Abstract
Global food security is threatened by damage to crop production by acid rain. To alleviate acid rain damage, we studied the effects of lanthanum (La3+) (0.06 and 0.12 mmol L−1) on plasma membrane H+-ATPase in rice seedlings under acid rain stress (pH 3.5 and 2.5). Relative growth rate, intracellular H+, ATP content, the activity, and gene expression of plasma membrane H+-ATPase were measured to validate the association of La3+ and plasma membrane H+-ATPase. We found that 0.06 mmol L−1 La3+ increased the plasma membrane H+-ATPase activity in rice treated with acid rain (pH 3.5) by increasing transcript levels of OSA 1,OSA 5, and OSA 7 genes, and thus, beneficial to pump excess H+ out of cells by supplying ATP energy. Thus, the decrease in relative growth rate was alleviated because of the application of 0.06 mmol L−1 La3+, showing an antagonistic interaction of acid rain and La3+ (0.06 mmol L−1). Contrarily, the application of 0.12 mmol L−1 La3+ aggravated the decrease in relative growth rate of rice under acid rain by decreasing the activity and expression of plasma membrane H+-ATPase, showing a synergistic interaction of acid rain and La3+ (0.12 mmol L−1). These results indicate that La3+ at the proper concentration can enhance the tolerance of rice seedlings to acid rain stress by increasing the activity of plasma membrane H+-ATPase, whereas La3+ at a higher concentration can aggravate the damage caused by acid rain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid rain is a global environmental problem constraining agricultural and forest productivity (Shukla and others 2013; Singh and Agrawal 2008). Since the 1970s, acid rain has devastated several regions of North America and Western Europe, and now it has become a threat to the developing countries, especially India and China (Abbasi and others 2013). Acid rain inhibits plant growth by damaging foliage, destroying membrane integrity, disordering intracellular homeostasis, and inducing oxidative stress (Chen and others 2013; Ramlall and others 2015; Wen and others 2011; Yi and others 2014). For example, acid rain has caused an estimated annual economic and ecological loss worth RMB 45.9 billion (about 7.1 billion US dollars) in China (Zhao and Hou 2010), and the extreme acidity of rainfall was at a pH of 2.54 in China in 2012 (Wang and others 2014b). Therefore, it is crucial to reduce losses to agricultural productivity caused by acid rain, while measures should be taken to control air pollution.
Lanthanum (La3+), one of the rare earth elements, is of particular importance as it has been applied as a fertilizer in agriculture and forestry since the 1970s to promote plant growth and productivity (Hu and others 2004; Tyler 2004). Application of La3+ can improve the tolerance of plants to abiotic stresses such as low temperature, UV-B radiation, heavy metal toxicity, and acid rain (Liang and others 2006; Sun and others 2013; Wang and others 2012; Zhang and others 2006). The mechanisms on La3+ enhancing plant tolerance to abiotic stresses involve maintaining the integrity of the plasma membrane, regulating endogenous hormone contents, promoting photosynthetic capacity, and increasing expression of proteins including antioxidant enzymes, phytochelatin synthase, and metallothionein to regulate the network of reactions in response to abiotic stress (He and others 2005; Shen and Yan 2002; Wang and others 2014a; Yang and others 2014).
Plasma membrane H+-ATPase, a tightly bound and integral transmembrane protein, plays a role in adaptation of plants to abiotic stresses including salinity, drought, heavy metals, and low temperature (Janicka-Russak 2011; Zhou and others 2016). Our previous study proved that plasma membrane H+-ATPase also plays an important role in adaptation of rice to acid rain because the higher activity of plasma membrane H+-ATPase pumps excess H+ out of cells to maintain substance stability in cells (Liang and others 2015; Zhang and others 2017). Zhu and others (2009) reported that the up-regulation of genes encoding plasma membrane H+-ATPase is responsible for the adaptation of rice to low pH. Thus, we presume that the regulation of La3+ on the activity of plasma membrane H+-ATPase could be important to clarify the mechanism of La3+ to enhance the tolerance in plants to acid rain. So far, few studies have considered the change in plasma membrane H+-ATPase activity in response to La3+ and acid rain (Li and others 2003). Regulation of La3+ on the activity of plasma membrane H+-ATPase in plants under acid rain stress in unclear. Therefore, we tried to clarify the regulation of La3+ on the activity of plasma membrane H+-ATPase and its gene expression at the transcriptional level, validated by analyzing other plant-growth related parameters. The parameters include relative growth rate, the activity of plasma membrane H+-ATPase, intracellular H+, ATP content, and gene expression of plasma membrane H+-ATPase at the transcript level in rice seedlings treated with acid rain at different pH and La3+ at different concentrations.
Based on these findings, the application of La3+ for eliminating the negative effects of acid rain will be possible.
