Abstract
The response of Dendrobium officinale Kimura et Migo (D. officinale) to continuous UV-B irradiation at different carbon to nitrogen ratios (C/N ratios) was investigated. Seedlings grown for 60 days were incubated under aseptic conditions with UV-B irradiation (15.6 µW cm−2) at different C/N ratios: control group (CK; C/N 30 without UV-B), UV-B + CK (C/N 30 with UV-B irradiation, similarly hereafter), UV-B + C/N 120, UV-B + C/N 60, UV-B + C/N 15, UV-B + C/N 10, UV-B + C/N 7.5. Growth parameters (the defoliation rate and the sprout number), photosynthetic pigments (carotenoids, chlorophyll a and chlorophyll b), total polysaccharides, total alkaloids, and activities of antioxidant enzymes were determined following 4, 8, 12, and 16 days of continuous UV-B exposure. Results indicated that UV-B irradiation increased the defoliation rate and the content of carotenoids, total polysaccharides and total alkaloids, as well as the activities of antioxidant enzymes. Conversely, UV-B irradiation reduced the sprout number and chlorophyll content in D. officinale. Compared with UV-B + CK, lower C/N ratio treatments (UV-B + C/N 15, UV-B + C/N 10 and UV-B + C/N 7.5) enhanced the defoliation rate and sprout number, but decreased antioxidant enzyme activities and total polysaccharide content during the whole period, and reduced total alkaloid content after 4 days of UV-B exposure. Following initial UV-B irradiation, lower C/N ratios increased the contents of carotenoid and chlorophyll b, while after 8 days, a reversal in carotenoid content was observed, and after 12 days, a reversal in chlorophyll b content was observed. Optimizing the C/N ratio (C/N 60) resulted in lower defoliation rate, higher photosynthetic pigments and total polysaccharides, and increased activities of antioxidant enzymes, whereas no significant change in sprout number and total alkaloid content was recorded under long-term UV-B irradiation. Furthermore, the UV-B + C/N 120 treatment negatively affected D. officinale in terms of an increased defoliation rate and reduced sprout number, photosynthetic pigments, and total alkaloids. Therefore, results suggested that an appropriate C/N ratio (C/N 60) could ameliorate the adverse effects of continuous UV-B irradiation on D. officinale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solar ultraviolet radiation (UV; 100–400 nm) is known as one of the most damaging agents that living organisms are exposed to (Grandahl et al. 2018). According to wavelength and biological effects, UV can be divided into UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm). Although UV-B radiation is a minor portion of the solar UV radiation, it is possibly the most damaging component (Mazza et al. 2013). Currently, depletion of the stratospheric ozone layer, caused by the release of destructive chemicals, such as chlorofluorocarbons (CFCs), results in enhanced UV-B radiation reaching the Earth’s surface (Fu and Shen 2017). Previous investigations have shown that increased UV-B could reduce growth and photosynthesis of plants; cause DNA damage and increase oxidative stress by inducing reactive oxygen species (ROS) synthesis, such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide (Araujo et al. 2016; Cong and Li 2018; Singh et al. 2011a). There has been substantial research pertaining to the effects of elevated UV-B radiation on inhibition of growth and biomass of cotton (Dehariya et al. 2011), potato (Singh et al. 2011b), Chrysanthemum (Li et al. 2011), soybean (Ma et al. 2016), spinach and amaranthus (Singh et al. 2013). Recently the effects of UV-B irradiation on plant secondary metabolites have drawn great attention (Alonso et al. 2015; Kumari and Agrawal 2010; Kumari and Prasad 2013; Zhang and Bjorn 2009). For instance, Manaf et al. (2016) stated that a moderate extension of UV-B exposure time had a beneficial impact on the contents of caffeic acid and total phenols in coneflower (Echinacea purpurea (L.) Monech). Furthermore, the effects of UV-B irradiation on growth and the major active compounds might be attributed to protective mechanisms of plant, which can synthesize protective UV-B incorporating compounds, such as phenols and flavonoids, and initiate the activity of a free-radical scavenging system, such as increased antioxidase activities and induced low-molecular antioxidants (Dai et al. 2011; Martz et al. 2010; Singh et al. 2011a). It is also well documented that polysaccharides can act as free radical scavengers and inhibit DNA damage (Wong et al. 2011; Yang et al. 2017). The majority of previous studies have concentrated on the effects of short-term UV-B irradiation, while only a few have focused on continuous UV-B exposure (Schmidt et al. 2010). Carbon and nitrogen are the two essential nutrient elements for organisms, especially, nitrogen is the main constituent of amino acids, nucleotides, chlorophyll, alkaloids, and numerous other N-containing components (Zaid and Mohammad 2018). As for carbon to nitrogen (C/N) ratio, it has significant effects on the biomass productivity and nutritional components of microalgae (Lu et al. 2016) and carotenoids production of yeast (Braunwald et al. 2013), while effects on the plants have been less reported. Moreover, little is known about the responses of plants to continuous UV-B irradiation at different C/N ratios.
The dried stem of D. officinale, a prized traditional Chinese herb, has a long history of medicinal application, as recorded in a famous traditional Chinese medicine pharmacopeia, Shen Nong’s Herbal Classic. It can improve immunity, and nourish the stomach and lungs (Ng et al. 2012). Previous reports indicate that polysaccharides and alkaloids, the major active compounds of D. officinale, have immune-regulation, hepato-protective and antioxidant functions; or anticancer; and neuroprotective effects (Ng et al. 2012; Xu et al. 2013). The biosynthesis and accumulation of polysaccharides and alkaloids in D. officinale is related to numerous environmental factors, such as water, climate, mineral nutrition, and light (Lin et al. 2010; Zheng et al. 2012a, b). UV-B irradiation has enormous implications on field cultivation of D. officinale, but few studies have verified this. Furthermore, no studies have elucidated the effects of different C/N ratios on D. officinale under UV-B irradiation. Thus, the present research investigated the effects of continuous UV-B irradiation on D. officinale at different C/N ratios.
