Abstract
Borosilicate glasses were prepared via melt quench technique with different concentration of Nd3+ ions. X-ray diffraction (XRD) was performed for the prepared glass to study the amorphous structure. The glass stability was studied by Thermo-gravimetric Analysis (TGA). Spectroscopic analysis of the prepared glasses was done through absorption, excitation, emission and decay measurements. Absorption spectra was used to evaluate the J-O parameters. Radiative parameters are evaluated with the help of application of J-O theory. Emission spectra show three prominent bands in NIR region arising from 4F3/2 → 4I9/2, 4I11/2 and 4I13/2 centered around 910, 1068 and 1339 nm accordingly, with the most intense being the one at 1068 nm. Stimulated emission cross section, branching ratio and quantum efficiency for 4F3/2 → 4I11/2 transition is very high and is suitable for lasing applications. Dipole–dipole interaction is observed among activator ions on the basis of Dexter theory. The optimized Nd3+ doped glass (NBS10) possess maximum value for all radiative parameters and hence can be best suited for applications in the area of lasers and fiber amplifiers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hyaline substances doped with a rare earth (RE) ions find vast usage in numerous optoelectronic devices like light emitting diodes (LEDs), fiber amplifiers, solid state lasers, optical waveguides, temperature sensors, etc. [1,2,3,4,5]. Intense lasing emissions in NIR range for Nd3+ places it as most famous RE ion for luminescence based characteristics [6, 7]. The transition at 1068 nm is sharpest and hence, is vastly explored for usage in high power lasers. Also, it can be observed easily at room temperature [8, 9]. While the one associated with 1340 nm finds huge utility in enhancing the O-band optical signal and provides a window in telecommunication transition, the emission centered around 900 nm provides a window for forging a device that could produce blue emission via monolithic integration of emission from a powerful diode laser [10, 11].
Various glassy systems like silicate, borates, phosphates, tellurites, chalcogenides and fluorides have been explored for the NIR range luminescence via Nd3+ ions [7, 10, 12,13,14,15]. Among these, borosilicate, a combination of borate and silicate glass systems, provides fine RE ion solubility, good mechanical strength, thermal and chemical durability [16]. Further, luminescence characteristics can be enhanced by reducing the phonon energy in the glass host, which in turn produces further utility for fiber amplifiers and high efficiency lasers [16, 17]. Phonon energy in this system can be lowered upon introduction of heavy metal oxide (PbO) which also increases the refractive index for the glass system. It further helps in reduction of transition temperature and allows for a wider spectral transmission window. Incorporation of ZnO, a nontoxic and non-hygroscopic oxide in the host matrix paves the way for better optical, magnetic and electrical characteristics. ZnO also possess some unique properties like direct wide band gap, large intrinsic emitting and higher exciting binding energy which in turn helps the glass system to find applications in solar energy converters, optoelectronic devices and gas sensors. By taking up the tetrahedral and octahedral positions in the host matrix, the addition of Al2O3 enhances its chemical and thermal stability as well as its mechanical strength, emission characteristics, and solubility of RE ions. It also decreases crystallization. The inclusion of alkali oxides (Li2O, Na2O, etc.) in the borate matrix can change the three coordinated boron atoms in the system into four coordinated tetrahedral structure, which improves chemical stability and causes non-bridging oxygens (NBO) to be produced [18,19,20,21,22]. As compared to phosphors, glasses possess easier fabricating technique and lower melting temperature leading to better incorporation in the optical devices [10, 23].
Nd3+ ions populate the 4F5/2 level via pumping and later decay to 4F3/2 state via non-radiative transition (multi phonon relaxation). Subsequently, the ions decay to 4I13/2, 4I11/2 and 4I9/2 levels from 4F3/2 emitting radiation in NIR range at 900, 1068 and 1340 nm respectively. These NIR range emissions have two major setbacks. Firstly, nearby Nd3+ ions tend to suffer cross relaxation mechanism, leading to quenching thereby mandating an optimized concentration of Nd3+ ions [24, 25]. Secondly, to acquire a rapid multi phonon relaxation rate of Nd3+ ions from the 4F5/2 (2H9/2) to 4F3/2 level when excited by 585 nm, the ambient phonon energy possessed by glass host should be close to the bridge energy gap (~1000 cm−1). Minimum non radiative transition produced by multiphonon relaxation provides a good window for quantum yield and hence can be better used as laser amplifiers.
