Abstract
Gibberellins (GAs), as one of the important hormones in regulating the growth and development of higher plants, can significantly promote cell elongation and expansion. Celery is a widely grown leafy vegetable crop with rich nutritional value. However, the effect of gibberellins on celery leaves is unclear. In this paper, the celery variety “Jinnan Shiqin” plants were treated with gibberellic acid (GA3) and paclobutrazol (PBZ, a gibberellin inhibitor). Our results showed that GA3 treatment promoted the growth of celery leaves and caused lignification of celery leaf tissue. In addition, the transcript levels of genes associated with gibberellins, auxin, cytokinins, ethylene, jasmonic acid, abscisic acid, and brassinolide were altered in response to increased or decreased exogenous gibberellins or inhibitor. GA3 may regulate celery growth by interacting with other hormones through crosstalk mechanisms. This study provided a reference for further study of the regulation mechanism of gibberellins metabolism, and exerted effects on understanding the role of gibberellins in the growth and development of celery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant hormones play an important role in plant growth and stress response. Among them, gibberellin is a kind of biguanide compound, which is one of the essential hormones for plant growth and development (Achard et al. 2009; Ubeda-Tomás et al. 2009; Gao et al. 2011; Wang et al. 2015a; Zhuang et al. 2015). Up to now, more than 130 different types of gibberellins have been discovered and identified from different plants, but only a few have physiological activity (Silverstone and Sun 2000). The regulation of exogenous gibberellins and its mechanism provide a theoretical basis for crop yield increase and quality improvement.
Gibberellins synthesis and metabolic pathways have been extensively studied. In higher plants, isopentenyl pyrophosphate (IPP) synthesizes the precursor of gibberellin (GA) biosynthesis geranylgeranyl diphosphate (GGPP) (Hedden and Phillips 2000). The GA synthesis process is mainly divided into three phases, completing in different subcellular structures. The first stage is completed in the plastid, and GGPP is catalyzed by ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS) to the ent-kaurene, the precursor of gibberellin. In the second stage, ent-kaurene synthesizes GA12-aldehyde under the action of ent-kaurene oxidase (KO) and ent-kaurenoic acid oxidase (KAO). In the final stage, GA12-aldehyde was converted to other GA species by the action of GA20-oxidase (GA20ox), GA3-oxidase (GA3ox), and GA2-oxidase (GA2ox) (Hedden 2001; Wang, et al. 2015b). Commercially available GA, which is widely used in agricultural production and horticulture, is mainly a mixture of GA3 and GA7 isolated from gibberella.
The CPS and KS of Arabidopsis thaliana are encoded by a single copy of the GA1 and GA2 genes, and the KO is encoded by the GA3 gene. GA deletion mutant results in a severely dwarfed phenotype of Arabidopsis (Helliwell et al. 2001). GA3ox catalyzes GA9 and GA20 into biologically active GA4 and GA1, respectively (Hedden and Phillips 2000). Paclobutrazol (PBZ) is a type of GA biosynthesis inhibitor that primarily delays the activity of KO and some monooxygenases. As one of the important hormones regulating higher plants, GA is affected by many factors and is strictly controlled in time and space (Ross and O’Neill 2002; Wolbang et al. 2004).
Previous studies have shown that other hormones are also involved in the regulation of GA biosynthesis. Auxin was reported to regulate the growth and development of plants by regulating the biosynthesis of GA (Olszewski et al. 2002). Studies in peas and tobacco have found that treatment with exogenous auxin transport inhibitors reduces the amount of GA1 in stems (Wolbang and Ross 2001; Ross et al. 2010). Exogenous GA treatment can partially restore the height phenotype of ethylene mutant ctr1 plant (Achard et al. 2006). GA promotes cell elongation required BR (brassinosteroid) signaling and BR-activated BRASSINAZOLE RESISTANT1 (BZR1). BR mutant lacks intranuclear BZR1; GA-induced DELLA degradation does not increase BZR1 activity and thus does not promote cell elongation (Gallego-Bartolomé et al. 2012; Bai et al. 2012). BR also plays a positive role in the regulation of GA20ox1 expression level at special stage of plant growth and development.