In the present study, we hypothesize that continuous UV-B irradiation may affect the growth of D. officinale in many ways, such as increasing of the defoliation rate; reducing the sprout number; altering the photosynthetic parameters; increasing the activity of antioxidant enzymes, and the amount of total polysaccharides and total alkaloids. Moreover, different C/N ratios may mitigate or exacerbate the effects of UV-B irradiation on D. officinale. Thus, it is beneficial to identify a moderate C/N ratio that may exert protective effects on D. officinale under UV-B exposure. In order to test the above hypothesis, we investigated the effects of artificial UV-B irradiation of 15.6 µW cm−2 on D. officinale at different C/N ratios under sterile conditions by determining their effects on growth; the activities of antioxidant enzymes; the content of photosynthetic pigments, total polysaccharides, and total alkaloids. Finally, we elucidated the optimal C/N ratio to alleviate the damage caused by continuous UV-B irradiation.
Materials and Methods
Surface sterilized seedlings of D. officinale, grown for 60 days, were provided by our laboratory (Key Laboratory of State Administration of Traditional Chinese Medicine for Production & Development of Cantonese Medicinal Materials). The optimal growth medium for seedlings was a basic solid 1/2 Murashige and Skoog medium (MS medium) with half-strength macronutrients and 30 g/L sucrose, supplemented with 2.0 mg/L naphthalene acetic acid (NAA). Sucrose served as the carbohydrate source and inorganic nitrogen (ammonium nitrate and potassium nitrate) served as the nitrogen source. The first treatment utilized 1/2 MS medium with a C/N ratio of 30, which contained 30 g/L sucrose, 0.289 g/L ammonium nitrate and 0.132 g/L potassium nitrate. The other treatments were designed by varying the nitrate nitrogen content of this basic medium while maintaining a consistent carbon content (Table 1). Seedlings characterized as green and healthy, with an average height of 5–6 cm, were chosen for incubation using the above treatment medium. All cultures were incubated at 25 ± 2 °C, under the intensity of 2000 lx from white fluorescent tubes (TL5- 28 W/1162 mm, Philips, China) with a 12: 12-h light: dark cycle. The distance between the seedlings and the white fluorescent tubes was 30 cm.
After 60 days for consistency, these cultures were divided into two groups: the control group (CK) and the experimental group. The control group was treated with C/N 30 without UV-B irradiation, while the experimental groups had differing C/N ratios with 15.6 μW cm−2 UV-B irradiation: UV-B + C/N 120 (C/N 120; similarly hereafter), UV-B + C/N 60, UV-B + CK (C/N 30), UV-B + C/N 15, UV-B + C/N 10, UV-B + C/N 7.5. Supplemental UV-B was provided in doses of 15.6 μW cm−2 (UV-B-313, LongPro co., LTD, China), for 4 h per day (from 12:00–16:00 h) for a sequential 16 days, imitating the exposure time of maximum UV-B intensity in shade in Guangdong province, during the D. officinale growth and development season. Each treatment had three replications. At 4, 8, 12, and 16 days after UV-B treatment, the followings were determined:
Growth Parameters
Growth parameters were determined in terms of brown leaves, the number of sprouts and the defoliation rate. A brown leaf was defined as a leaf in which greater than 50% of the leaf area turned brown. The number of sprouts was determined by the quantitative difference between the total numbers of buds before and after irradiation. The defoliation rate was calculated as follows: defoliation rate = (number of deciduous leaves + number of brown leaves)/total number of leaves.
Determination of Photosynthetic Pigments
Carotenoids (Car), chlorophyll a (Chl a), and chlorophyll b (Chl b) were extracted from 0.1 g blade with 5 mL 80% acetone. The extract was separated by centrifugation at 6000×g for 10 min, and absorbances were recorded at 470, 646 and 663 nm by ultraviolet and visible spectrophotometer (UV755B, Shanghai APL instrument co., LTD, China). Contents of Car, Chl a, and Chl b were determined according to the formulas below (Wellburn and Lichtenthaler 1984):
A = absorbance.
Determination of Antioxidant Enzyme Activities
Fresh leaf blades (0.5 g) were ground with 5 mL 0.1 M phosphate buffer (pH 6.8) on an ice-bath. The homogenate was centrifuged at 12,000×g for 15 min at 4 °C. The supernatant was used as the enzyme extract for assays for superoxide dismutase, guaiacol peroxidase (POD) and catalase (CAT) activity. SOD activity was determined following the method of Beyer and Fridovich (1987). Briefly, the reaction solution contained 50 mM sodium phosphate buffer, 2.1 mM methionine, 100 μM ethylene diamine tetraacetic acid (EDTA), 1.72 mM nitroblue tetrazolium chloride (NBT), 0.24 mM riboflavin, and 50 μL enzyme extract. The solution was allowed to react for 20 min under fluorescent light in 4000 lx and then kept in the dark immediately. The reference solution in the absence of enzyme was placed in the dark as a control group (CK). Finally, the absorbance was determined at 560 nm, SOD activity was calculated as follows:
ACK = absorbance of the reference solution, AR = absorbance of the reaction solution, V = volume of the extracting solution, VR = volume of the reaction solution used for determination at 560 nm, W = weight of the fresh leaf blades.