Great interest by numerous researchers has been observed in studying the physical, absorption and fluorescence properties of Nd3+ doped glass matrix and it can be improvised by selecting appropriate glassy system [26,27,28]. In the present study, we aim to prepare Nd3+ ions doped borosilicate glass and hence to identify the improvised NIR lasing band obtained upon the introduction of optimized Nd3+ content. On account of above scientific parameters, we prepared a borosilicate glass with Li2O, PbO, ZnO and Al2O3 as intermediates for enhanced thermal and mechanical strength. Dwelling upon the aforementioned utilities, we intend to state a pertinent examination of lithium lead zinc alumino borosilicate glass doped via various concentrations of Nd3+ ions figuring out optimized content of the RE ion for intense laser emission at 1068 nm.
2 Experimental procedure
With the help of melt quench technique Nd3+ doped glasses were prepared according to the following glass composition:
10Li2O–5PbO–(5−x)ZnO–10Al2O3–10SiO2–60B2O3–xNd2O3, where, x = 0.1, 0.5, 1.0, 1.5, 2.0 and 2.5 mol%. For the ease of recognition, these samples were tagged as NBS01, NBS05, NBS10, NBS15, NBS20 and NBS25 accordingly. The starting reagents i.e. H3BO3, SiO2, PbO, ZnO, Al2O3, Li2O and Nd2O3 of high purity (98–99.99%) were taken. A batch of 7.0 g of ingredient chemicals were measured and then grounded finely via agate mortar so as to obtain homogeneous mixture. This mixture was transferred to an alumina crucible and then placed in an electric furnace at 1100 °C for 3 h. The melt so obtained was suddenly quenched by decanting it on a preheated brass plate and pressing with a similar one. The obtained glass samples were further annealed in a muffle furnace at 350 °C for about 4 h. This process helps in releasing internal mechanical stress which avoids cracking and bubbles formation in the glass samples. Figure 1 shows the photograph of Nd3+ ions doped NBS glasses. These samples were then characterized at room temperature (RT). Archimedes’ principle and Brewster’s method was used to estimate the densities and refractive indices respectively of NBS glasses using xylene as the immersion liquid. Using an XPERT-PRO diffractometer, the XRD spectral recordings were made with a step size of 0.02 and a 2θ angle that ranged from 10° to 75°. JASCO V-670 model UV–vis–NIR spectrophotometer was used to record the optical absorption spectra of the samples. Hitachi-F7000 fluorescence spectrofluorometer was used to record photoluminescence (PL) excitation, emission spectra and fluorescence decay.
3 Results and discussion
3.1 Physical properties and XRD
Density is a useful tool for examining changes in coordination, the degree of structural compactness, the geometrical topologies of the glass network, and the dimensions of interstitial holes. The values of density measured for the present glass system are 2.64, 2.66, 2.67, 2.69, 2.73 and 2.76 g/cm3 as the mol% of Nd3+ ions increased from 0.1 to 2.5 mol% respectively. The refractive index of optical materials is another crucial parameter. The values of refractive index measured for the 0.1 to 2.5 mol% of Nd3+ ions doped NBS glasses are 2.42, 2.47, 2.51, 2.54, 2.59 and 2.62 respectively. The calculated values of molar volume for the 0.1 to 2.5 mol% of Nd3+ ions doped NBS glasses are 3062, 3077, 3114, 3138, 3139 and 3151 cm3 respectively. Figure 2 shows that the refractive index, density and molar volume of the glasses which increase as the concentration of Nd3+ ions rises, signifying the addition of Nd2O3 in place of B2O3. These increasing values of density and molar volume suggest that there are more non-bridging oxygen molecules (NBOs) in the glass network. Since Nd2O3 enters the glass network as a network modifier, occupies an interstitial space within the network and ultimately produces NBOs in the glass structure, the glass network grows with the addition of Nd2O3.
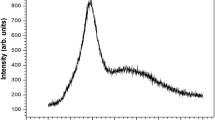
Figure 3 depicts the XRD pattern of the un-doped NBS glass. It shows the amorphous behaviour of the as-synthesized glass. The broad hump signifies absence of long range order in the glass lattice.