Materials and Methods
Plant Material and Culture Conditions
Seeds of rice (Oryza sativa) “Huaidao 8” (Wuxi Seed CO., Ltd., China) were surface-sterilized with HgCl2 (0.1%, w/v) solution for 5 min and rinsed with distilled water several times. After being soaked in distilled water for 12 h, the seeds were placed in a dish under-laid with three layers of filter paper and germinated in an incubator at 25 °C. When the radicle length was approximately 2 cm, germinating seeds were transplanted into plastic boxes of 6.88 L filled with vermiculite. After the second true leaf had developed, the seedlings were cultivated in 1/2 routine nutrition solution at a greenhouse with a light/dark regime of 14/10 h and a light intensity of 300 mol m−2 s−1 photosynthetically active radiation, temperature of 25/20 °C, and relative humidity of 70/80% (day/night). Distilled water was added every day to maintain the solution volume, the nutrient solution was renewed every 3 days to stabilize pH, electronic air pumps were used to provide fresh oxygen. When the third true leaf of rice appeared, seedlings were treated with La3+ and simulated acid rain (SAR).
Treatment of La3+ Solution and SAR
In a pre-experiment (results not shown here), we used LaCl3 solution at different concentrations of 0 (control), 0.04, 0.06, 0.08, and 0.12 mmol L−1 to treat rice seedling leaves for 24 h, and then measured relative growth rate, chlorophyll content, and membrane permeability in rice seedling leaves. La3+ at 0.06 mmol L−1 caused an obvious increase in relative growth rate and chlorophyll content and a significant decrease in membrane permeability, whereas La3+ at 0.12 mmol L−1 caused an obvious decrease in relative growth rate and chlorophyll content and a significant increase in membrane permeability, compared to the control (p < 0.05). La3+ at 0.04 and 0.08 mmol L−1 did not affect significantly the three indices mentioned above in rice seedlings. Hence, we chose La3+ at 0.06 and 0.12 mmol L−1 for our further experiments. La3+solution (0.06 and 0.12 mmol L−1) was prepared as described by Averbuch-Pouchot and Durif (1996). A stock solution of SAR at pH 1.0 was prepared with H2SO4 and HNO3 in a ratio of 3:1 (v/v), according to the general anion composition of rainfall in South China (Chen and others 2010). Then the stock solution was diluted to pH 3.5 and 2.5 as the spraying solution of SAR. Table 1 gives an overview of all treatments. The SAR solution (pH 3.5 and 2.5) was sprayed at 24 h intervals on the leaves of rice seedlings until drops began to fall. As the control, the same amount of distilled water (pH 7.0) was applied to rice leaves. Rice seedlings were sprayed with the solution of La3+ (La 1/0.06 mmol L−1 or La 2/0.12 mmol L−1) on leaves of rice until drops began to fall. All treatments were performed in triple. Half of rice seedlings were collected for detecting after a 5-day exposure, and the remaining seedlings were cultured under the control conditions for another 5 days and then collected for detecting.
Relative Growth Rate Measurement
Relative growth rate was calculated through the formula as follows (Poorter and Remkes 1990):
t 1 The day before exposure (recovery) treatment
t 2 The fifth day of exposure (recovery) period
W 1 The dry mass measured at t 1
W 2 The dry mass measured at t 2
Plasma membrane isolation and hydrolytic activity of plasma membrane H+-ATPase.
Plasma membrane vesicles were isolated from rice leaves by two-phase partitioning according to Larsson and others (1987). Protein obtained by this method was measured according to the method described by Bradford (1976). The hydrolytic activity of plasma membrane H+-ATPase was measured by determining the Pi amount after 30 min of hydrolysis according to the method described by Liang and others (2015). A standard curve of phosphate in the reaction mixture was induced for each assay. The hydrolytic activity of the plasma membrane was expressed in μmol mg−1 min−1.
Intracellular H+ Measurement
Protoplasts were isolated from rice leaves according to the method of Liang and others (2015). Rice leaves (0.1 g) were cut into pieces and then put into test tubes containing enzyme solution [2% cellulose, 5% macerase, 0.1% pectolyase, 5 mmol L−1 2-(4-morpholino) ethane sulfonic acid, and 0.45 mmol L−1 mannitol]. The reaction mixture was shaken at 40 rpm at 30 °C in the dark for 3 h before being filtered with plugs (400 meshes). The filtrate was centrifuged at 1000 ×g for 5 min, and then the precipitate was washed twice with solution (3% sucrose and 0.4 mol L−1 mannitol). The washing solution was collected and passed through 50 μm-pore-size nylon mesh filter to collect protoplasts. The concentration of protoplasts obtained by this procedure was adjusted to 5 × 106 mL−1 with a microscope and hemocytometer for detecting intracellular H+.
Intracellular H+ was measured by a flow cytometry instrument according to Undem and others (2012). Protoplasts isolated from rice leaves were incubated with the pH-sensitive fluorescent dye [2′, 7′-bis (carboxyethyl)-5(6)-carboxy] at 37 °C for 60 min under an atmosphere of 21% O2–5% CO2. The concentration of intracellular H+ was expressed by relative fluorescence of fluorescent dye.
ATP Extraction and Measurement
ATP was extracted from rice leaves according to the method of Yang and others (2002). Rice leaves (2 g) were homogenized to powder in liquid nitrogen. Then the powder was dissolved with perchloric acid (0.6 mol L−1) in an ice bath for 1 min, before being centrifuged at 6000×g for 10 min at 4 °C. The supernatant (6 mL) was taken and quickly neutralized to pH 6.5 with KOH solution (1 mol L−1). The neutralized supernatant was stored for 30 min in an ice bath to precipitate most of the potassium perchlorate. The filtrate solution was filtered through a 0.45 μm filter. The final filtrate solution was made up to 8 mL for detecting ATP content. ATP content was measured by high performance liquid chromatography according to the method described by Zhang and others (2016a).