Celery is a biennial herb of the Apiaceae family, which is a nutrient-rich leafy vegetable (Li et al. 2018). The molecular research of celery is lagging, and there are few reports on the expression of genes related to gibberellins metabolism and signal transduction. In this study, morphological, anatomical characteristics, and hormonal interactions were analyzed to fully elucidate the effects of applied GA3 on leaf growth. This study helped further elucidate the roles of GA in celery growth.
Materials and methods
Plant material and GA3 and PBZ treatments
The seeds of celery “Jinnan Shiqin” were planted in the climate-controlled chamber at the state key laboratory of crop genetics and germplasm enhancement in Nanjing Agricultural University (32° 04′ N, 118° 85′ E). The climate chamber was set at 25 °C for 16 h during the day and at 18 °C for 8 h during the night. The celery plants were planted in a plastic pot (18 × 18 × 16 cm) mixed with vermiculite, organic soil, and perlite compounds (volume ratio of 2:2:1). After 70 DAS (days after sowing), 100 mL of GA3 (150 mg L−1), PBZ (20 mg L−1), or a mixture of the both was sprayed onto the plants respectively. Plants sprayed with aqueous solution used as the control. The treatments were performed every 4 days for a total of four treatments. Three biological replicates were tested and samples were collected and stored at − 80 °C.
Anatomical structure analysis
The fresh sample was cut into slices and stored in phosphate buffer solution (pH 7.2) including 2.5% glutaraldehyde at 4 °C. The samples were subjected to safranin-O/fast green staining, and the morphological structure of the plant cells was observed, and the lignified parts were stained red (Wang, et al. 2016). The lignified cell wall was autofluorescent under ultraviolet (UV) fluorescence microscopy; the degree of lignification of celery leaves was observed under different treatments. Three independent biological replicates of each celery plant sample were prepared for histochemical staining.
Total RNA isolation and cDNA synthesis
Total RNA from celery leaf blades and petioles was extracted according to the requirements of the RNA extraction kit (Tiangen, Beijing, China). RNA was reverse transcripted into cDNA using a reverse transcription kit (Vazyme Biotech, Nanjing, China), and the cDNA was diluted 15-fold in deionized water for RT-qPCR analysis.
Gene expression analysis by RT-qPCR
Genes involved in GA, auxin, cytokinins, ethylene, jasmonic acid, abscisic acid, and brassinolide pathways were selected from CeleryDB (Feng et al. 2018). Specific primers were designed using Primer Premier 6.0 for RT-qPCR and submitted to Nanjing Genscript Inc. for synthesis. AgACTIN was used as a standard analysis of the results of the celery reference gene (Li et al. 2014, 2016). RT-qPCR analysis was performed according to TaKaRa SYBR Premix Ex Taq (Takara, Dalian, China) using a 15-μL system with 1.5 μL of diluted cDNA, 0.3 μL of forward and reverse primer pairs, 7.5 μL of SYBR Premix Ex Taq, and 5.4 μL of ddH2O. Each reaction performed in three biological repeats. The operating procedure for RT-qPCR is 95 °C for 30 s, 40 cycles for 5 s at 95 °C, followed by 60 °C for 30 s, and melting curve analysis, increasing 0.5 °C at 5 s intervals. The relative expression of selected gene was calculated by formula 2−ΔΔCt method (Pfaffl 2001). The primer sequences of the genes are shown in Tables 1 and 2.
Statistical analysis
Duncan method was applied to analyze difference at the 0.05 significance level using the SPSS statistics software.
Results
Effects of GA3 or PBZ treatment on the growth of celery plants
In order to investigate the effects of exogenous GA3 or PBZ treatment on the growth of celery, the celery leaves treated with GA3 or PBZ were collected. The phenotypes of “Jinnan Shiqin” under different treatments were shown in Fig. 1. The number of celery leaf blades treated with GA3 was the highest, which was 1.035-fold than that of the control. The number of celery leaves treated with PBZ was the lowest, which were 1.035- and 0.779-fold than that of the control (Fig. 2). The co-treatment with GA3 and PBZ caused a phenotype similar to that of the control, which was the intermediate between the GA3 and the PBZ-treated plants. Compare with other treatments, the area and the number of celery leaf blade treated with GA3 were the highest, which were 1.028- and 1.035-fold than that of the control, respectively (Fig. 3).