3.2 Thermal analysis
The TGA curve was utilized to calculate the weight loss and identify the weight loss mechanisms of the powder precursors to form pure NBS glass. As seen in Fig. 4, the TGA curve illustrates weight loss across three separate stages from 21 to 800 °C. Evaporation of the absorbed water from the sample's surface causes a weight loss of 4.09% during the first stage, which is observed between 21 and 102 °C. The exhaustion and breakdown of remaining oxides in the sample is represented by the second stage (102–334 °C), which has a weight loss of 8.59%. The presence of combustibles in the sample is the reason of the third stage, which is between 334 and 768 °C and has a weight loss of 3.83%. The evaporation of various gases and the exhaustion of organic species cause a total weight loss of around 16.51%. Therefore, it is evident from Fig. 4 that the glass sample have a lesser mass loss at high temperatures and is thermally more stable.
3.3 Absorption spectrum and Judd–Ofelt parameters
Absorption spectra for Nd3+ doped NBS glass is shown in Fig. 5 for the wavelength range of 300–1100 nm. The figure comprises of various peaks of f–f electronic transitions belonging to Nd3+ ions from their ground state. The transitions found in the UV–vis–NIR range are from 4I9/2 → 4F3/2, 4F5/2, 4F7/2 + 4S3/2, 4F9/2, 2H11/2, 4G5/2, 4G7/2, 4G9/2 + 2(D+P)3/2 and 2P1/2 which are centered at 876, 800, 743, 680, 628, 582, 525, 470 and 429 nm respectively. These transitions are quite identical to other reported Nd3+ doped glasses excluding the minor changes in band positions and respective intensities, on account of the present glass system possessing diverse ligand fields having corresponding nature [7, 29]. As the intensity for all bands increases on increasing the Nd3+ content in the glass, it can be concluded that the Nd3+ ions are very well homogeneously dissolved in the glass matrix.
Nephelauxetic ratio (β) and bonding parameter (δ%) help in determination of the type of Nd3+ ligand bond [16].
here, \({\Upsilon}_{a}\), \({\Upsilon}_{b}\) represents the energies for the transitions in complex and free ion or aqua ion respectively as given in Carnall paper [30]. From all calculated β values respective to the transitions occurring in absorption spectra, \(\overline{\upbeta }\) the average value was calculated, which further helps in determination of the bonding parameters by using following expression:
Bonding parameter value is either positive or negative corresponding to covalent or ionic bonding of the RE ions with the local host in glassy system. Hence, as the values obtained for the bonding parameters are positive for all Nd3+ doped NBS glasses, we can conclude that the bonding between the ions is covalent in nature. The values of nephelauxetic ratios and bonding parameters of all the samples are tabulated in Table 1.
Various important parameters like experimental oscillator strength, Judd–Ofelt parameters, various radiative properties such as transition probability, radiative lifetime and fluorescence branching ratio rely upon the data from the absorption spectra. The area under the curve for each transition occurring in absorption band is used to estimate the experimental oscillator strength (fexp) of f–f induced electric -dipole transitions by the following expression [1, 8]:
Here, for the wavenumber (cm−1), \(\varepsilon (\nu )\) represents the molar extinction coefficient while \(d\nu\) represents the half-bandwidth corresponding to the absorption band. If Gaussian shapes do occur for the absorption bands, then for such circumstances oscillator strength could be easily determined by half width technique according to the relation [1, 8]:
Here, \(\Delta \nu\) (cm−1) represents the width of the band at half the intensity of the peak. However Gaussian shape does not occur for glasses generally and bands suffer from inhomogeneous broadening which forces us to not use the latter technique, as it would give inaccurate results. For the present study, intensities for all absorption bands have been determined via area method. Calculated oscillator strength (fcal) are evaluated in accordance with the Judd–Ofelt (J–O) theory, for f–f intensity model with the help of least square fit analysis from initial to final state [31]. Proper relation for estimating the fcal was used from literature [32, 33]. Root mean square deviation (δrms) is used to estimate the goodness of fit among fexp and fcal by the equation:
