after 120 h of EGCG treatment. Seeds were germinated with either ddH2O or 5 μM GA and two levels of EGCG (0.5 M and 1.0 mM) in Petri dishes. Each Petri dish contains 100 seeds on filter papers moistened with the aforementioned solutions. Data are the averages of four replicates and the error bars indicate ± standard deviations. Mean denoted by different letters indicate statistically significant differences according to Tukey’s test at P < 0.05
ABA deficiency mitigates EGCG-induced growth inhibition in tomato seedlings. a Root length, and b fresh weight after 120 h of EGCG treatment. Seeds of Ailsa craig (wild-type) and its ABA biosynthetic mutant notabilis (not) were germinated with either ddH2O or two levels of EGCG (0.5 M and 1.0 mM) in Petri dishes. Each Petri dish contains 100 seeds on filter papers moistened with the aforementioned solutions. Data are the averages of four replicates and the error bars indicate ± standard deviations. Mean denoted by different letters indicate statistically significant differences according to Tukey’s test at P < 0.05
In conclusion, we found that high concentrations of EGCG increased ABA concentration and decreased GA concentration in tomato (Fig. 7). Since ABA inhibits and GA promotes seed germination and seedling growth (Tuan et al. 2018), EGCG-induced reduction in GA/ABA ratio results in an attenuated seed germination rate and seedling growth. Either exogenous GA application or endogenous ABA deficiency could alleviate EGCG-retarded seed germination and seedling growth, further confirming the antagonistic interaction between ABA and GA in mediating EGCG-inhibited seed germination and post-germination seedling establishment (Fig. 7). These results deepen our understanding of the effect of high concentrations of EGCG on seed germination and could be useful to further explore the role of flavonoids in controlling seed dormancy to avoid offseason seed germination.
A hypothetical model depicting potential mechanisms of EGCG-induced inhibition in seed germination and seedling growth. High concentrations of EGCG not only increase ABA concentration but also decrease gibberellin (GA) concentrations. While exogenous GA could alleviate the EGCG-induced growth retardation, ABA deficiency could also mitigate the EGCG-induced inhibition in early seedling growth, suggesting that ABA and GA act antagonistically to mediate the EGCG-retarded seed germination and seedling growth in tomato
References
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76. https://doi.org/10.1016/j.plantsci.2012.07.014
Ahammed GJ, Li Y, Li X, Han W-Y, Chen S (2018) Epigallocatechin-3-gallate alleviates salinity-retarded seed germination and oxidative stress in tomato. J Plant Growth Regul 37:1349–1356. https://doi.org/10.1007/s00344-018-9849-0
Ahammed GJ, Wang Y, Mao Q, Wu M, Yan Y, Ren J, Wang X, Liu A, Chen S (2020a) Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ Pollut 259:113957. https://doi.org/10.1016/j.envpol.2020.113957
Ahammed GJ, Wu M, Wang Y, Yan Y, Mao Q, Ren J, Ma R, Liu A, Chen S (2020b) Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci Hortic 265:109205. https://doi.org/10.1016/j.scienta.2020.109205
Bailly C (2019) The signalling role of ROS in the regulation of seed germination and dormancy. Biochem J 476(20):3019–3032. https://doi.org/10.1042/BCJ20190159
Bais HP, Venkatachalam L, Biedrzycki ML (2014) Stimulation or inhibition conflicting evidence for (±)-catechin’s role as a chemical facilitator and disease protecting agent. Plant Signal Behav 5:239–246. https://doi.org/10.4161/psb.5.3.10573
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Seeds: physiology of development, germination and dormancy. Springer, New York
Bicalho EM, Santos TRSD, Garcia QS (2018) Abscisic acid and the antioxidant system are involved in germination of Butia capitata seeds. Acta Bot Bras 33:174–178. https://doi.org/10.1590/0102-33062018abb0193