The celery leaves were evaluated by measuring the lengths and fresh weights (Figs. 4 and 5). The length of the petiole between the different treatments was not obviously different. The length of the petiole under GA3 + PBZ treatment was 1.077-fold than that of the PBZ treatment. Compared with the control, the length of the petiole under GA3 treatment increased, and the effect was also observed when GA3 was applied together with PBZ. The length of the petiole under GA3, PBZ, and the GA3 + PBZ were 1.013-, 0.991-, and 1.066-fold than that of the control, respectively. Treatment with GA3 increased the leaf weight of celery, but this phenomenon was alleviated when GA3 and PBZ were applied simultaneously. The root weights of the GA3 + PBZ-treated plants were the intermediate between the GA3 and the PBZ-treated plants, which were 0.381- and 1.305-fold than that of GA3 and PBZ, respectively. In general, treatment with GA3 stimulated the leaf growth of celery.
Anatomical structure analysis of celery under GA3 treatments
In order to clarify the effect of exogenous GA3 or PBZ treatment on the development of celery leaf tissue, the anatomical structure of celery leaves was visualized by safranin-O/fast green staining and observed by microscopic technique (Fig. 6). Among them, the distribution of epidermal cells, palisade tissue, sponge tissue, and vascular bundles were clearly observed. Lignin is mainly distributed in the epidermis, collenchyma, and vascular bundles. The palisade tissue and sponge tissue composed the tissue structure of celery leaves. In this study, the degrees of lignification in celery leaves were distinct under different treatment conditions. The accumulation of lignin in vascular bundles was most obvious under GA3 treatment, especially in petiole, showing a distinct purple-red color. Sponge tissue (St) and palisade tissue (Pt) were also arranged more closely, and these results were consistent with observations under UV fluorescence (Fig. 7). Anatomical structure analysis showed that the applied GA3 resulted in significant lignification of the xylem of celery leaves.
Fluorescence micrographs of transverse sections of celery leaves (leaf blades and petioles) treated with GA3, PBZ or GA3 + PBZ. C, collenchyma; Ep, epidermis; X, xylem; V, vascular bundles; Pt, palisade tissue; St, spongy tissue. Scale bars in the figure are 20 μm in length. White lines in the lower right corner of the figure a–h panels are 20 μm in length
Effects of GA3 treatment on the expression levels of GA biosynthetic genes
AgKS, AgKO, AgKAO, AgGA20ox1, AgGA20ox2, AgGA3ox1, AgGA2ox1, AgGA2ox2, and AgGA2ox3 genes were annotated as GA biosynthetic pathway-related genes based on celery genome database. To illustrate the effects of applied GA3 on GA biosynthesis, we selected these genes and detected the changes in the expression profiles of these genes by RT-qPCR (Table 1; Fig. 8). Compared with the control, exogenous GA3 treatment promoted the transcription levels of AgKO, AgKAO, AgGA20ox1, AgGA20ox2, AgGA3ox1, AgGA2ox1, and AgGA2ox3 in leaf blades, but decreased the transcription levels of AgKS and AgGA2ox2. Similarly, under the treatment of GA3 + PBZ, the expression levels of AgKS decreased, and the expression levels of AgKAO, AgGA20ox1, AgGA20ox2, AgGA3ox1, AgGA2ox1, AgGA2ox2, and AgGA2ox3 increased. Under PBZ treatment, the expression levels of AgKS, AgKO, AgGA3ox1, and AgGA2ox2 decreased. Among them, AgKO and AgGA3ox1 showed upregulation under GA3 treatment and reached the highest expression levels, which were 2.532- and 2.182-fold than that of the GA3 + PBZ, respectively.
In the petiole, exogenous GA3 upregulated the expression levels of AgGA20ox1, AgGA20ox2, AgGA2ox, and AgGA2ox3 and downregulated the mRNA abundance of AgKS, AgKO, AgKAO, AgGA3ox1, and AgGA2ox1. Under GA3 + PBZ treatment, the expression levels of AgGA20ox2 in leaf blade and petiole markedly increased to the highest value, which was 11.844- and 16.495-fold than that of the control, respectively. In addition, the expression levels of the AgGA20ox1 and AgGA20ox2 were high and peaked at the treatment of GA3 + PBZ treatment, which were 7.989- and 9.940-fold than that of the GA3, respectively. Under different treatments, the expression levels of AgGA20ox1 and AgGA20ox2 in leaves showed consistent characteristics. Overall, for most GA biosynthetic genes, exogenous GA3 treatment upregulated its expression in leaves, and on the other hand, treatment with GA3 + PBZ promoted its expression in the petiole.