One unit of SOD was defined as the quantity of enzyme required to inhibit the rate of NBT photoreduction by 50%. POD and CAT activities were estimated by the method of Chance and Maehly (1955), with some modification: the POD reaction liquid consisted of 50 mM sodium phosphate buffer (pH 7.8, Tianjin damao chemical reagent factory, China), 200 mM hydrogen peroxide (H2O2, Tianjin zhiyuan chemical reagent factory, China), 50 mM guaiacol (CAS: 90-05-1, Aladdin, China), and enzyme extract. The absorbance of the reaction liquid was recorded at 470 nm within 3 min. The POD activity was calculated as follows:
ΔA470 = changes in absorbance during the reaction time, V = volume of the extracting liquid, VR = volume of the reaction liquid used for determination at 470 nm, W = weight of fresh leaf blades, t = reaction time.
One unit of POD was defined as the change in the absorbance of the reaction liquid by 0.01 at 470 nm. The CAT reaction solution contained 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 200 mM H2O2 and enzyme extract. The absorbance was recorded at 240 nm. CAT activity was determined as follows:
ΔA240 = the change in absorbance during the reaction time, V = volume of the extracting solution, VR = volume of the reaction solution used for determination at 240 nm, W = weight of fresh leaf blades, t = reaction time.
One unit of CAT was defined as the change in the absorbance of the reaction solution by 0.01 at 240 nm.
Determination of Total Polysaccharides and Total Alkaloids
Total polysaccharides were determined according to the China Pharmacopeia Committee (2015). Dry stem (2.0 g) was crushed in 2 mL distilled water with a mortar and pestle. The homogenate, with 200 mL distilled water, was refluxed for 2 h and allowed to cool to ambient temperature. The extract was filtrated and transferred to a 250-mL volumetric flask. A 6-mL aliquot from the 250-mL crude extract was centrifuged (4000×g) with 30 mL ethanol for 30 min. The precipitate was collected and washed twice with 80% ethanol (24 mL) and centrifuged again. The resulting precipitate was redissolved in boiling water and transferred to a 10-mL volumetric flask as purified extract. A 1-mL aliquot of the extracts was used for total polysaccharides determination at 488 nm.
Total alkaloids were measured according to Zheng et al. (2012a). Briefly, 0.4 g dry stem was soaked in ammonia solution (30 min) and refluxed with 10 mL chloroform for 2 h. The extract was filtered, and then 5 mL of the filtrate was transferred to a separatory funnel with 5 mL chloroform, 5 mL buffer (pH 4.5) and 1 mL 0.04% bromocresol green solution. Afterward, the upper fraction from the separatory funnel was filtered. A 5-mL aliquot of the filtrate was contained with 1 mL 0.01 mol/L NaOH for determination. The absorbance was recorded at 620 nm.
Statistical Analysis
All experiments were replicated three times. The data were subjected to one-way analysis of variance (ANOVA) using SPSS statistical software package (ver. 23.0). Differences of treatment means were compared by the Duncan’s multiple range tests at P < 0.05.
Results
Growth Parameters
Compared with CK, UV-B irradiation caused damage to D. officinale blades in terms of the increased defoliation rate under UV-B + CK, with an increasing defoliation rate observed upon prolonged UV-B exposure (Figs. 1a, 2). Treatment with C/N 60 to UV-B treated D. officinale seedlings (UV-B + C/N 60) decreased the defoliation rate in comparison with UV-B + CK, while other treatments increased the defoliation rate even further. Especially under lower C/N ratio treatments (UV-B + C/N 15, UV-B + C/N 10 and UV-B + C/N 7.5), the defoliation rate was higher as treatment C/N ratios decreased over the entire UV-B irradiation period. After 16 days of irradiation, the defoliation rate was 60.67, 32.73, 44.64, 61.65, 60.10 and 84.07% for C/N ratios from 120 to 7.5 (UV-B + C/N 120, UV-B + C/N 60, UV-B + CK, UV-B + C/N 15, UV-B + C/N 10 and UV-B + C/N 7.5), respectively. This result showed that UV-B + C/N 60 had a lower defoliation rate and more positive effect on UV-B treated plants during the whole period.
Effects of UV-B irradiation on the defoliation rate (a) and the number of sprouts (b) of D. officinale seedlings at different C/N ratios. Data expressed as mean ± SD (n = 3). Data followed by different letters indicate significant differences between means within each parameter (4, 8, 12, and 16 days) according to the Duncan’s multiple range tests (P < 0.05)
The number of sprouts decreased after exposure to UV-B irradiation, with the decrease in sprouts becoming more pronounced as exposure time increased. Treatment UV-B + C/N 60 showed no significant differences with the UV-B + CK during the whole period. However, lower C/N ratio treatments (UV-B + C/N 15, UV-B + C/N 10 and UV-B + C/N 7.5) led to an increase in the number of sprouts, when compared to UV-B + CK, regardless of the UV-B exposure duration (4, 8, 12 or 16 days) (Figs. 1b, 2). After 16 days of UV-B exposure, the average number of sprouts was 0.500, 0.905, 0.857, 1.143, 1.333, and 1.147, for C/N ratios from 120 to 7.5 (UV-B + C/N 120, UV-B + C/N 60, UV-B + CK, UV-B + C/N 15, UV-B + C/N 10 and UV-B + C/N 7.5), respectively.