Preparation of Total RNA and Real-Time PCR
Total RNA was extracted with Trizol reagent. A NanoDrop Spectrophotometer ND-1000 (Thermo Scientific, Wilmington, DE) was used to determine total RNA yield and purity. To avoid any DNA contamination, the RNA samples were treated with RNase-free DNase I (Fermentas) and then reverse-transcribed into first-strand cDNA with the High-Capacity cDNA Reverse Transcription Kit (Sangon Biotech, Inc, Shanghai, China) according to the manufacturer’s instructions. The cDNA was then used as the template for PCR amplification with the 2× SG Fast qPCR Master Mix (Sangon Biotech, Inc, Shanghai, China). Gene specific primers used for PCR and the amplifications conditions were synthesized according to Liang and others (2015).
Data Analysis
Evaluations on the Combined Effects of SAR and La3+
The evaluations on the combined effects of SAR and La3+ were done according to the Abott’s formula (Gisi 1996; Zhang and others 2016a). In this formula, the excepted efficacy of a mixture, expressed as percent control (C exp), can be predicted as:
where A and B are the degree of inhibition of the single factor A and B. The synergy factor (SF) for combination of A and B can be calculated as follows:
where C obs is the experimentally observed efficacy of the components. The biological interaction between SAR and La3+ can be evaluated by the SF value. If SF > 1.5, there is synergism; if 0.5 < SF < 1.5, there is additivity; if SF < 0.5, there is antagonism.
Statistical Analysis
The measurements mentioned before were all done in triplicate. Data are shown as mean ± standard deviation. The one-way analysis of variance was used to analyze the significant differences between treatments by using SPSS 11.5.
Results
Effects of SAR and La3+ on the Hydrolytic Activity of Plasma Membrane H+-ATPase
The single SAR 1 (pH 3.5) or the single La 1 (0.06 mmol L−1) increased the hydrolytic activity of plasma membrane H+-ATPase, whereas the single SAR 2 (pH 2.5) or the single La 2 (0.12 mmol L−1) decreased the hydrolytic activity of plasma membrane H+-ATPase compared with that of the control (Fig. 1a). In the rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1), the hydrolytic activity of plasma membrane H+-ATPase was higher than that of the control, and also higher than that treated with the single SAR (1) Although the hydrolytic activity of plasma membrane in rice leaves treated with the combination of SAR 2 (pH 2.5) and La 1 (0.06 mmol L−1) was lower than that of the control, it was higher than that treated with the single SAR (2). However, the hydrolytic activity of plasma membrane H+-ATPase in rice treated with the combination of SAR 1 (pH 3.5) and La 2 (0.12 mmol L−1) or the combination of SAR2 (pH 2.5) and La 2 (0.12 mmol L−1) was decreased, and the decreased degree was even larger than that treated with the single SAR or the single La 2. After a 5-day recovery, the hydrolytic activity of plasma membrane H+-ATPase in rice leaves treated with the single SAR 1 (pH 3.5) was still higher than that of the control, but the hydrolytic activity of plasma membrane H+-ATPase in rice leaves treated with the single La 1 (0.06 mmol L−1) or the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) was not different from the control (p < 0.05) (Fig. 1b). However, the hydrolytic activity of plasma membrane H+-ATPase treated with the single La 2 (0.12 mmol L−1), the combination of SAR 2 (pH 2.5) and La 1 (0.06 mmol L−1) as well as the combination of SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol L−1) was all still lower than that of the control. In addition, the decreased degree in hydrolytic activity of plasma membrane H+-ATPase treated with the combination of SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol L−1) was even lower than that treated with the single SAR (pH 3.5 or 2.5).
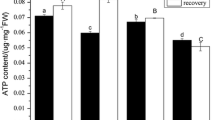
Effects of SAR and La3+ on the ATP Content in Rice Seedlings
As shown in Fig. 2a, the ATP content was decreased in rice seedlings treated with the single SAR (pH 3.5 or 2.5), single La (0.06 or 0.12 mmol L−1) as well as the combination of SAR (pH 3.5 or 2.5) and La (0.06 or 0.12 mmol L−1) compared to the control, respectively. In addition, the decreased degree in ATP content in rice treated with the combination of SAR and La3+ was larger than that treated with the single SAR. After a 5-day recovery, ATP content in rice seedlings treated with the single SAR 1 (pH 3.5) or the single SAR 2 (pH 2.5) was still lower than that of the control. However, ATP content in the rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) recovered to the control levels. Although ATP content in rice seedlings treated with the single La 1 (0.06 mmol L−1) or the single La 2 (0.12 mmol L−1) was recovered to the level of the control, ATP content in rice seedlings treated with a combination of SAR 2 and La (0.06 mmol L−1 or 0.12 mmol L−1) was still lower than that of the control, and even lower than that treated with the single SAR.