The number of energy levels in totality for present fitting technique are represented by p. The experimental and calculated oscillator strengths along with δrms are shown in Table 1 for all Nd3+ doped NBS glasses. Smaller values of δrms signifies the precision of estimation used. Applying least square fitting procedure provided in literature [34], the three J–O intensity parameters Ωt (t = 2, 4, 6) for Nd3+ ions doped NBS glasses have been determined and are listed in Table 2 with other reported JO parameters [35,36,37,38]. The trend followed by JO parameters for Nd3+ doped NBS glasses is Ω6 > Ω2 > Ω4 and these values increase upto 1 mol%. Beyond this concentration, the values for these three parameters decrease. As similar dopant sites are occupied, the JO intensity characteristics ideally do not need to vary with concentration in the same host. However, if the clustering of dopant ions occurs, then variation in JO intensity parameters is observable, like the one noticed in the present work [39]. These three parameters are concerned with numerous physical and structural characteristics of glasses. Ω2 is highly sensitive to the RE ions surroundings and is associated with the lack of symmetry with respect to coordination structure, type of bonding and polarizability of ligand ions. Since Ω2 is correlated with the asymmetry of the immediate environment around the Nd3+ sites, it demonstrates the reliance of the covalence between Nd3+ ions and ligand anions. The ion site becomes less centro symmetrical and forms a stronger ionic chemical interaction with the ligands when the Ω2 parameter values increase. The current investigation reveals that the values of Ω2 changes from 5.8593 to 5.7406 and then increases to 6.4213 as the concentration of Nd3+ ions increases from 0.1, to 0.5 and to 1.0 mol%, respectively, indicating a rise in the covalent nature of the Nd–O bond. The values obtained in the present study are higher than the values reported in the different composition, presented in the Table 2 [35,36,37,38]. Ω4 is the parameter which defines the bulk characteristics such as rigidity and viscosity of the host glass system comprising of the RE ions. Ω6 is inversely proportional to the covalency of Nd–O bonds and this can be altered on modification of the host glass structure or composition [40, 41]. J-O intensity parameters for NBS10 glass shows the highest value among the six prepared glasses by varying Nd3+ ion content, so we can say that NBS10 possess more asymmetry, high covalency, and maximum rigidity as compared with the other Nd3+ doped NBS glasses.
Hypersensitive transition for Nd3+ doped NBS glasses is 4I9/2 → 4G5/2 situated around 582 nm in Fig. 5. This transition is entirely influenced by the RE ion-ligand surrounding and obeys the selection rule ΔS = 0, ΔL ≤ 2 and ΔJ ≤ 2. Along with this, the 4I9/2 → 4G5/2 transition possess maximum values for oscillator strengths and matrix elements ||U2||2, showing an association with hypersensitivity and Ω2 parameter (both are directly proportional). Ω4 and Ω6 help in calculating the radiative analysis like stimulated emission cross section, transition probability and branching ratio for the active ions. Ω4 and Ω6 are quite impactful in considering the neodymium emission intensity. For the present case, 4F3/2 → 4I9/2 transition is dependent upon Ω4 while Ω6 is accountable for 4F3/2 → 4I11/2 transition. Spectroscopic quality factor (χ), a ratio between Ω4 and Ω6, thats helps in recognizing the path way for the excited metastable state 4F3/2 to decay to ground state. Smaller the value of χ, better will be the probability for lasing in NIR corresponding to 4F3/2 → 4I11/2 transition. According to relation between χ and NIR transitions (4F3/2 → 4I9/2 and 4I11/2), if its values are less than 1 then 4F3/2 → 4I11/2 transition has higher efficiency and is better for lasing. While if its values are more than one then, 4F3/2 → 4I11/2 transition efficiency is reduced and 4F3/2 → 4I9/2 transition efficiency is increased [42]. For the present case study, NBS10 possess minimum value of χ as 0.168 (however, usual reported values are in the range 0.22–1.5) and is shown in Table 2 [43].