Photosynthetic Pigments
Compared with CK, treatment UV-B + CK induced a reduction in the Chl a content by 18.38, 7.66, 13.57, and 21.25% after 4, 8, 12, and 16 days of UV-B irradiation, respectively (Fig. 3 a). During 8 days of UV-B exposure, with the exception of UV-B + C/N 120, there was no significant increase under the other treatments as compared to UV-B + CK. However, after 16 days of UV-B exposure, plants under treatment UV-B + C/N 60 improved Chl a content by 15.52% with respect to that of the plants under UV-B + CK (Fig. 3a).
Effects of UV-B irradiation on the content of chlorophyll a (a), chlorophyll b (b) and carotenoid (c) in D. officinale seedlings at different C/N ratios. Data expressed as mean ± SD (n = 3). Data followed by different letters indicate significant differences between means within each parameter (4, 8, 12, and 16 days) according to the Duncan’s multiple range tests (P < 0.05)
The Chl b content was also negatively influenced by UV-B irradiation and it significantly decreased by 29.11, 15.81, 31.70, and 28.66% after 4, 8, 12, and 16 days of UV-B exposure, respectively, in treatment UV-B + CK as compared to that of CK (Fig. 3b). However, this effect could be ameliorated by treatment UV-B + C/N 60 after 4 days (4.75%), 8 days (4.02%), 12 days (18.35%), and 16 days (26.62%) of UV-B exposure relative to UV-B + CK. Furthermore, lower C/N ratio treatments (UV-B + C/N 15, UV-B + C/N 10, and UV-B + C/N 7.5) increased the Chl b content during 12 days of UV-B exposure and then decreased at 16 days, but treatment UV-B + C/N 120 decreased the Chl b content during the whole period, compared with UV-B + CK (Fig. 3b).
UV-B irradiation in UV-B + CK enhanced the Car content by 21.40, 25.48, 17.09, and 24.21% after 4, 8, 12, and 16 days of UV-B exposure, respectively, compared with CK (Fig. 3c). Lower C/N ratio treatments (UV-B + C/N 15, UV-B + C/N 10 and UV-B + C/N 7.5) increased the Car content during 8 days of UV-B exposure and then decreased at the following days as compared to UV-B + CK. During the initial stage of UV-B irradiation, the Car content showed a non-significant change under treatment UV-B + C/N 60, while after 8 days, it increased by 16.31% (12 days) and 9.52% (16 days),compared with UV-B + CK (Fig. 3c). These results showed that a suitable C/N ratio (i.e., C/N 60) was probably beneficial to chlorophyll synthesis under prolonged UV-B irradiation and may aid in resisting UV-B damage to a maximum degree (Fig.3a–c).
Activities of Antioxidant Enzymes
UV-B irradiation enhanced the activity of SOD by 147.77, 32.65, 2.93, and 4.70% after 4, 8, 12, and 16 days of UV-B irradiation in treatment UV-B + CK when compared with the control (Fig. 4a). Lower C/N ratio treatments (UV-B + C/N 15, UV-B + C/N 10, and UV-B + C/N 7.5) caused decrease in SOD activity during the whole period, with respect to that of the UV-B + CK. However, treatments UV-B + C/N 60 and UV-B + C/N 120 increased the SOD activities by 96.34 and 71.98%, respectively, after 12 days of UV-B exposure, and by 63.67% and 21.32%, respectively, after 16 d of UV-B exposure, as compared to UV-B + CK (Fig. 4a).
Effects of UV-B irradiation on the activity of SOD (a), POD (b) and CAT (c) of D. officinale seedlings at different C/N ratios. Data expressed as mean ± SD (n = 3). Data followed by different letters indicate significant differences between means within each parameter (4, 8, 12, and 16 days) according to the Duncan’s multiple range tests (P < 0.05)
POD activity also significantly increased by 171.30, 93.48, 58.52, and 96.67% in response to 4, 8, 12, and 16 days of UV-B exposure in treatment UV-B + CK compared to that of the corresponding control (Fig. 4b). Treatment UV-B + C/N 60 further increased the POD activity by 6.69, 7.07, and 6.15% after 8, 12, and 16 days of UV-B irradiation, respectively, whereas the POD activity was reduced under the other C/N ratio treatments, compared with UV-B + CK (Fig. 4b).
With the exception of a non-significant change at 8 days UV-B exposure, CAT activity was enhanced by 5.84, 25.45, and 16.67%, respectively, in response to 4, 12, and 16 days of UV-B irradiation in treatment UV-B + CK as compared with that of control plants (Fig. 4c). Moreover, the CAT activities decreased under treatments UV-B + C/N 10 and UV-B + C/N 7.5 but increased under other treatments (UV-B + C/N 120, UV-B + C/N 60, and UV-B + C/N 15) in comparison with treatment UV-B + CK. Especially, treatment UV-B + C/N 60 resulted in the maximum increase in CAT activity by 29.01, 2.10, 24.21, and 48.79%, respectively, after 4, 8, 12, and 16 days of UV-B exposure relative to treatment UV-B + CK (Fig. 4c).
Total Polysaccharides and Total Alkaloids
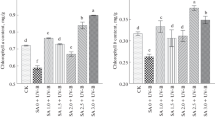
UV-B irradiation enhanced the accumulation of polysaccharides by 35.45, 18.29, 115.65, and 19.55%, respectively, after 4, 8, 12, and 16 days of UV-B exposure in treatment UV-B + CK with respect to CK (Table 2). Moreover, total polysaccharides were further enhanced in higher C/N ratio treatments (UV-B + C/N 120 and UV-B + C/N 60) but not in lower C/N ratio treatments (UV-B + C/N 15, UV-B + C/N 10, and UV-B + C/N 7.5), as compared to UV-B + CK. A maximum content of total polysaccharides was observed in treatment UV-B + C/N 60, after 16 days of UV-B irradiation (Table 2).