Effects of SAR and La3+ on the Intracellular H+ in Rice Seedlings
The single SAR 1 (pH 3.5) increased intracellular H+ in rice seedlings, whereas the single SAR 2 (pH 2.5) decreased intracellular H+ compared with that of the control during the exposure period (Fig. 3a). The intracellular H+ in rice seedlings treated with the combination of SAR (pH 3.5 or 2.5) and La 1 (0.06 mmol L−1) was higher than that of the control. In addition, the increased degree of intracellular H+ in rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) was lower than that of the single SAR 1. The single La3+ increased intracellular H+ in rice seedlings compared with that of the control. The combination of SAR 1 (pH 3.5) and La 2 (0.12 mmol L−1) increased the intracellular H+, whereas the combination of SAR 2 (pH 2.5) and La 2 (0.12 mmol L−1) decreased the intracellular H+. In addition, the degree of change in the intracellular H+ in rice seedlings treated with the combination of SAR and La3+ was higher than that treated with the single SAR. After a 5-day recovery, the intracellular H+ in rice seedlings treated with the single SAR 1 (pH 3.5), the single La 1 (0.06 mmol L−1) as well as the combination of SAR 1 (pH 3.5), and La 1 (0.06 mmol L−1) recovered to the level of the control (Fig. 3b). However, the intracellular H+ in rice seedlings treated with the combination of SAR 2 (pH 2.5) and La 1 (0.06 mmol L−1) was still higher than that of the control. On the contrary, the intracellular H+ in rice seedlings treated with the single SAR 2 (pH 2.5) as well as the combination of SAR (pH 2.5) and La 2 (0.12 mmol L−1) were still lower than that of the control. In addition, the decreased degree of the intracellular H+ in rice seedlings treated with the combination of SAR (pH 2.5) and La 2 (0.12 mmol L−1) was larger than that treated with the single SAR 2 (pH 2.5).
Effects of SAR and La3+ on Gene Expression of Plasma Membrane H+-ATPase
A total of 10 genes encoding plasma membrane H+-ATPase were all expressed after a 5-day exposure (Fig. 4a). The single La 1 (0.06 mmol L−1) increased the transcript level of all genes by 37–97% except OSA 3 and OSA 6, whereas the single La 2 (0.12 mmol L−1) decreased the transcript level of all genes by 9–290% except OSA 2, OSA 3, and OSA 10. The combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) increased the transcript level of all genes by 11–390%, and the up-regulation on the transcript level of genes OSA 1, OSA 5, OSA 7, OSA 8, and OSA 9 was higher than that treated with the single SAR (1). Although the combination of SAR 2 (pH 2.5) and La 1 (0.06 mmol L−1) decreased the transcript level of all genes by 42–318% except OSA 2 and OSA 3, the down-regulation on the transcript level of OSA 1, OSA 5, and OSA 7 was lower than that treated with the single SAR (2). The combination of SAR 1 (pH 3.5) and La 2 (0.12 mmol L−1) decreased the transcript level of genes OSA 1, OSA 3, OSA 4, OSA 5, OSA 6, OSA 7, and OSA 10 by 79–169%, and the down-regulation on the transcript levels of OSA 1, OSA 4, OSA 5, OSA 7, and OSA 10 was larger than that treated with the single SAR 1. In addition, the combination of SAR 2 (pH 2.5) and La 2 (0.12 mmol L−1) decreased the transcript level of all genes by 17–306% except OSA 2 and OSA 3, and the down-regulation of OSA 1, OSA 5, OSA 6, OSA 7, and OSA 9 was larger than that treated with the single SAR 2.
After a 5-day recovery (Fig. 2b), the transcript level of OSA 1 in the rice seedlings treated with the single La 1 (0.06 mmol L−1) recovered to the level of the control. The up-regulation of OSA 4 was lower than that measured during the exposure period, whereas the rest of the genes were lower than the control. For the rice seedlings treated with the single La 2 (0.12 mmol L−1), the transcript level of all genes except OSA 1, OSA 2, and OSA 7 was lower than the control, and the decreased degree of OSA 5, OSA 6, and OSA 9 was even lower than those detected during the exposure period. The transcript levels of genes OSA 1, OSA 3, OSA 7, and OSA 8 in the rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) were still higher than the control, whereas the rest of the genes were lower than the control. The transcript levels of all genes except OSA 7 in the rice seedlings treated with the combination of SAR 2 (pH 2.5) and La 1 (0.06 mmol L−1) were still lower than those of the control. The down-regulation on the transcript level of genes OSA 1, OSA 3, OSA 5 was even lower than those treated with the single SAR 2. The transcript levels of all genes in rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 2 (0.12 mmol L−1) or the combination of SAR 2 (pH 2.5) and La 2 (0.12 mmol L−1) were still lower than those of the control. In addition, the down-regulation on the transcript level of genes OSA 1 and OSA 6 in rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 2 (0.12 mmol L−1) was even larger than that treated with the single SAR 1. Similarly, the down-regulation on the transcript level of genes OSA 1, OSA 4, OSA 5, OSA 7, and OSA 9 in rice seedlings treated with the combination of SAR 2 (pH 2.5) and La 2 (0.12 mmol L−1) was even larger than those treated with the single SAR 2.