3.4 Emission and radiative characteristics
Photoluminescene excitation spectra for 1 mol% (NBS10) Nd3+ doped NBS glass at 1068 nm emission wavelength is shown in Fig. 6. Eleven bands arising from 4I9/2 → 2I11/2, 4D3/2, 2P1/2, 2K15/2, (4G9/2, 2K13/2), (4G7/2, 4G5/2), 2H11/2, 4F9/2, (4S3/2, 4F7/2), 2H9/2 and 4F5/2 which are centered around 293, 358, 431, 475, 526, 585, 627, 683, 738, 800 and 825 nm in UV–vis–NIR range can be seen. Among all the transitions, 585 nm was the most intense peaks observed. Therefore, the emission spectra of all the prepared glass samples were recorded at this excitation wavelength. Figure 7 shows the emission spectra with three peaks in NIR range, arising from the transitions 4F3/2 → 4I9/2, 4I11/2 and 4I13/2 centered around 910, 1068 and 1339 nm respectively. Among these bands, the most intense one is at 1068 nm and so, is best suited for laser transition. In the inset diagram of Fig. 7 the variation of intensity for 4F3/2 → 4I11/2 transition with the concentration of Nd3+ ions as shown in the form of bar graphs. The observed intensity of 4F3/2 → 4I11/2 transition increases till 1 mol% of the RE ion beyond which, the intensity of the band decreases. This decrement is ascribed due to increased interaction among Nd3+—Nd3+ ions and also among Nd3+-host defects, resulting in the cross-relaxation process in the effective ion centres through concentration quenching. Figure 8 depicts the diagram for energy level transitions of the Nd3+ ions in the NBS glasses emphasizing upon the excitation, emission and cross- relaxation processes. As the Nd3+ ions are excited from 4I9/2 ground state to 4F5/2 excited state, they decay to a metastable state 4F3/2 via a non-radiative decay. This 4F3/2 level gets strongly populated by Nd3+ ions resulting in three radiative transitions: 4F3/2 → 4I9/2, 4I11/2 and 4I13/2. The transition corresponding to 4I11/2 is most intense. When the Nd3+ ions content is increased in the host glass system, it leads to reduction in the separation between Nd3+ ions resulting in increased cross- relaxation mechanism among the Nd3+ ions. These processes cumulatively depopulate the Nd3+ ions situated at 4F3/2 energy level. Cross relaxation channels involved in the process is 4F3/2 + 4I9/2 → 4I15/2 + 4I15/2 result in loss of energy through non-radiative mechanism. The non radiative energy transfer can occur by either exchange interaction or multipolar interaction. The kind of inter-ionic interaction between the dopant ions can be accessed via Dexter Theory. The interactions can be grouped as dipole–dipole (d–d), dipole–quadrupole (d–q) and quadrupole—quadrupole (q–q). Based upon the relation between emission intensity and concentration of the activator ions, a graph can be plotted using the following relation [16]:
Here I, c, f and d are emission intensity, concentration of activator ions, a constant which is independent of activator ions concentration and dimension of the compound (=3) respectively. Interaction between the closest neighbouring ions is predicted by s, where s = 6, 8 and 10 for d-d, d-q and q-q interaction respectively [16]. The plot between log (I/c) and log (c) is presented in Fig. 9 which shows the value of slope (s/d) to be 1.86, hence the calculated data for s = 1.86 *3 = 5.6. Here the obtained data for s is approximately equal to 6 indicating towards dipole–dipole interaction among the activator ions. Hence in the NBS glasses, dipole–dipole interaction is responsible for non-radiative energy transfer among Nd3+ ions. Moreover, this non radiative energy transfer leads to the concentration quenching effect in the emission spectra.
The radiative parameters like transition probability (AR), total transition probability (AT), radiative lifetime (τR) and branching ratio (βR) corresponding to 4F3/2 → 4I9/2, 4I11/2 and 4I13/2 transitions for Nd3+ ions doped NBS glasses with the help of relations provided in literature were also calculated [44] and are represented in Table 3. From the data represented in Table 3, we can observe that the values of branching ratio (βR) for NBS10 glass are maximum among all prepared Nd3+ doped NBS glasses corresponding to 4F3/2 → 4I11/2 transition. The values for total radiative transition probability increase upto 1 mol% of Nd3+ ions and then decrease upon further increment of the RE ions concentration. The total radiative transition probability for 4F3/2 → 4I11/2 is highest which is the most intense peaks. The fluorescence branching ratio is an important parameter in identifying the intensity for laser transition ascribed to electric dipole transitions of various excited and lower energy levels. If the value of βR is ≥ 0.5 then it is very well suited for the lasing job. In the present study the value of βR is above 0.5 for 4F3/2 → 4I11/2 energy level for all Nd3+ ions doped NBS making it as possible candidate for the lasing field applications. For further analyzing the utility as an appropriate lasing host, stimulated emission cross-section (σse) of the glass should be of high value. The value of stimulated emission cross-section suggests the behavior of energy extraction from optical material and is evaluated with the help of relation [1, 11]:
where, the \({\lambda }_{p}\) and \(\Delta {\lambda }_{p}\) represent the wavelength corresponding to most intense band and linewidth (FWHM). Table 4 shows all the calculated values for stimulated emission cross-sections (\({\upsigma }_{se}\)), emission peak wavelength (\({\lambda }_{P}\)), line widths (\(\Delta {\lambda }_{P}\)), gain band width (\({\upsigma }_{se}\times \Delta {\lambda }_{P}\)) and optical gain parameter (\({\upsigma }_{se}\times {\uptau }_{\text{R}}\)) parameters for the emission transitions for Nd3+ ions in NBS glasses. The value of stimulated emission cross-section for NBS10 glass is maximum, making it superior host for lasing and optical fiber amplifier applications.