The total alkaloid content of D. officinale significantly increased by 77.68, 212.38, 101.30, and 52.84%, respectively, after 4, 8, 12, and 16 days of UV-B exposure in treatment UV-B + CK compared with CK (Table 3). Following initial UV-B exposure (4 days of UV-B exposure), an increase of total alkaloid content by 117.80, 48.51, and 64.39% was found under lower C/N ratio treatments (UV-B + C/N 15, UV-B + C/N 10, and UV-B + C/N 7.5), respectively, after which the total alkaloid content decreased relative to UV-B + CK. There was a significant (P < 0.05) reduction of total alkaloid content between UV-B + C/N 120 and UV-B + CK, whereas no significant difference (P > 0.05) was noticed between UV-B + C/N 60 and UV-B + CK (Table 3).
Discussion
UV-B irradiation has been shown, in the majority of studies, to significantly affect growth and synthesis of secondary metabolites (Interdonato et al. 2011; Kumari et al. 2009; Matsuura et al. 2013; Takshak and Agrawal 2014). Our experiments show that UV-B exposure inhibited growth of D. officinale as depicted by the browning of leaves, increased defoliation and reduced number of sprouts. These inhibitory effects were more prominent with prolonged UV-B irradiation (Fig. 1). It is possible that foliage, as the most sensitive organ of plant, turns to brown and deciduous to reduce UV-B penetration into leaf. This result is consistent with earlier studies on cotton plants where plant height and leaf area decreased under ambient UV-B irradiation (Dehariya et al. 2011). However, little is known about the combined effects of C/N ratios and UV-B irradiation on the growth and quality of D. officinale. Our research demonstrates that different C/N ratios might alter the deleterious effects of UV-B irradiation. A decrease in the defoliation rate and a non-significant change of the number of sprouts was observed in treatment C/N 60 under continuous UV-B exposure (Fig. 1). Thus, these results suggest that a moderate C/N ratio could counteract the adverse effects of UV-B exposure on D. officinale growth. More detailed discussions are required on the physiological and biochemical aspects to determine the exact mechanism of mitigation associated with the moderate C/N ratio.
UV-B irradiation is known to cause significant effects, not only on growth, but also on other physiological characteristics, such as photosynthetic pigments and antioxidant enzymes (Baroniya et al. 2013; Gonzalez et al. 2009; Xu et al. 2008). In the present study, UV-B exposure reduced the content of chlorophyll but enhanced the content of carotenoids, which is consistent with previous studies on Cymbopogon citratus (D.C.) Staph, Prunus dulicis, amaranthus, and spinach (Kumari and Agrawal 2010; Ranjbarfordoei et al. 2011; Singh et al. 2013). Several explanations could be given for the reduction of chlorophyll content, including degradation of chlorophyll due to the destruction of related enzymes; inhibition of photosynthetic pathway; damage to the photosynthetic capacity of chloroplasts; and decrease in the seedlings’ height and leaf area (Dehariya et al. 2011; Ranjbarfordoei et al. 2011). In addition, carotenoids protect the photosynthetic system from photo-damage by absorbing excess excitation energy, thus increased carotenoid content could alleviate the damage caused by UV-B exposure on chlorophyll (Agrawal and Rathore 2007). The response of D. officinale to continuous UV-B irradiation on the photosynthetic pigments was variable for seedlings incubated at different C/N ratios. Nitrogen, as an essential factor for the photosynthetic pigments, can stimulate pigment synthesis by facilitating N metabolism (Xu et al. 2014). In the present investigation, the photosynthetic pigment contents were augmented in treatments with lower C/N ratios (UV-B + C/N 15, UV-B + C/N 10 and UV-B + C/N 7.5) during 8 days of UV-B exposure, while following prolonged exposure, a reduction in pigments was observed. Conversely, chlorophyll and carotenoid contents increased in the UV-B + C/N 60 treatment under long-term UV-B exposure, which suggests that suitable C/N 60 ratio contributed to mitigation of UV-B-induced damage on D. officinale (Fig. 3).
Antioxidant enzymes, which scavenge free radicals and help alleviate oxidative stress, were affected by UV-B irradiation. Synthesis of antioxidant enzymes might be provoked by UV-B to maintain the metabolic stability of plants in the presence of increased ROS production (Wang et al. 2015). This was validated by our results, in which higher activities of antioxidant enzymes (SOD, POD, and CAT) were observed after exposure of D. officinale to UV-B radiation (Fig. 4). Similar findings were also reported by Takshak and Agrawal (2014) in Withania somnifera, Wang et al. (2015) in Oryza sativa L. and Yannarelli et al. (2006) in Helianthus annuus L. Furthermore, different C/N ratio treatments also had differing effects on the activities of these antioxidant enzymes. Earlier investigations reported that the activities of SOD and CAT were markedly higher under low nitrogen conditions in coffee leaves (Pompelli et al. 2010). Our study also showed that the activities of antioxidant enzymes were higher under continuous UV-B irradiation with higher C/N ratios, while the C/N 60 treatment ameliorated the oxidative damage caused by long-term UV-B irradiation to the highest degree (Fig. 4).