Effects of GA3 or PBZ treatment on the expression levels of GA response genes
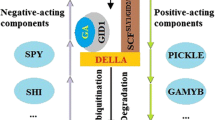
There is a molecular network of GA signal transduction in plants. When the gibberellin receptor senses the GA signal, it activates the signal transduction pathway and regulates the expression of downstream genes, thereby affecting plant growth and morphogenesis. The genes encoding gibberellin receptors and important components of signal transduction, such as AgGID1b, AgGID1c, AgDELLA, AgSLY1, AgSPY, AgGAMYB, and AgSHI, were examined in the celery. These genes were used to initially analyze the effect of GA3 or PBZ treatment on the response of GA signals (Table 1; Fig. 9). In the leaf blade, exogenous GA3 downregulated the expression levels of AgGID1b, AgGID1c, AgSPY, and AgGAMYB and upregulated the expression levels of AgDELLA, AgSHI, and AgSLY1. In the petiole, the expression levels of AgGID1b, AgGID1c, AgSPY, and AgGAMYB under GA3 treatment decreased, and the expressions levels of AgDELLA, AgSLY1, AgGAMYB, AgSPY, and AgSHI reached the highest value under GA3 + PBZ treatment. The expression levels of AgDELLA and AgSLY1 under PBZ treatment were significantly reduced, and GA3 treatment downregulated the expression of GA response genes in petiole at different degrees. The expression levels of different GA response genes in celery leaves were distinct under gibberellin treatment. In some aspects, the expression of these genes can be used to analyze the effect of GA3 treatment on signal transduction.
Effects of GA3 or PBZ treatment on the expression levels of genes implicated in other hormone pathway
In order to verify the effects of other plant hormone regulation on gibberellin synthesis and metabolism, we selected seven related genes AgABAH1, AgTSB, AgDWF7, AgLOG1, AgLOG3, AgSAMS2, and AgJM from the auxin, cytokinins, ethylene, jasmonic acid, abscisic acid, and brassinolide biosynthesis synthetic pathways and analyzed their response to GA3 or PBZ treatment (Table 2; Fig. 10). Under GA3 treatment, the expression levels of seven selected genes in the leaf blades were downregulated. In the leaf blades, under the PBZ treatment, the expression profiles of AgDWF7 and LOG1 were significantly upregulated, and transcription was inhibited when GA3 was applied, which was ameliorated by application of GA3 + PBZ treatment. Similarly, ABAH1 and AgSAMS2 in the petiole were alleviated under GA3 + PBZ treatment. The transcription levels of AgSAMS2 was 1.040-fold than that of the control in the leaf blades under GA3 + PBZ treatment. In addition, in the petiole, the transcription levels of AgSAMS2 were 1.046- and 8.940-fold than that of the GA3 + PBZ and GA3 respectively under PBZ treatment. Treatment with GA3 or PBZ resulted in changes in the expression levels of hormone-related genes in celery plants.
Effects of GA3, PBZ, or GA3 + PBZ on the expression levels of genes involved in auxin, cytokinins, ethylene, jasmonic acid, abscisic acid, and brassinolide biosynthesis synthetic pathways. Bars represent the mean values of three biological replicates ± standard deviation. Different letters in columns indicate statistically significant differences (P < 0.05)
Discussion
GAs, one type of plant hormones, have been widely used to regulate plant growth in horticultural and agricultural production. The most obvious physiological effect of GA is promoting stem growth. In addition, it also plays roles in seed germination, male flower formation of some dioecious plants, and induction of aleurone in aleurone (Mikihiro et al. 2003; Achard et al. 2009; Susana et al. 2009; Chen et al. 2016).