Effects of SAR and La3+ on Relative Growth Rate of Rice Seedlings
After a 5-day exposure, the single SAR at pH 3.5 or 2.5 decreased the relative growth rate of rice seedlings compared with that of the control (Fig. 5a). The single La 1 (0.06 mmol L−1) increased the relative growth rate, and the relative growth rate of rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) was higher than that treated with the single SAR, showing no difference from that of control (p < 0.05). When rice seedlings were treated with the combination of SAR 2 (pH 2.5) and La 1 (0.06 mmol L−1), the relative growth rate of rice seedlings was lower than that of control, but was not different from that treated with the single SAR 2 (p < 0.05). The single La 2 (0.12 mmol L−1) decreased the relative growth rate of rice seedlings, and the relative growth rate of rice seedlings treated with the combination of SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol L−1) was lower than that of the control, and even lower than that treated with the single SAR. After a 5-day recovery, the relative growth rate of rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) was still not different from that of controls, but higher than that treated with the single SAR (1). In the rice seedlings treated with the combination of SAR 2 (pH 2.5) and La 1 (0.06 mmol L−1), the relative growth rate was still lower than that of the control, but the decreased degree was smaller than that treated with the single SAR (2). However, the relative growth rate of rice seedlings treated with the combination of SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol L−1) was still lower than that of the control, and even lower than that treated with the single SAR (pH 3.5 or 2.5).
Evaluation on Biological Interaction Between SAR and La3+
The biological interaction between SAR and La3+ was evaluated according to the synergy factor (SF) value as shown in Table 2. The combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol.L−1) had an antagonistic effect on relative growth and intracellular H+ because the SF values were <0.5, and had synergistic effects on membrane plasma H+-ATPase and ATP content because SF values were >1.5. For the combination of SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol L−1), all SF values were >1.5, indicating that the interaction between SAR and the high concentration of La was synergistic.
Discussion
Effect of La3+ on Adaptation of Plasma Membrane H+-ATPase in Rice Seedlings to SAR
The plasma membrane H+-ATPase directly couples ATP hydrolysis to transport excess H+ out of the cell, creating pH and electrical potential differences across the plasma membrane and providing the proton-motive force for transporting many solutes (ions, metabolites, and so on) into and out of the cell, thus play an important role in regulating plant growth (Briskin and Hanson 1992). Making a comparison between the SAR 1 and the combination of SAR 1 and La 1, we found that the application of La 1 (0.06 mmol L−1) increased the activity of plasma membrane H+-ATPase, and decreased the intracellular H+ and ATP content in rice seedlings under SAR 1 stress (Figs. 1a, 2a, and 3a). The plasma membrane H+-ATPase activated by La 1 can be beneficial to pumping excessive H+ out of cells and maintaining the proton-motive force for transporting many solutes (ions, metabolites, etc) into and out of the cell (Zhang and others 2016b), thus alleviated the inhibition on rice growth caused by acid rain (pH 3.5) (Fig. 4a). On the other hand, it may be also because the application of La 3+ can alleviate the inhibition on photosynthetic capacity, nutrition absorption, and the antioxidant system in plants caused by acid rain (Liang and others 2011; Rui and others 2007; Sun and others 2013). Making a comparison between the combination of La 1 and SAR 1 and the combination of La 1 and SAR 2, we found that the alleviating effect of La 1 on the activity of plasma membrane H+-ATPase and growth in rice seedlings under SAR 1 stress was better than that on rice seedlings under SAR 2 stress. It indicates that the regulation of La 1 on the growth of rice under acid rain stress depended on the intensity of acid rain. On the contrary, the application of La 2 (0.12 mmol L−1) decreased the activity of plasma membrane H+-ATPase and ATP content compared with that treated with the SAR (Figs. 1a, 2a). It means that proton transport on the membrane could be hindered, uptake of nutrients properly affected, and then, the inhibition on growth of rice seedlings was aggravated. Moreover, the intracellular H+ was increased in rice seedlings treated with the combination of SAR 1 and La 2, whereas it was decreased in rice seedlings treated with the combination of SAR 2 and La 2 compared with that treated with the SAR (Fig. 3a). The change in intracellular H+ may result from two aspects. One could be that the high concentration La3+ (La 2) aggravated the inhibition on activity of plasma membrane H+-ATPase and destabilized intracellular H+ in cells. The other could be that the high concentration La3+ (La 2) aggravated membrane damage and led to more H+ outflow. Our previous studies also found that a low concentration of La3+ (0.06 mmol L−1) did not affect the activity of antioxidant enzymes (catalase and peroxidase) in soybean seedlings, whereas a high concentration of La3+ (0.18 mmol L−1) did. The joint stress of La3+ (0.18 mmol L−1) and acid rain (pH 4.5 and 3.0) more severely affected the activity of catalase and peroxidase (Liang and Wang 2013). The combination of La3+ at high concentration (0.4 and 1.2 mmol L−1) and acid rain (pH 3.5 and 4.5) can obviously destroy the chloroplast ultrastructure of the cell and aggravate the harmful effect of the single La3+ and acid rain on photosynthetic capacity and growth in soybean seedlings (Wen and others 2011). The degree of decrease in growth of soybean depends on the increases in the concentration of La3+ and acid rain (H+) when they are treated with acid rain (pH 3.0, 3.5, 4. 5) and La3+ (0.24, 0.40, and 1.2 mmol L−1) (Liang and others 2010). Besides considering the concentration of La3+ and the acidity of acid rain, the response degree of plants to La3+ and acid rain also depended on the species of plant, the growth stage of the plant, and the La treatment method (Hu and others 2016; Zhang and others 2016c). According to the synergy factor (SF) between SAR and La3+ (Table 2), we found that the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol.L−1) had antagonistic effects on relative growth and intracellular H+ and had a synergistic increase on membrane plasma H+-ATPase and ATP content. However, the combination of SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol.L−1) had a synergistic inhibition on all parameters. That also proved that La3+ at the proper concentration can enhance the tolerance of rice seedlings to acid rain stress by increasing the activity of plasma membrane H+-ATPase, whereas La3+ at a higher concentration can aggravate the damage caused by acid rain.