Polysaccharides and alkaloids are the main bioactive compounds of D. officinale (Zheng et al. 2012a). Previous studies indicate that the quality of medicinal plants could be enhanced under UV-B exposure (Sun et al. 2010). In our study, UV-B irradiation improved the total polysaccharide and alkaloid contents. Compared with CK, a highest increase in total polysaccharide content was found at 12 days exposure to UV-B (Table 2). The increase in polysaccharide accumulation might be related to its anti-UV-B effects (Wong et al. 2011). UV-B irradiation also led to an augmentation of the alkaloid synthesis, which might be due to its effective quenching of ROS induced by UV-B (Paranhos et al. 2009). This result agrees with (Pi et al. 2010), where an 11-fold enhancement in camptothecin content was found under UV-B irradiation of Camptotheca acuminata. Additionally, (Zu et al. (2010)) reported that long-time UV-B exposure significantly increased the content of taxol in Taxus chinensis var. mairei. Moreover, the total polysaccharide and alkaloid contents in D. officinale were also altered under different C/N ratios. Total polysaccharides increased with higher C/N ratios under long-term UV-B irradiation, with a maximum content observed at C/N 60 after 16 days. Conversely, total alkaloids showed a non-significant change with C/N 60 ratio under continuous UV-B exposure, with maximum content being observed at C/N 15 after 16 days of UV-B exposure. Polysaccharides are complex carbohydrates and are derived as a part of plant primary metabolism while alkaloids are biosynthesized as a part of plant secondary metabolism. The inverse trend of total polysaccharides and alkaloids might suggest a trade-off between plant primary and secondary metabolism.
In conclusion, UV-B exposure led to a reduction in growth (the increasing defoliation rate and the decreasing sprout number) and photosynthesis (chlorophyll content) compared with CK, while increased the content of total polysaccharides and total alkaloids, as well as the activities of antioxidant enzymes (SOD, POD, and CAT). The treatment UV-B + C/N 120 might aggravate the negative effects on D. officinale, such as increasing the defoliation rate and decreasing the sprout number, the activities of antioxidant enzymes, the content of photosynthetic pigments, and total alkaloids compared with UV-B + CK during the whole period. Lower C/N ratios (UV-B + C/N 15, UV-B + C/N 10 and UV-B + C/N 7.5) increased the defoliation rate and sprout number but decreased the content of total polysaccharides and activities of antioxidant enzymes compared with UV-B + CK over the entire UV-B irradiation period. At the beginning, lower C/N ratios increased the content of photosynthetic pigments and total alkaloids, while following prolonged exposure, a reduction was found in total alkaloid content after 4 days, in carotenoid content after 8 days, and in chlorophyll content after 12 days. However, a moderate C/N ratio (C/N 60) could alleviate the negative effects on D. officinale. The protective strategies associated with C/N 60 under UV-B stress mainly include reduction of defoliation; increment of chlorophyll and carotenoid contents; enhanced activities of antioxidant enzymes (SOD, POD, and CAT); accumulation of total polysaccharides; and the maintenance of total alkaloids. Further studies should be conducted to find the optimal cultivation conditions to enhance the yield and quality of D. officinale.
References
Agrawal S, Rathore D (2007) Changes in oxidative stress defense system in wheat (Triticum aestivum L.) and mung bean (Vigna radiata L.) cultivars grown with and without mineral nutrients and irradiated by supplemental ultraviolet-B. Environ Exp Bot 59:21–33. https://doi.org/10.1016/j.envexpbot.2005.09.009
Alonso R, Berli FJ, Bottini R, Piccoli P (2015) Acclimation mechanisms elicited by sprayed abscisic acid, solar UV-B and water deficit in leaf tissues of field-grown grapevines. Plant Physiol Biochem PPB 91:56–60. https://doi.org/10.1016/j.plaphy.2015.03.011
Araujo M, Santos C, Costa M, Moutinho-Pereira J, Correia C, Dias MC (2016) Plasticity of young Moringa oleifera L. plants to face water deficit and UVB radiation challenges. J Photochem Photobiol, B 162:278–285. https://doi.org/10.1016/j.jphotobiol.2016.06.048
Baroniya SS, Kataria S, Pandey GP, Guruprasad KN (2013) Intraspecific variations in antioxidant defense responses and sensitivity of soybean varieties to ambient UV radiation. Acta Physiol Plant 35:1521–1530. https://doi.org/10.1007/s11738-012-1193-6
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Braunwald T, Schwemmlein L, Graeff-Honninger S, French WT, Hernandez R, Holmes WE, Claupein W (2013) Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl Microbiol Biotechnol 97:6581–6588. https://doi.org/10.1007/s00253-013-5005-8
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
Committee CP (2015) Chinese pharmacopoeia. China Medical Science Press, Beijing, pp 282–283
Cong W, Li X (2018) The importance of short-term ultraviolet-B radiation in biomass and photosynthetic productivity of Eichhornia crassipes (Mart.) Solms. J Plant Growth Regul 37:896–910. https://doi.org/10.1007/s00344-018-9785-z