Celery is a leafy vegetable crop widely cultivated worldwide (Li et al. 2018). It is important to increase the yield by regulating the growth of celery leaves. In this experiment, GA3 were found promoting the length of petioles and inhibiting root growth. Previous studies have shown that gibberellin treatment significantly affects the distribution of dry matter in the aboveground and underground parts of plants (Wang et al. 2015a).
GA regulates many key enzymes of the GA synthesis pathway through feedforward or feedback. Transcription level of AtKAO gene is negatively regulated by active GA during seed germination (Helliwell et al. 1998, 1999). Overexpression of GA20ox can increase the endogenous activity of gibberellin in plants, allowing Arabidopsis to flower in advance (Huang et al. 1998). Excessive GA20ox can lead to excessive synthesis of GA and significantly promote plant growth (Huang et al. 1998). GA20ox is a key rate-limiting process in GA synthesis, and GA30ox is also a key factor regulating the synthesis of active GA during seed germination (Yukika et al. 2004). The expression levels of GA20ox and GA3ox are negatively regulated by feedback of GA, while GA2ox gene expression is positively regulated by feedforward of GA (Coles et al. 1999; Hedden and Phillips 2000). Previous research have shown that the transcription of GA2ox1 was promoted when treated with active GA in Arabidopsis (Phillips et al. 1995; Thomas et al. 1999).
Our results indicated that the expression of KAO gene was positively and negatively regulated by GA3 in the growth process of celery leaf blade and petiole respectively, showing an obvious tissue-specific. GA2ox3 was induced under excessive GA treatment, and GA2ox1 and GA2ox2 also exhibited tissue specificity. The expression trends of GA20ox1 and GA20ox2 were same under different treatments in the same period, and the expression of GA20ox1 and GA20ox2 was the highest under the GA3 + PBZ treatment. Plant GA synthesis and metabolic pathways responding to active GA are carried out in two ways. Steady-state equilibrium of active GA levels in plants is achieved by altering the GA synthesis reaction (upregulating or downregulating the expression levels of the GA20ox and GA3ox genes) or the GA passivation reaction (downregulating or upregulating the expression level of GA2ox).
Gibberellins also have synergistic or antagonistic effects with auxin, abscisic acid, cytokinins, ethylene, and brassinolide. SPINDLY (Spy) is a negative regulator of gibberellin signaling, but the Arabidopsis spy mutant also exhibits insensitivity to exogenous cytokinins. In the leaves, exogenous GA3 suppressed AgSPY but increased DcSPY in carrot (Wang et al. 2015b). This indicated that there was a difference in the SPY expression in different species. Spy mutations in Arabidopsis and gibberellin treatment inhibit the typical cytokinins phenotype (Yaarit et al. 2005). And whether cytokinin is involved in the regulation of gibberellin synthesis and signal transduction remains inconclusive (Jasinski et al. 2005; Yaarit et al. 2005; Yanai et al. 2005). Ethylene treatment can delay the degradation of DELLA protein mediated by root tip GA in Arabidopsis thaliana, leading to high levels of DELLA protein accumulation and inhibition of root growth (Achard et al. 2003). We found that exogenous gibberellin treatment significantly reduced the transcriptional abundance of the ethylene signal gene SAMS2 in the petiole of celery, and decreased the transcript level of DELLA significantly, but increased the transcription level of DELLA in the leaf blade significantly. ABA and GA have antagonistic effects (Zentella et al. 2007). Similarly, GA3 treatment reduced the expression level of ABA-related gene ABAH1 in celery leaves. ABA synthesis-deficient mutant aba2 passes gibberellin synthesis ability through GA3ox1 and GA3ox2 (Eunkyoo et al. 2007). At the seedling stage, ABA treatment can reduce the transcription level of GA2ox1 gene (Zentella et al. 2007).
The biosynthesis of GA is affected by the regulation of other different hormones, which is a common phenomenon. But the type of specific genes regulated may vary from species to species. By applying exogenous GA3 or PBZ to change the gibberellin content in plants, it may also change the interaction with other hormones to jointly control the growth of celery (Eunkyoo et al. 2007). The synthesis and metabolism of GA are strictly controlled in time and space. Due to the complexity of hormone action and the limitations of research methods, the understanding of the interaction between these plant hormones is still very limited.