3.5 Decay spectral examination
Figure 10 shows the fluorescence decay curves for the Nd3+ ions in NBS glasses at 1068 nm emission wavelength for 4F3/2 → 4I11/2 transition under 585 nm excitation. The measured decay curves for the Nd3+ doped NBS glasses show single exponential fit behavior for all concentrations. The experimental decay time \({(\uptau }_{\text{exp}}\)) for the Nd3+ doped NBS glasses are calculated initially by applying e-folding time of the intensity for decay curves and with the help of equation provided in literature which are shown in Table 5 [8, 11, 22]. The values of \({\uptau }_{\text{exp}}\) are found to be decreasing with increment in concentration of Nd3+ ions for NBS glasses which may be attributed to concentration quenching effect among the Nd3+ ions. The \({\uptau }_{\text{exp}}\) values are also lower than the corresponding \({\uptau }_{\text{R}}\) obtained via J–O theory, which may be ascribed due to specific the non-radiative decay mechanism (WNR). Quantum efficiency (\(\eta\)), which is the ratio between emitted and absorbed light intensity, is an important parameter that describes the utility of the material for lasing applications. Higher the value of quantum efficiency, better will be the lasing properties of studied glass matrix. Following equations are used to determine the values of \(\eta\) and WNR given in literature and are shown in Table 5 [8, 11]:
From the obtained values we can see that the highest quantum efficiency and lowest non radiative decay rate is for 1 mol% of Nd3+ ions doped NBS glasses, which further ascertains its suitability for NIR laser emission at 1068 nm [45, 46].
The values of important parameters, such as stimulated emission cross-section, gain band width, optical gain, experiment decay time, quantum efficiency and non-radiative decay rate, of NBS glass are compared to other Nd3+-doped glasses and are shown in Table 6, along with the other reported glasses doped with Nd3+ ions. The measured values for optimized NBS10 glasses are maximum from all the other prepared glass as well as other reported values, which marks towards more suitability in various lasing applications.
4 Conclusions
Nd3+doped NBS glasses were prepared via a melt quench technique. The X-ray diffraction pattern shows the amorphous behavior of the undoped glass. The Thermo-gravimetric analysis shows stability of the glass. A thorough spectroscopic analysis of the samples was done via characterization techniques like UV–VIS-NIR absorption, photoluminescence excitation and emission and time decay measurements. Judd–Ofelt analysis was conducted and three intensity parameters Ω2, Ω4 and Ω6 were obtained for all the prepared glass samples from the absorption data. Various important radiative parameters were calculated to help assess the suitability of the glasses in lasing applications. Emission of the Nd3+ doped NBS glasses was found in NIR range with three prominent transitions corresponding to 4F3/2 → 4I9/2, 4I11/2 and 4I13/2, wherein 4F3/2 → 4I11/2 transition centered at 1068 nm had the maximum intensity. This transition possesses the maximum value of spectroscopic quality factor, branching ratio, stimulated emission cross section and quantum efficiency for the optimized glass (NBS10). Interaction among the activator ions was predicted to be of dipole–dipole nature by utilizing the Dexter theory. The decay curves for NBS glasses show single exponential for all concentrations. The aforementioned results are encouraging enough to ascertain that the 1 mol% of Nd3+ ions doped NBS glass is ideal for laser and fiber amplifier applications.