Dai J, Ma H, Fan J, Li Y, Wang J, Ni H (2011) Crude polysaccharide from an anti-UVB cell clone of Bupleurum scorzonerifolium protect HaCaT cells against UVB-induced oxidative stress. Cytotechnology 63:599–607. https://doi.org/10.1007/s10616-011-9381-6
Dehariya P, Kataria S, Pandey GP, Guruprasad KN (2011) Assessment of impact of solar UV components on growth and antioxidant enzyme activity in cotton plant. Physiol Mol Biol Plants 17:223–229. https://doi.org/10.1007/s12298-011-0071-9
Fu G, Shen Z-X (2017) Effects of enhanced UV-B radiation on plant physiology and growth on the Tibetan Plateau: a meta-analysis. Acta Physiol Plant. https://doi.org/10.1007/s11738-017-2387-8
Gonzalez JA, Rosa M, Parrado MF, Hilal M, Prado FE (2009) Morphological and physiological responses of two varieties of a highland species (Chenopodium quinoa Willd.) growing under near-ambient and strongly reduced solar UV-B in a lowland location. J Photochem Photobiol, B 96:144–151. https://doi.org/10.1016/j.jphotobiol.2009.05.003
Grandahl K, Eriksen P, Ibler KS, Bonde JP, Mortensen OS (2018) Measurements of solar ultraviolet radiation exposure at work and at leisure in Danish workers. Photochem Photobiol 94:807–814. https://doi.org/10.1111/php.12920
Interdonato R, Rosa M, Nieva CB, González JA, Hilal M, Prado FE (2011) Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons. Environ Exp Bot 70:204–211. https://doi.org/10.1016/j.envexpbot.2010.09.006
Kumari R, Agrawal SB (2010) Supplemental UV-B induced changes in leaf morphology, physiology and secondary metabolites of an Indian aromatic plant Cymbopogon citratus (D.C.) Staph under natural field conditions. Int J Environ Stud 67:655–675. https://doi.org/10.1080/00207233.2010.513828
Kumari R, Prasad MNV (2013) Medicinal plant active compounds produced by UV-B exposure. Sustainable agriculture reviews, vol 12. Springer, Netherlands, pp 225–254. https://doi.org/10.1007/978-94-007-5961-9_8
Kumari R, Singh S, Agrawal S (2009) Effects of supplemental ultraviolet-B radiation on growth and physiology of Acorus calamus L. (sweet flag). Acta Biol Crac Ser Bot 51:19–27
Li X, Zhang L, Li Y, Ma L, Bu N, Ma C (2011) Changes in photosynthesis, antioxidant enzymes and lipid peroxidation in soybean seedlings exposed to UV-B radiation and/or Cd. Plant Soil 352:377–387. https://doi.org/10.1007/s11104-011-1003-8
Lin Y, Li J, Li B, He T, Chun Z (2010) Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell, Tissue Organ Cult (PCTOC) 105:329–335. https://doi.org/10.1007/s11240-010-9871-9
Lu L, Wang J, Yang G, Zhu B, Pan K (2016) Heterotrophic growth and nutrient productivities of Tetraselmis chuii using glucose as a under different C/N ratios. J Appl Phycol 29:15–21. https://doi.org/10.1007/s10811-016-0919-z
Ma CH, Chu JZ, Shi XF, Liu CQ, Yao XQ (2016) Effects of enhanced UV-B radiation on the nutritional and active ingredient contents during the floral development of medicinal Chrysanthemum. J Photochem Photobiol, B 158:228–234. https://doi.org/10.1016/j.jphotobiol.2016.02.019
Manaf HH, Rabie KAE, Abd El-Aal MS (2016) Impact of UV-B radiation on some biochemical changes and growth parameters in Echinacea purpurea callus and suspension culture. Ann Agric Sci 61:207–216. https://doi.org/10.1016/j.aoas.2016.08.001
Martz F, Turunen M, Julkunen-Tiitto R, Suokanerva H, Sutinen M-L (2010) Different response of two reindeer forage plants to enhanced UV-B radiation: modification of the phenolic composition. Polar Biol 34:411–420. https://doi.org/10.1007/s00300-010-0896-7
Matsuura HN, de Costa F, Yendo ACA, Fett-Neto AG (2013) Photoelicitation of bioactive secondary metabolites by ultraviolet radiation: mechanisms, strategies, and applications. Biotechnology for medicinal plants. Springer, Berlin, pp 171–190. https://doi.org/10.1007/978-3-642-29974-2_7
Mazza CA, Gimenez PI, Kantolic AG, Ballare CL (2013) Beneficial effects of solar UV-B radiation on soybean yield mediated by reduced insect herbivory under field conditions. Physiol Plant 147:307–315. https://doi.org/10.1111/j.1399-3054.2012.01661.x
Ng TB, Liu J, Wong JH, Ye X, Wing Sze SC, Tong Y, Zhang KY (2012) Review of research on Dendrobium, a prized folk medicine. Appl Microbiol Biotechnol 93:1795–1803. https://doi.org/10.1007/s00253-011-3829-7
Paranhos JT, Fragoso V, da Silveira VC, Henriques AT, Fett-Neto AG (2009) Organ-specific and environmental control of accumulation of psychollatine, a major indole alkaloid glucoside from Psychotria umbellata. Biochem Syst Ecol 37:707–715. https://doi.org/10.1016/j.bse.2009.12.003
Pi Y, Jiang K, Hou R, Gong Y, Lin J, Sun X, Tang K (2010) Examination of camptothecin and 10-hydroxycamptothecin in Camptotheca acuminata plant and cell culture, and the affected yields under several cell culture treatments. Biocell 34:139–143
Pompelli MF, Martins SC, Antunes WC, Chaves AR, DaMatta FM (2010) Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J Plant Physiol 167:1052–1060. https://doi.org/10.1016/j.jplph.2010.03.001