Abbreviations
- CPS:
-
ent-copalyl diphosphate synthase
- GA:
-
Gibberellin
- GA3 :
-
Gibberellic acid
- GA20ox:
-
GA20-oxidase
- GA2ox:
-
GA2-oxidase
- GA3ox:
-
GA3-oxidase
- GGPP:
-
Geranylgeranyl diphosphate
- GID1:
-
Gibberellin insensitive dwarf1
- IPP:
-
Isopentenyl pyrophosphate
- KAO:
-
ent-kaurenoic acid oxidase
- KO:
-
ent-kaurene oxidase
- KS:
-
ent-kaurene synthase
- PBZ:
-
Paclobutrazol
- RT-qPCR:
-
Quantitative real-time polymerase chain reaction
- UV:
-
Ultraviolet
- SHI:
-
Short internode
- SLY1:
-
Sleepy1
- SPY:
-
Spindly
References
Achard P, Vriezen WH, Van Der Straeten D, Harberd NP (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15(12):2816–2825
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311(5757):91–94
Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GTS, Genschik P (2009) Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr Biol 19(14):1188–1193
Bai MY, Shang JX, Oh E, Fan M, Bai Y, Zentella R, Sun TP, Wang ZY (2012) Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat Cell Biol 14(8):810–817
Chen S, Wang XJ, Zhang LY, Lin SS, Liu DC, Wang QZ, Cai SY, El-Tanbouly R, Gan LJ, Wu H, Li Y (2016) Identification and characterization of tomato gibberellin 2-oxidases (GA2oxs) and effects of fruit-specific SlGA2ox1 overexpression on fruit and seed growth and development. Hortic Res 3:16059
Coles JP, Phillips AL, Croker SJ, García-Lepe R, Lewis MJ, Hedden P (1999) Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J 17(5):547–556
Eunkyoo O, Shinjiro Y, Jianhong H, Jikumaru Y, Byunghyuck J, Inyup P, Hee-Seung L, Tai-Ping S, Yuji K, Giltsu C (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19(4):1192–1208
Feng K, Hou XL, Li MY, Jiang Q, Xu ZS, Liu JX, Xiong AS (2018) CeleryDB: a genomic database for celery. Database (Oxford) 2018. https://doi.org/10.1093/database/bay070
Gallego-Bartolomé J, Minguet EG, Grau-Enguix F, Abbas M, Locascio A, Thomas SG, Alabadí D, Blázquez MA (2012) Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proc Natl Acad Sci U S A 109(33):13446–13451
Gao XH, Xiao SL, Yao QF, Wang YJ, Fu XD (2011) An updated GA signaling ‘relief of repression’ regulatory model. Mol Plant 4(4):601–606
Hedden P (2001) Gibberellin metabolism and its regulation. J Plant Growth Regul 20(4):317–318
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5(12):523–530
Helliwell CA, Sheldon CC, Olive MR, Walker AR, Zeevaart JA, Peacock WJ, Dennis ES (1998) Cloning of the Arabidopsis ent-kaurene oxidase gene GA3. Proc Natl Acad Sci U S A 95(15):9019–9024
Helliwell CA, Poole A, Peacock WJ, Dennis ES (1999) Arabidopsis ent-kaurene oxidase catalyzes three steps of gibberellin biosynthesis. Plant Physiol 119(2):507–510
Helliwell CA, Chandler PM, Poole A, Dennis ES, Peacock WJ (2001) The CYP88A cytochrome P450, ent-kaurenoic acid oxidase, catalyzes three steps of the gibberellin biosynthesis pathway. Proc Natl Acad Sci U S A 98(4):2065–2070
Huang S, Raman AS, Ream JE, Fujiwara H, Cerny RE, Brown SM (1998) Overexpression of 20-oxidase confers a gibberellin-overproduction phenotype in Arabidopsis. Plant Physiol 118(3):773–781
Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M (2005) KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol 15(17):1560–1565
Li MY, Wang F, Jiang Q, Ma J, Xiong AS (2014) Identification of SSRs and differentially expressed genes in two cultivars of celery (Apium graveolens L.) by deep transcriptome sequencing. Hortic Res 1:10
Li MY, Wang F, Jiang Q, Wang GL, Tian C, Xiong AS (2016) Validation and comparison of reference genes for qPCR normalization of celery (Apium graveolens) at different development stages. Front Plant Sci 7:313
Li MY, Hou XL, Wang F, Tan GF, Xu ZS, Xiong AS (2018) Advances in the research of celery, an important Apiaceae vegetable crop. Crit Rev Biotechnol 38(2):172–183
Mikihiro O, Atsushi H, Yukika Y, Ayuko K, Yuji K, Shinjiro Y (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15(7):1591–1604