After a 5-day recovery, the activity of plasma membrane H+-ATPase, ATP content, and intracellular H+ in rice seedlings treated with the combination of SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) all recovered to the level of control, but the ATP content in rice seedlings treated with SAR 1 (pH 3.5) was still lower than that of the control. Although three indices in rice seedlings treated with the combination of SAR 2 (pH 2.5) and La 1 (0.06 mmol L−1) were still worse than those of the control, but better than those treated with the single SAR 2. These results indicate that the application of low concentration La3+ can promote the recovery of rice seedlings following the withdrawal of the SAR, and the degree of recovery was related to the pH of acid rain. However, the activity of plasma membrane H+-ATPase, intracellular H+, and ATP content in rice seedlings treated with SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol L−1) were not recovered, and even worse than that measured during the stress period. This indicates that the application of high concentration La3+ prolongs the recovery time of rice seedlings treated with acid rain.
Effect of La3+ on Gene Expression of Plasma Membrane H+-ATPase in Rice Seedlings Under SAR
Rice genome sequencing reveals the existence of ten plasma membrane H+-ATPase isoforms divided into five subfamilies: I (OSA 1, 2, 3), II (OSA 5, 7), III (OSA 9), IV (OSA 4, 6, 10), and V (OSA 8) (Loss Sperandio and others 2011; Michelet and Boutry 1995). In this study, La3+ (La1/La2) can up-/down- regulate expression of OSA 1, OSA 4, OSA 5, OSA 7, OSA 8, and OSA 9 (Fig. 5a) to mediate mainly the activity of plasma membrane H+-ATPase (Fig. 1a). Among the isoforms that are mediated by La3+, the expression of OSA 1, OSA 5, and OSA 7 in rice leaves treated with SAR (pH 3.5 or 2.5) and La 1 (0.06 mmol L−1) was higher than that treated with the single SAR, in line with the increase in the activity of plasma membrane H+-ATPase. Moreover, the transcript level of OSA 3 decreased in rice leaves treated with the single La 1, whereas it increased in rice leaves treated with the combination of SAR (pH 3.5 or 2.5) and La 1. This phenomenon shows that La 1 activated the plasma membrane H+-ATPase in leaves under SAR stress by increasing the OSA 1, OSA 3, OSA 5, and OSA 7. These four isoforms belong to subfamilies I and II, which are usually expressed highly and widely in plants (Arango and others 2003). The distinct response of transcript level among individual isoforms may result in a shift in the enzyme composition of individual H+-ATPase in the plasma membrane (Zhu and others 2009). As it has been proven that different isoforms of H+-ATPase have different enzyme kinetics (Palmgren and Christensen 1994), we speculated that OSA 1, OSA 3, OSA 5, and OSA 7 may express the isoenzymes of plasma membrane H+-ATPase which have a high affinity for the substrate. When rice was exposed to the combination of SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol L−1), the down-regulation on the transcript level of OSA 1, OSA 5, and OSA 7 was higher than that treated with the single SAR. It may be one of the reasons resulting in the decrease of plasma membrane H+-ATPase activity in rice leaves treated with SAR (pH 3.5 or 2.5) and La 2 compared to that in leaves treated with the single SAR.
After a 5-day recovery, expression of all genes in the rice seedlings treated with SAR 1 (pH 3.5) and La 1 (0.06 mmol L−1) was lower than in those measured during the exposure period, resulting in the recovery of plasma membrane H+-ATPase activity. In the rice seedlings treated with the combination of SAR 2 (pH 2.5) and La 1, the degree in down-regulation of OSA 1, OSA 3, and OSA 5 was smaller than in those treated with the single SAR 2. Analyzing the recovery of the plasma membrane H+-ATPase activity in rice seedlings treated with the combination of SAR 2 and La 1 (Fig. 1b), we inferred that the low concentration of La3+ (La 1) can increase expression of OSA 1, OSA 3, and OSA 5 at the transcription level to promote the recovery of plasma membrane H+-ATPase activity, which could be beneficial to maintain the normal intracellular pH and uptake of nutrients. The expression of 10 genes at the transcriptional level in rice leaves treated with SAR (pH 3.5 or 2.5) and La 2 (0.12 mmol L−1) was still lower those of the controls, and the decreased degree in expression of OSA 1, OSA 4, OSA 5, OSA 7 was larger than in those treated with the single SAR or measured during the stress period. This may be responsible for the unrecovered plasma membrane H+-ATPase activity in rice seedlings under such combined stresses.