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brunetti C, Di Ferdinando M, Fini A, Pollastri S, Tattini M (2013) Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci 14:3540–3555. https://doi.org/10.3390/ijms14023540
Cao H, Han Y, Li J, Ding M, Li Y, Li X, Chen F, Soppe WJJ, Liu Y (2019) Arabidopsis thaliana Seed dormancy 4-like regulates seed dormancy and germination by mediating the gibberellin pathway. J Exp Bot 71:919–933. https://doi.org/10.1093/jxb/erz471
Du L, Li J, Zhang X, Wang L, Zhang W (2018) Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of MAPKs activation. J Funct Foods 43:62–69. https://doi.org/10.1016/j.jff.2018.01.028
Fan J, Zhu W, Kang H, Ma H, Tao G (2012) Flavonoid constituents and antioxidant capacity in flowers of different Zhongyuan tree penoy cultivars. J Funct Foods 4(1):147–157. https://doi.org/10.1016/j.jff.2011.09.006
Fei P, Ali MA, Gong S, Sun Q, Bi X, Liu S, Guo L (2018) Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control 94:289–294. https://doi.org/10.1016/j.foodcont.2018.07.022
Hasan MK, Ahammed GJ, Sun S, Li M, Yin H, Zhou J (2019) Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J Agric Food Chem 38:10563–10576. https://doi.org/10.1021/acs.jafc.9b02404
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207(4):604–611. https://doi.org/10.2307/23385611
Hong G, Wang J, Hochstetter D, Gao Y, Xu P, Wang Y (2015) Epigallocatechin-3-gallate functions as a physiological regulator by modulating the jasmonic acid pathway. Physiol Plantarum 153:432–439. https://doi.org/10.1111/ppl.12256
Landi M (2017) Commentary to: "Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds" by Hodges Planta, et al 1999 207:604–611. Planta 245:1067. https://doi.org/10.1007/s00425-017-2699-3
Li H, Torres-Garcia J, Latrasse D, Benhamed M, Schilderink S, Zhou W, Kulikova O, Hirt H, Bisseling T (2017a) Plant-specific histone deacetylases HDT1/2 regulate GIBBERELLIN 2-OXIDASE2 expression to control arabidopsis root meristem cell number. Plant Cell 29:2183–2196. https://doi.org/10.1105/tpc.17.00366
Li Z, Xu J, Gao Y, Wang C, Guo G, Luo Y, Huang Y, Hu W, Sheteiwy MS, Guan Y, Hu J (2017b) The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front Plant Sci 8:1153. https://doi.org/10.3389/fpls.2017.01153
Li X, Zhong Y, Pang X, Yuan Y, Liu Y, Zhang Z (2018) Trypsin and ascorbic acid have a synergistic effect on the quality of apple processing by protecting apple cells from oxidative damage. J Food Biochem 42:e12582. https://doi.org/10.1111/jfbc.12582
Li X, Li Y, Ahammed GJ, Zhang X-N, Ying L, Zhang L, Yan P, Zhang L-P, Li Q-Y, Han W-Y (2019) RBOH1-dependent apoplastic H2O2 mediates epigallocatechin-3-gallate-induced abiotic stress tolerance in Solanum lycopersicum L. Environ Exp Bot 161:357–366. https://doi.org/10.1016/j.envexpbot.2018.11.013
Liu CC, Ahammed GJ, Wang GT, Xu CJ, Chen KS, Zhou YH, Yu JQ (2018) Tomato CRY1a plays a critical role in the regulation of phytohormone homeostasis, plant development, and carotenoid metabolism in fruits. Plant Cell Environ 41:354–366. https://doi.org/10.1111/pce.13092
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Shi J, Shi G, Tian Z (2016) Effect of exogenous hydrogen peroxide or ascorbic acid on senescence in cut flowers of tree peony (Paeonia suffruticose Andr.). J Hortic Sci Biotechnol 90:689–694. https://doi.org/10.1080/14620316.2015.11668732
Shu K, Qi Y, Chen F, Meng Y, Luo X, Shuai H, Zhou W, Ding J, Du J, Liu J, Yang F, Wang Q, Liu W, Yong T, Wang X, Feng Y, Yang W (2017) Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front Plant Sci 8:1372. https://doi.org/10.3389/fpls.2017.01372
Shu K, Zhou W, Chen F, Luo X, Yang W (2018) Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Front Plant Sci 9:416. https://doi.org/10.3389/fpls.2018.00416