Ranjbarfordoei A, Samson R, Van Damme P (2011) Photosynthesis performance in sweet almond [Prunus dulcis (Mill) D. Webb] exposed to supplemental UV-B radiation. Photosynthetica 49:107–111. https://doi.org/10.1007/s11099-011-0017-z
Schmidt ÉC, Maraschin M, Bouzon ZL (2010) Effects of UVB radiation on the carragenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales): changes in ultrastructure, growth, and photosynthetic pigments. Hydrobiologia 649:171–182. https://doi.org/10.1007/s10750-010-0243-6
Singh M, Singh S, Agrawal SB (2011a) Intraspecific responses of six cultivars of wheat (Triticum aestivum L.) to supplemental ultraviolet-B radiation under field conditions. Acta Physiol Plant 34:65–74. https://doi.org/10.1007/s11738-011-0805-x
Singh S, Kumari R, Agrawal M, Agrawal SB (2011b) Growth, yield and tuber quality of Solanum tuberosum L. under supplemental ultraviolet-B radiation at different NPK levels. Plant biol 13:508–516. https://doi.org/10.1111/j.1438-8677.2010.00395.x
Singh S, Agrawal M, Agrawal SB (2013) Differential sensitivity of spinach and amaranthus to enhanced UV-B at varying soil nutrient levels: association with gas exchange, UV-B-absorbing compounds and membrane damage. Photosynth Res 115:123–138. https://doi.org/10.1007/s11120-013-9841-2
Sun M et al (2010) Change of secondary metabolites in leaves of Ginkgo biloba L. in response to UV-B induction. Innov Food Sci Emerg Technol 11:672–676. https://doi.org/10.1016/j.ifset.2010.08.006
Takshak S, Agrawal SB (2014) Effect of ultraviolet-B radiation on biomass production, lipid peroxidation, reactive oxygen species, and antioxidants in Withania somnifera. Biol Plant 58:328–334. https://doi.org/10.1007/s10535-014-0390-0
Wang Y, Yu G, Li K, Wu M, Ma J, Xu J, Chen G (2015) Responses of photosynthetic properties and antioxidant enzymes in high-yield rice flag leaves to supplemental UV-B radiation during senescence stage. Environ Sci Pollut Res Int 22:4695–4705. https://doi.org/10.1007/s11356-014-3714-x
Wellburn A, Lichtenthaler H (1984) Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Advances in photosynthesis research. Springer, Dordrecht, pp 9–12
Wong WC, Wu JY, Benzie IF (2011) Photoprotective potential of Cordyceps polysaccharides against ultraviolet B radiation-induced DNA damage to human skin cells. Br J Dermatol 164:980–986. https://doi.org/10.1111/j.1365-2133.2010.10201.x
Xu C, Natarajan S, Sullivan JH (2008) Impact of solar ultraviolet-B radiation on the antioxidant defense system in soybean lines differing in flavonoid contents. Environ Exp Bot 63:39–48. https://doi.org/10.1016/j.envexpbot.2007.10.029
Xu J, Han Q-B, Li S-L, Chen X-J, Wang X-N, Zhao Z-Z, Chen H-B (2013) Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem Rev 12:341–367. https://doi.org/10.1007/s11101-013-9310-8
Xu Z, Wu H, Zhan D, Sun F, Sun J, Wang G (2014) Combined effects of light intensity and NH4+ -enrichment on growth, pigmentation, and photosynthetic performance of Ulva prolifera (Chlorophyta). Chin J Oceanol Limnol 32:1016–1023. https://doi.org/10.1007/s00343-014-3332-y
Yang J, Zhang H-F, Cao X-Y, Yang X-H, Wang F-Z, Guo Q, Sun C-Q (2017) Enzymatic water extraction of polysaccharides from Epimedium brevicornu and their antioxidant activity and protective effect against DNA damage. J Food Biochem 41:e12298. https://doi.org/10.1111/jfbc.12298
Yannarelli GG, Gallego SM, Tomaro ML (2006) Effect of UV-B radiation on the activity and isoforms of enzymes with peroxidase activity in sunflower cotyledons. Environ Exp Bot 56:174–181. https://doi.org/10.1016/j.envexpbot.2005.01.015
Zaid A, Mohammad F (2018) Methyl jasmonate and nitrogen interact to alleviate cadmium stress in Mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J Plant Growth Regul. https://doi.org/10.1007/s00344-018-9854-3
Zhang WJ, Bjorn LO (2009) The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia 80:207–218. https://doi.org/10.1016/j.fitote.2009.02.006
Zheng Y, Jiang W, Silva EN, Mao L, Hannaway DB, Lu H (2012a) Optimization of shade condition and harvest time for Dendrobium candidum plants based on leaf gas exchange, alkaloids and polysaccharides contents. Plant Omics 5:253–260
Zheng YP, Jiang W, Liao FL (2012b) Optimization of light quality for production of alkaloid and polysaccharide in Dendrobium candidum Wall. ex Lindl. J Med Plants Res 6:560–565. https://doi.org/10.5897/jmpr10.544
Zu YG, Pang HH, Yu JH, Li DW, Wei XX, Gao YX, Tong L (2010) Responses in the morphology, physiology and biochemistry of Taxus chinensis var. mairei grown under supplementary UV-B radiation. J Photochem Photobiol, B 98:152–158. https://doi.org/10.1016/j.jphotobiol.2009.12.001
Acknowledgments
This work was supported by Guangdong Science and Technology Department, China (Nos. 2016B01012014 & 2014A020221098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, D., Mo, Y., Zeng, L. et al. Response of Dendrobium officinale Kimura et Migo, a Prized Medicinal Plant, to Continuous UV-B Irradiation at Different C/N Ratios. J Plant Growth Regul 39, 358–369 (2020). https://doi.org/10.1007/s00344-019-09987-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09987-w