Conclusion
Our study confirms that a low concentration of La3+ (0.06 mmol L−1) can alleviate the inhibition caused by acid rain on the growth in rice seedlings by increasing the activity of plasma membrane H+-ATPase to pump excess H+ out of the cells. However, a high concentration of La3+ (0.12 mmol L−1) aggravated the inhibition caused by acid rain on the growth of rice seedlings due to decreased activity of plasma membrane H+-ATPase and destabilization of intracellular H+. These results will help us understand that the application of La3+ at the proper concentration could be one way to eliminate the damage caused by acid rain to plants through regulating the activity of plasma membrane H+-ATPase. On the other hand, caution should be used because application of La3+ at high concentrations could have harmful effects on plants, and such negative effects could be even worse because of the occurrence of acid rain.
References
Abbasi T, Poornima P, Kannadasan T, Abbasi SA (2013) Acid rain: past, present, and future. Int J Environ Eng 5(3):229–272
Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216(3):355–365
Averbuch-Pouchot M, Durif A (1996) Topics in phosphate chemistry. World Scientific, Singapore
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Briskin DP, Hanson JB (1992) How dose the plant plasma membrane H+-ATPase pump protons. J Exp Bot 43:269–289
Chen J, Li W, Gao F (2010) Biogeochemical effects of forest vegetation on acid precipitation-related water chemistry: a case study in southwest China. J Environ Monitor 12(10):1799–1806
Chen J, Wang W, Liu T, Wu F, Zheng H (2013) Photosynthetic and antioxidant responses of Liquidambar formosana and Schima superba seedlings to sulfuric-rich and nitric-rich simulated acid rain. Plant Physiol Biochem 64:41–51
Gisi U (1996) Synergistic interaction of fungicides in mixture. Phytopathology 11(86):1273–1279
He Z, Li J, Zhang H, Ma M (2005) Different effects of calcium and lanthanum on the expression of phytochelatin synthase gene and cadmium absorption in Lactuca sativa. Plant Sci 168(2):309–318
Hu Z, Richter H, Sparovek G, Schnug E (2004) Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: a review. J Plant Nutr 27(1):183–220
Hu H, Wang L, Zhou Q, Huang X (2016) Combined effects of simulated acid rain and lanthanum chloride on chloroplast structure and functional elements in rice. Environ Sci Pollut Res 23(9):8902–8916
Janicka-Russak MG (2011) Plant plasma membrane H+-ATPase in adaptation of plants to abiotic stresses. Abiotic stress response in plants-physiological, biochemical and genetic perspectives. InTech 1:197–218
Larsson C, Widell S, Kjellbom P (1987) Preparation of high-purity plasma membranes. Methods Enzymol 148:558–568
Li YH, Yan CL, Liu JC, Chen YH, Hu J, Xue B (2003) Effects of La3+ on ATPase activities of plasma membrane vesicles isolated from Casuarina equisetifolia seedlings under acid rain stress. J Rare Earths 21(6):675–679
Liang C, Wang W (2013) Antioxidant response of soybean seedlings to joint stress of lanthanum and acid rain. Environ Sci Pollut Res 20(11):8182–8191
Liang CJ, Huang XH, Tao WY, Zhou Q (2006) Effect of rare earths on plant under supplementary ultraviolet-B radiation II: effect of cerium on antioxidant defense system in rape seedlings under supplementary ultraviolet-B radiation. J Rare Earths 24:364–368
Liang C, Pan D, Xu Q, Zhou Q (2010) Combined injured effects of acid rain and lanthanum on growth of soybean seedling. Chin J Environ Sci 31(7):1652–1656
Liang CJ, Zhang GS, Zhou Q (2011) Effect of cerium on photosynthetic pigments and photochemical reaction activity in soybean seedling under ultraviolet-B radiation stress. Biol Trace Elem Res 142(3):796–806
Liang C, Ge Y, Su L, Bu J (2015) Response of plasma membrane H+-ATPase in rice (Oryza sativa) seedlings to simulated acid rain. Environ Sci Pollut Res 22(1):535–545
Loss Sperandio MV, Santos LA, Bucher CA, Fernandes MS, de Souza SR (2011) Isoforms of plasma membrane H+-ATPase in rice root and shoot are differentially induced by starvation and resupply of NO3 − or NH4 +. Plant Sci 180(2):251–258
Michelet B, Boutry M (1995) The plasma-membrane H+-ATPase-A highly regulated enzyme with multiple physiological functions. Plant Physiol 108(1):1–6
MT AP (1996) Topics in phosphate chemistry. Singapore: World Scientific
Palmgren MG, Christensen G (1994) Functional comparisons between plant plasma membrane H+-ATPase isoforms expressed in yeast. J Biol Chem 269(4):3027–3033
Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83(4):553–559
Ramlall C, Varghese B, Ramdhani S, Pammenter N, Bhatt A, Berjak P, Sershen (2015) Effects of simulated acid rain on germination, seedling growth and oxidative metabolism of recalcitrant-seeded Trichilia dregeana grown in its natural seed bank. Physiol Plant 153(1):149–160