Tuan PA, Kumar R, Rehal PK, Toora PK, Ayele BT (2018) Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front Plant Sci 9:668. https://doi.org/10.3389/fpls.2018.00668
Vishal B, Kumar PP (2018) Regulation of Seed Germination and abiotic stresses by gibberellins and abscisic acid. Front Plant Sci 9:838. https://doi.org/10.3389/fpls.2018.00838
Wang HZ, Zhang LZ, Ma J, Li XY, Li Y, Zhang RP, Wang RQ (2010) Effects of water stress on reactive oxygen species generation and protection system in rice during grain-filling stage. Agric Sci China 9:633–641. https://doi.org/10.1016/s1671-2927(09)60138-3
Wang F, Guo ZX, Li HZ, Wang MM, Onac E, Zhou J, Xia XJ, Shi K, Yu JQ, Zhou YH (2016) Phytochrome A and B function antagonistically to regulate cold tolerance via abscisic acid-dependent jasmonate signaling. Plant Physiol 170(1):459–471. https://doi.org/10.1104/pp.15.01171
Wang B, Wei H, Xue Z, Zhang WH (2017) Gibberellins regulate iron deficiency-response by influencing iron transport and translocation in rice seedlings (Oryza sativa). Ann Bot 119:945–956. https://doi.org/10.1093/aob/mcw250
Xiong LG, Chen YJ, Tong JW, Huang JA, Li J, Gong YS, Liu ZH (2017) Tea polyphenol epigallocatechin gallate inhibits Escherichia coli by increasing endogenous oxidative stress. Food Chem 217:196–204. https://doi.org/10.1016/j.foodchem.2016.08.098
Xu D, Cao H, Fang W, Pan J, Chen J, Zhang J, Shen W (2017) Linking hydrogen-enhanced rice aluminum tolerance with the reestablishment of GA/ABA balance and miRNA-modulated gene expression: a case study on germination. Ecotoxicol Environ Saf 145:303–312. https://doi.org/10.1016/j.ecoenv.2017.07.055
Yiu JC, Tseng MJ, Liu CW (2011) Exogenous catechin increases antioxidant enzyme activity and promotes flooding tolerance in tomato (Solanum lycopersicum L.). Plant Soil 344(1–2):213–225. https://doi.org/10.1007/s11104-011-0741-y
Zhao S, Li J, Wang L, Wu X (2016) Pomegranate peel polyphenols inhibit lipid accumulation and enhance cholesterol efflux in raw264.7 macrophages. Food Funct 7:3201–3210. https://doi.org/10.1039/c6fo00347h
Zhang Y, Liang Y, Zhao X, Jin X, Hou L, Shi Y, Ahammed GJ (2019) Silicon compensates phosphorus deficit-induced growth inhibition by improving photosynthetic capacity, antioxidant potential, and nutrient homeostasis in tomato. Agronomy 9:733. https://doi.org/10.3390/agronomy9110733
Zhang Z, Wu P, Zhang W, Yang Z, Liu H, Ahammed GJ, Cui J (2020) Calcium is involved in exogenous NO-induced enhancement of photosynthesis in cucumber (Cucumis sativus L.) seedlings under low temperature. Sci Hortic 261:108953. https://doi.org/10.1016/j.scienta.2019.108953
Acknowledgements
This research was partially supported by the National Natural Science Foundation of China (31950410555); the National Key Research and Development Program of China (2018YFD1000800); the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2014-TRICAAS); and Henan University of Science and Technology (HAUST) Research Start-up Fund for New Faculty (13480058). We are grateful to the Tomato Genetics Resource Center at the California University for tomato (Ailsa Craig and notabilis) seeds.
Author information
Authors and Affiliations
Contributions
GJA: Conceptualization, Methodology, Formal analysis, Investigation, Writing—original draft, Writing—review & editing, Supervision, Funding acquisition. YL: Formal analysis, Investigation. AL: Supervision, Resources, Funding acquisition. SC: Supervision, Resources, Funding acquisition. XL: Writing—review & editing, Supervision, Resources, Funding acquisition, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahammed, G.J., Li, Y., Cheng, Y. et al. Abscisic Acid and Gibberellins Act Antagonistically to Mediate Epigallocatechin-3-Gallate-Retarded Seed Germination and Early Seedling Growth in Tomato. J Plant Growth Regul 39, 1414–1424 (2020). https://doi.org/10.1007/s00344-020-10089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10089-1