Data availability
The data that support the findings of this study are available on request from the corresponding author Dr. Yasha Tayal. The data are not publicly available due to restrictions, e.g. their containing information that could compromise the privacy of research participants.
References
F. Zaman, G. Rooh, N. Srisittipokakun, S. Ruengsri, H.J. Kim, J. Kaewkhao, J. Non-Cryst, Solids 452, 307 (2016)
C.M. da Silva Jr, L.A. Bueno, A.S. Gouveia-Neto, J. Non- Cryst. Solids 410, 151 (2015)
M.D. Dramićanin, Methods Appl. Fluoresc. 4, 042001 (2016)
C.X. Liu, X.L. Shen, H.T. Guo, W.N. Li, W. Wei, Optik 131, 132 (2017)
N.K. Goel, G. Pickrell, R. Stolen, Opt. Fiber Technol. 20, 325 (2014)
F. Elan, E.L. Falcão-Filho, M.E. Camilo, J.A.M. Garcia, L.R.P. Kassab, C. de Araújo, Opt. Mater. 60, 313 (2016)
S. Mohan, K.S. Thind, Opt. Mater. 57, 134 (2016)
C.R. Kesavulu, H.J. Kim, S.W. Lee, J. Kaewkhao, N. Wantana, E. Kaewnuam, S. Kothan, S. Kaewjaeng, J. Alloys Compd. 695, 590 (2017)
L.R.P. Kassab, D.M. Silva, J.A.M. Garcia, D.S. da Silva, C.B. de Araújo, Opt. Mater. 60, 25 (2016)
H.K. Dan, D.C. Zhou, Z.W. Yang, Z.G. Song, X. Yu, J.B. Qiu, J. Non-Cryst, Solids 414, 21 (2015)
T.F. Xue, L.Y. Zhang, J.J. Hu, M.S. Liao, L.L. Hu, Opt. Mater. 47, 24 (2015)
A. Pandey, S. Som, V. Kumar, V. Kumar, K. Kumar, V. KumarRai, H.C. Swart, Sensors Actuators B 202, 1305 (2014)
D.D. Ramteke, R.E. Kroon, H.C. Swart, J. Non-Cryst, Solids 457, 157 (2017)
L.Y. Zhang, B.H. Yang, L.L. Hu, J. Quant. Spectrosc. Radiat. Transf. 147, 47 (2014)
S.M. Lima, J.A. Sampaio, T. Catunda, A.S.S. de Camargo, L.A.O. Nunes, M.L. Baesso, D.W. Hewak, J. Non-Cryst. Solids 284, 274 (2001)
Y. Tayal, A.S. Rao, Opt. Mat. 107, 110070 (2020)
J.A. Savage, Mater. Sci. Rep. 2, 99 (1987)
H. Yamasaki, K. Minato, D. Nezaki, T. Okamoto, A. Kawamoto, M. Takata, Solid State Ionics 172, 349 (2004)
Y. Tayal, A.S. Rao, Opt. Mat. 117, 111112 (2021)
Y.I. Alivov, D.C. Look, B.M. Ataev, M.V. Chukichev, V.V. Mamedov, V.I. Zineko, Y.A. Agafonov, A.N. Pustovit, Solid State Electron. 48, 2343 (2004)
B.L. Zhu, C.S. Xie, D.W. Zeng, W.L. Song, A.H. Wang, Mater. Chem. Phys. 89, 148 (2005)
Y. Tayal, A.S. Rao, S. Kaur, Solid State Sci. 125, 106834 (2022)
X.F. Wang, Q. Liu, Y.Y. Bu, C.S. Liu, T. Liu, X.H. Yan, RSC Adv. 5, 86219 (2015)
S. Rai, A.L. Fanai, J. Lumin. 170, 325 (2016)
C. Tian, X. Chen, Y. Shuibao, Solid State Sci. 48, 171 (2015)
B. Klimesz, G. Dominiak-Dzik, P. Solarz, M. Zelechower, W. RybaRomanowski, J. Alloys Compd. 403, 76 (2005)
S. Mohan, K.S. Thind, G. Sharma, L. Gerward, Spectro chim. Acta Part A 70, 1173 (2008)
Y. Tayal, R.A. Talewar, S.K. Mahamuda, A. Prasad, K. Maheshwari, M. Kumar, A.S. Rao, Opt. Mater. 142, 114049 (2023)
K. Linganna, R. Narro-García, H. Desirena, E. De la Rosa, Ch. Basavapoornima, V. Venkatramu, C.K. Jayasankar, J. Alloys Compd. 684, 322 (2016)