Rui C, Huang XH, Qing Z, Cheng XY (2007) Effects of lanthanum(III) on nitrogen metabolism of soybean seedlings under elevated UV-B radiation. J Environ Sci-China 19(11):1361–1366
Shen H, Yan X (2002) Membrane permeability in roots of crotalaria seedlings as affected by low temperature and low phosphorus stress. J Plant Nutr 25(5):1033–1047
Shukla J, Sundar S, Naresh R (2013) Modeling and analysis of the acid rain formation due to precipitation and its effect on plant species. Nat Res Model 26(1):53–65
Singh A, Agrawal M (2008) Acid rain and its ecological consequences. J Environ Biol 29(1):15–24
Sun Z, Wang L, Zhou Q, Huang X (2013) Effects and mechanisms of the combined pollution of lanthanum and acid rain on the root phenotype of soybean seedlings. Chemosphere 93(2):344–352
Tyler G (2004) Rare earth elements in soil and plant systems—a review. Plant Soil 267(1–2):191–206
Undem C, Rios EJ, Maylor J, Shimoda LA (2012) Endothelin-1 augments Na+/H+ exchange activity in murine pulmonary arterial smooth muscle cells via Rho kinase. PLoS ONE 7(9):e46303
Wang C, Luo X, Tian Y, Xie Y, Wang S, Li Y, Tian L, Wang X (2012) Biphasic effects of lanthanum on Vicia faba L. seedlings under cadmium stress, implicating finite antioxidation and potential ecological risk. Chemosphere 86(5):530–537
Wang C, Wang Q, Tian Y, Zhang J, Li Z, Cao P, Zhu M, Li T (2014a) Lanthanum ions intervened in enzymatic production and elimination of reactive oxygen species in leaves of rice seedlings under cadmium stress. Environ Toxicol Chem 33(7):1656–1664
Wang L, Wang W, Zhou Q, Huang X (2014b) Combined effects of lanthanum (III) chloride and acid rain on photosynthetic parameters in rice. Chemosphere 112:355–361
Wen K, Liang C, Wang L, Hu G, Zhou Q (2011) Combined effects of lanthanum ion and acid rain on growth, photosynthesis and chloroplast ultrastructure in soybean seedlings. Chemosphere 84(5):601–608
Yang NC, Ho WM, Chen YH, Hu ML (2002) A convenient one-step extraction of cellular ATP using boiling water for the luciferin-luciferase assay of ATP. Anal Biochem 306(2):323–327
Yang S, Wei M, Yang X, Wang H, He L, Li C (2014) A novel metallothionein gene from mussel, hyriopsis cumingii: identification and expression under lanthanum exposure. J World Aquac Soc 45(4):454–460
Yi L, Liu M, Yu S, Yu F, Yin X (2014) Effects of simulated acid rain stress on chlorophyll fluorescence characteristics and growth in leaves of Lithocarpus glaber and Schima superba seedlings. Asian J Chem 26(15):4619–4622
Zhang J-H, Liu Y-P, Pan Q-H, Zhan J-C, Wang X-Q, Huang W-D (2006) Changes in membrane-associated H+-ATPase activities and amounts in young grape plants during the cross adaptation to temperature stresses. Plant Sci 170(4):768–777
Zhang B, Bu J, Liang C (2016a) Regulation of nitrogen and phosphorus absorption by plasma membrane H+-ATPase in rice roots under simulated acid rain. Int J Environ Sci Technol 14:1–12
Zhang B, Bu J, Liang C (2016b) Root morphology and growth regulated by mineral nutrient absorption in rice roots exposed to simulated acid rain. Water Air Soil Pollut 227(12):457
Zhang X, Wang L, Zhou A, Zhou Q, Huang X (2016c) Alterations in cytosol free calcium in horseradish roots simultaneously exposed to lanthanum(III) and acid rain. Ecotoxicol Environ Saf 126:62–70
Zhang B, Bu J, Liang C (2017) Regulation of nitrogen and phosphorus absorption by plasma membrane H+-ATPase in rice roots under simulated acid rain. Int J Environ Sci Technol 14(1):101–112
Zhao Y, Hou Q (2010) Characteristics of the acid rain variation in China during 1993–2006 and associated causes. Acta Meteorologica Sinica 24(2):239–250
Zhou XH, Gu ZH, Xu HN, Chen LM, Tao GX, Yu YX, Li KZ (2016) The effects of exogenous ascorbic acid on the mechanism of physiological and biochemical responses to nitrate uptake in two rice cultivars (Oryza sativa L.) under aluminum stress. J Plant Growth Regul 35(4):1013–1024
Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ 32(10):1428–1440
Acknowledgements
The authors are grateful for the financial support from Natural Science Foundation of Jiangsu Province (No. BK20161131) and National Natural Science Foundation of China (No. 31370517). The authors would like to express their sincere gratitude to Bingjie Zhang and Xiaoqian Ren for their assistance to analyzing data of gene expression and drawing figures in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, C., Li, L. & Su, L. Effect of Lanthanum on Plasma Membrane H+-ATPase in Rice (Oryza sativa) Under Acid Rain Stress. J Plant Growth Regul 37, 380–390 (2018). https://doi.org/10.1007/s00344-017-9740-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9740-4