Olszewski N, Sun TP, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14:Suppl:S61–Suppl:S80
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Phillips AL, Ward DA, Uknes S, Appleford NE, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P (1995) Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol 108(3):1049–1057
Ross JJ, O’Neill DP (2002) Auxin regulation of the gibberellin pathway in pea. Plant Physiol 130(4):1974–1982
Ross JJ, O’Neill DP, Smith JJ, Kerckhoffs LH, Elliott RC (2010) Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J 21(6):547–552
Silverstone AL, Sun T (2000) Gibberellins and the green revolution. Trends Plant Sci 5(1):1–2
Susana UT, Fernán F, Ilda C, Beemster GTS, Rishikesh B, Ranjan S, Peter D, Jim H, Bennett MJ (2009) Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr Biol 19(14):1194–1199
Thomas SG, Phillips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2- oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci U S A 96(8):4698–4703
Ubeda-Tomás S, Federici F, Casimiro I, Beemster GTS, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett MJ (2009) Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr Biol 19(14):1194–1199
Wang GL, Feng Q, Xu ZS, Feng W, Xiong AS (2015a) Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol 15:290
Wang GL, Xiong F, Que F, Xu ZS, Wang F, Xiong AS (2015b) Morphological characteristics, anatomical structure, and gene expression: novel insights into gibberellin biosynthesis and perception during carrot growth and development. Hortic Res 2:15028
Wang GL, Huang Y, Zhang XY, Xu ZS, Wang F, Xiong AS (2016) Transcriptome-based identification of genes revealed differential expression profiles and lignin accumulation during root development in cultivated and wild carrots. Plant Cell Rep 35(8):1743–1755
Wolbang CM, Ross JJ (2001) Auxin promotes gibberellin biosynthesis in decapitated tobacco plants. Planta 214(1):153–157
Wolbang CM, Chandler PM, Smith JJ, Ross JJ (2004) Auxin from the developing inflorescence is required for the biosynthesis of active gibberellins in barley stems. Plant Physiol 134(2):769–776
Yaarit GW, Inbar M, Roy B, John A, Neil O, Naomi O, Yuval E, David W (2005) Cross talk between gibberellin and cytokinin: the Arabidopsis GA response inhibitor SPINDLY plays a positive role in cytokinin signaling. Plant Cell 17(1):92–102
Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, Samach A, Ori N (2005) Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol 15(17):1566–1571
Yukika Y, Mikihiro O, Ayuko K, Atsushi H, Yuji K, Shinjiro Y (2004) Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16(2):367–378
Zentella R, Zhang Z, Park M, Thomas SG, Endo A, Murase K, Fleet C, Jikumaru Y, Nambara E, Kamiya Y (2007) Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19(10):3037–3057
Zhuang WB, Gao ZH, Wen LH, Huo XM, Cai BH, Zhang Z (2015) Metabolic changes upon flower bud break in Japanese apricot are enhanced by exogenous GA4. Hortic Res 2:15046
Funding
The research was supported by Jiangsu Agricultural Science and Technology Innovation Fund [CX(18)2007], National Natural Science Foundation of China (31272175), and Priority Academic Program Development of Jiangsu Higher Education Institutions Project (PAPD).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: ASX AQD. Performed the experiments: AQD KF JXL ZSX. Analyzed the data: AQD KF ASX. Contributed reagents/materials/analysis tools: ASX. Wrote the paper: AQD. Revised the paper: ASX FQ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Klaus Harter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, AQ., Feng, K., Liu, JX. et al. Elevated gibberellin altered morphology, anatomical structure, and transcriptional regulatory networks of hormones in celery leaves. Protoplasma 256, 1507–1517 (2019). https://doi.org/10.1007/s00709-019-01396-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01396-w