Abstract
By changing the concentration of Nd3+ ions, the alkalicadmino borate glasses with molar composition of 15Na2O + 15Li2O + 20CdO + 50B2O3 (NaLiCdBNd) were fabricated by the suddenly melt-quenching technique. The XRD pattern and Raman spectroscopy indicate the amorphous nature and determinations of the various vibrational modes for undoped NaLiCdB glass, respectively. The fundamental radiative properties such as radiative emission probability (AR), branching ratio (βR), and radiative lifetime (τR), including effective line width (Δλeff) and stimulated emission cross-section (σemi), were calculated for Nd3+:4F3/2 → 4I9/2,11/2,13/2 transitions by following the Judd–Ofelt parameters (Ω2,4,6), and infrared (NIR) emission spectra. The optical bandgap energy corresponding to the direct, (αhυ)2 and indirect, (αhυ)1/2 allowed transitions was found to be decrease in the order of 3.92–3.86 eV and 3.51–3.36 eV by increasing of Nd3+ ions. Under 808 nm excitation, the NIR emission spectra show a strong 4F3/2 → 4I11/2 (1060 nm) transition, and two less intensity of 4F3/2 → 4I9/2(889 nm) and 4F3/2 → 4I13/2 (1332 nm) emission transitions. The lifetimes of 4F3/2 level are found to decrease with respect to increase in Nd3+ concentration and the decay curves of the title glasses exhibit non-exponential nature. The higher optical gain (175.2 × 10−25 cm2s) and low threshold (1.23 × 108 W/m2) together with superior stimulated emission cross-section (5.69 × 10−20 cm2) and 87% of quantum efficiency for NaLiCdBNd05 glass show the potential laser performance at 1.06 μm for high gain-low threshold laser materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rare-earths (RE3+) ions greatly influence the spectral properties of glass materials by playing a vital role in the field of optical devices like solid-state lasers, optical waveguides, fiber amplifiers, light-emitting diodes, and solar cells upconverters [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. Among the various RE3+ ions, neodymium (Nd3+) ion is the one to be studied as an attractive RE3+ for high power lasers at about 1.06 μm and O-band optical signal amplification at 1. 3 μm wavelength laser output in different glass matrices like silicate, borate, fluoride, phosphate, germanate, tellurite, and chalcogenide [5,6,7,8,9,10,11,12,13,14]. Also, the Nd3+ ions can be pumped using diode laser with the wavelength equal to 808 nm due to its large optical absorption cross-section. For science and technological application, the Nd3+-activated laser glasses mainly depend on high fluorescence branching ratio (βR), narrow emission bandwidth (Δλeff), large stimulated emission cross-section (σemi), longer (NIR emissions) lifetimes (τ), and lower threshold energy (Is).
In this regard, solid-state laser with high gain and low threshold at 1.06 μm (Nd3+:4F3/2 → 4I11/2) becomes a research hotspot in the fields of high energy and high-power lasers, laser communication, and optical amplifier devices [5,6,7,8,9,10,11,12,13,14]. To further improvement in the results of optical devices, the development of new borate glass is required to achieve high gain and low threshold lasers at 1.06 μm. In this respect, borate glass combining with the cadmium oxide (CdO) is beneficial for improving the laser properties of Nd3+ ions in glass. Similar multicomponent materials based on CdO [15,16,17,18] had shown promising applications for opto-electronic devices such as phototransistors, diodes, sensors, and solar cells. In addition, alkali elements like Li2O and Na2O were mixed into a borate glass so as to decrease the melting point of glass and modify the physical properties and structure of the glass [14, 19]. Therefore, the glasses with the combination of CdO and alkali elements have been demonstrated to be matrices with the advantages of both network former and network modifiers. More generally, borate glasses are abundant reliable than alternate glass former because of its low melting temperatures, sensible thermal stability and high optical transparency, chemical sturdiness, and moderate RE3+ particle solubility [7,8,9,10, 14,15,16,17,18,19,20,21]. Thereby, a new combination of CdO and alkali elements mixed borate glass is prepared for exploring its potential applications in high gain and low threshold laser materials.