W.T. Carnall, P.R. Fields, K. Rajnak, J. Chem. Phys. 49, 4424–4442 (1968)
G.S. Ofelt, J. Chem. Phys. 37, 511 (1962)
P. Babu, C.K. Jayasankar, Opt. Mater. 15, 65 (2000)
R. Praveena, V. Venkatramu, P. Babu, C.K. Jayasankar, Phys. B 403, 3527 (2008)
S. Tanabe, T. Ohyagi, N. Soga, T. Hanada, Phys. Rev. B 46, 3305 (1992)
T. Suzuki, H. Nasu, M. Hughes, S. Mizuno, K. Hasegawa, H. Ito, Y. Ohishi, J. Non Cryst. Solids 356, 2344 (2010)
M.B. Saisudha, J. Ramakrishna, Phys. Rev. B 53, 6186 (1996)
J.A. Pardo, J.I. Pena, R.I. Merino, R. Cases, A. Larrea, V.M. Orera, J. Non Cryst. Solids 298, 23 (2002)
H. Takebe, K. Morinaga, T. Izumitami, J. Non-Cryst, Solids 178, 58 (1994)
W.T. Carnall, H. Crosswhite, H.M. Crosswhite, Technical report, Argonne National. Laboratory (1997). https://doi.org/10.2172/6417825
D.D. Ramteke, A. Balakrishna, V. Kumar, H.C. Swart, Opt. Mater. 64, 171 (2017)
C.R. Kesavulu, H.J. Kim, S.W. Lee, J. Kaewkhao, N. Wantana, S. Kothan, S. Kaewjaeng, J. Alloys Compd. 683, 590 (2016)
J. Azevedo, J. Coelho, G. Hungerford, N.S. Hussain, Phys. B: Condens. Matter. 405, 4696 (2010)
M. Ajroud, M. Haouari, H.B. Ouada, H. Maaref, A. Brenier, C. Garapon, J. Phys. Condens. Matter 12, 3181 (2000)
R. Cases, M.A. Chamarro, R. Alcala, V.D. Rodriguez, J. Lumin. 48, 509 (1991)
Q.L. Chen, H. Wang, Q.P. Chen, J. Non-Cryst, Solids 391, 43 (2014)
D. Ramachari, L.R. Moorthy, C.K. Jayasankar, Opt. Mater. 36, 823 (2014)
N. Deopa, A.S. Rao, M. Gupta, G.V. Prakash, Opt. Mater. 75, 127–134 (2018)
H. Fares, I. Jlassi, S. Hraiech, H. Elhouichet, M. Ferid, J. Quant. Spectrosc. RA. 147, 224–232 (2014)
M. Venkateswarlu, S.K. Mahamuda, K. Swapna, M.V.V.K.S. Prasad, A.S. Rao, A.M. Babu, S. Shakya, G.V. Prakash, Opt. Mater. 39, 8–15 (2015)
Acknowledgements
One of the authors Dr. Yasha Tayal is grateful to the management of ABES Engineering College Ghaziabad Uttar Pradesh for providing a conducive work and research environment. She also acknowledges Prof. A.S. Rao for his impeccable research guidance that helped in achieving the desired results in this work.
Author information
Authors and Affiliations
Contributions
Y. Tayal: Conceptualization, Formal analysis, writing & editing, Investigation. R.A. Talewar: Formal analysis, review. Sk. Mahamuda: Formal analysis. K. Maheshwari: Formal analysis. S. Kumari: Formal analysis. R. Pilania: Formal analysis. M. Kumar: Formal analysis. A. Prasad: Formal analysis, review. A.S. Rao: Supervision, Writing-review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tayal, Y., Talewar, R.A., Mahamuda, S. et al. Enhanced emission in the NIR range by Nd3+ doped borosilicate glass for lasing applications. Appl. Phys. B 130, 157 (2024). https://doi.org/10.1007/s00340-024-08294-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-024-08294-x