The purpose of the present work is to prepare the alkalicadmino borate (NaLiCdB) glasses with different concentrations of Nd3+ ions by the melt-quenching technique and to examine its potentiality for 1.06 μm (4F3/2 → 4I11/2) laser emission applications. The amorphous nature and determinations of the various vibrational modes were analyzed by the XRD and Raman for the undoped NaLiCdB glass. To realize the interest in optical and laser properties, the Nd3+:NaLiCdB glasses were characterized through UV–visible absorption, NIR emissions and decay curves. By using absorption spectrum, the Judd–Ofelt (JO) intensity (Ω2,4,6) parameters and optical band gap energy (\(E_{g}^{\text{opt}}\)) values were calculated. These results are used in conjunction with NIR emission (λex = 808 nm) and decay times to obtain laser parameters such as long radiative (τrad, 308 μs) and experimental (τexp, 267 μs) lifetimes with good stimulated emission cross-section (σemi, 5.69 × 10−20 cm2), including higher laser gain (175.2 × 10−25 cm2s) and low threshold (1.23 × 108 W/m2) of the selected glasses. Moreover, in NaLiCdBNd05 glass, the high ~ 60% of βR with the bandwidth of 18.11 × 10−26 cm3 at Nd3+:4F3/2 → 4I11/2 transition is also important factors to design optical amplifier devices.
Experimental details
Glass preparation
Glasses of chemical composition 15Na2O + 15Li2O + (20−x)CdO + 50B2O3 + xNd2O3 with varying Nd3+ concentration (where x = 0.1, 0.5, and 1.0 mol%) were prepared by conventional melt-quenching technique and labelled as NaLiCdBNd01, NaLiCdBNd05, and NaLiCdBNd10, respectively. The homogeneously mixed chemical powders were melted in ceramic crucibles at 1000 °C for 3 h. The melts were then cast into a preheated stainless steel plate and rapidly pressed by using another steel plate for fast solidification. The glasses were annealed at 320 °C for 10 h for stress relaxation. The samples prepared were polished to study their physical and optical properties.
The annealing temperature was selected from the differential scanning calorimetry (DSC) as 320 °C for NaLiCdBNd glasses, and the same was reported in our earlier work [22].
Characterization techniques
The densities (g/cc) of Nd3+:NaLiCdB glasses were measured from the Archimedes principle utilizing water as the immersion liquid, and the refractive indices were measured by Abbe Refractometer using sodium wavelength with 1-bromonapthalene as the contact liquid. The X-ray diffraction studies of undoped NaLiCdB glass were recorded using the RIGAKU; Miniflex-600 with CuKα1 (1.54 Å) radiation. The Raman spectrum was recorded using the 632.15 nm line of argon ion laser in the range of 400–1400 cm−1 using a Horiba Jobin–Yvon Lab Ram HR 800 confocal Raman spectrometer. The absorption spectra were recorded in the range 300–1000 nm by a Perkins Elmer Lambda-950 UV–Vis–NIR spectrophotometer with the resolution 0.1 nm. The excitation, emission and decay measurements were obtained using an Edinburgh FLS-980 fluorescence spectrometer by the excitation of 808 nm laser diode with InGas detector with 0.02 nm wavelength accuracy and 0.05 nm minimum step size.
Results and discussion
X-ray diffraction (XRD) analysis
The XRD pattern of undoped alkalicadmino borate (NaLiCdB) glass is shown in Fig. 1. This XRD pattern clearly indicates that the X-rays have been spotted in several orders leading to a huge hump as a substitute of narrower peak with high intensity in a range 2 θ from 100 to 800, confirms the amorphous nature of undoped NaLiCdB glass.
Raman spectroscopy
Figure 2 shows the Raman spectrum of undoped NaLiCdB glass in the range of 200–1400 cm−1, and their bands were determined at 1143, 1072, 738, 534, and 346 cm−1. The vibration at 1143 cm−1 is attributed to the B–O bond stretching of BO4 units in tetraborate, and the other band near 1072 cm−1 is attributed to the same vibration but in diborate units [16,17,18,19,20]. Raman peak at 738 cm−1 is suggests that the presence of Na2+ in alkalicadmino borate (NaLiCdB) glass [16]. The evidence of Cd–O units was also confirmed by peak centered at 534 cm−1 [16,17,18,19]. A band around 346 cm−1 is assigned due to the vibrational frequencies of Li+ ions [16].
Physical parameters
The physical parameters such as density (ρ), thickness (t), average molecular weight (MW), molar volume (Vm), refractive index (n), molar refraction (Rm), Nd3+ ions concentration (N), polaron radius (rp), inter-ionic distance (ri), field strength (F), polarizability of glass oxide (αm), polarizability per unit volume (Rm/Vm), and metallization (M) were calculated for the Nd3+-doped alkalicadmino borate glasses by using the equations given in Refs. [20, 21] and are listed in Table 1.
The densities (ρ) and molar volumes (Vm) of the alkalicadmino borate glasses are plotted for different concentrations of Nd3+ ions in Fig. 3a. The relation between Vm and ρ was determined by using the equation,
here molecular weight (MW):the average mass of a molecule of a compound and calculated as the sum of the atomic weights of the constituent atoms selected in each glass sample. Density (ρ): mass of a glass from its volume, and the glass’s density was calculated using the weight of the glass in air (Wa), and weight of the glass when dipped in water (Ww) [21].
here ρw is the density of water (1 gm/cm3). As a result, Vm is the volume occupied by one mole of a chemical element or a chemical compound of the selected glass samples.
From Fig. 3a, the density (ρ) is increased upto 0.5 mol% and then decreased with further increasing in the Nd3+ concentration (1.0 mol%). This increasing value of density might have contributed by the replacement of lower molar mass of CdO (128.41 g/mol) with high molar mass of Nd2O3 (336.48 g/mol), resulting in to increase in the number of non-bridging oxygen (NBOs) atoms [23, 24]. In addition, the discrepancy in the density of NaLiCdBNd glasses is also because of the effect of different alkali oxides (Na, Li) presented in the glass host. Consequently, the molar volume (Vm) starts to decrease from 0.1 to 0.5 mol% glasses and then increases for further addition of 1.0 mol% of Nd3+ ions. This is because of the addition of more Nd2O3 in the glass host that causes to break the glass network and form NBOs, which increase the free spatial distances resulting in the increase in Vm of the present glass systems [21]. The obtained results of ρ and Vm for NaLiCdBNd glasses are following the trend as predicted in eq. (1), whereas the ρ increases and the Vm will decrease with respect to increase in Nd3+ concentration. Furthermore, the inter-ionic distance decreases due to increase in the field strength with Nd3+ concentration which is shown in Fig. 3b.
Optical absorption spectrum-Judd–Ofelt parameters
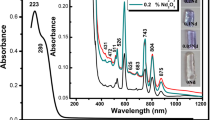
For NaLiCdBNd05 glass, the absorption spectrum shown in Fig. 4 consists of 12 transitions from the ground state 4I9/2 to various excited states: 4D3/2, (2P, 2D)3/2, 2P1/2, 2G9/2 + 2K15/2, 4G9/2,7/2,5/2, 2H11/2, 4F9/2,7/2,5/2,3/2 corresponding to 358, 391, 431, 474, 513, 526, 585, 626, 683, 748, 806, and 879 nm, respectively [14, 19]. NIR transition absorption bands, particularly the 806 nm corresponding to 4I9/2 → 4F5/2 transition hold great promise for the optical pumping of neodymium-based lasers either by flash lamps or by semiconductor GaAs laser diodes.
From the absorption spectrum, the oscillator strength [25,26,27,28,29] of each absorption band was determined experimentally (fexp) and theoretically (fcal) which are presented in Table 2. The fitting error estimated by root-mean-square deviation (δrms) between the oscillator strengths is found to be ± 0.19 × 10−6, and this small error reveals the more reliable fitting of NaLiCdBNd05 glass. The Judd–Ofelt parameters of 0.5 mol% Nd3+-activated NaLiCdB glass were found to be Ω2 = 3.35, Ω4 = 4.99, and Ω6 = 3.89 × 10−20 cm2 with the least square fitting between fexp and fcal, indicate a higher symmetry and rigidity of the matrix environment around Nd3+ ions [30,31,32] and are presented in Table 2. From Table 2, the value of spectroscopic quality factor (χ = Ω4/Ω6) for the NaLiCdBNd05 glass is found to be 1.28 and is more or less similar to those glass hosts reported NaKBNd (1.00) [28], NKZLSNd10 (1.05) [32] and CdO–P2O5–Nd (0.86) [33], which is important for a laser medium.
Optical band gap and Urbach’s energy
The absorption coefficient (α(ν)) as a function of photon energy (hν) was determined for all glass samples using the equation given below,
where A is the band tailing parameter, \(E_{g}^{\text{opt}}\) is the optical band gap energy and the exponent q determines the nature of allowed direct (q = 2) and indirect (q = ½) transitions, respectively [33,34,35]. As shown in Fig. 5a, b, the Tauc’s plot for direct and indirect transitions gives the straight line with intercept equal to the optical band gap energy. The optical band gap and band tailing parameter values of Nd3+:NaLiCdB glasses are presented in Table 3. The optical band gap energy of Nd3+: NaLiCdB glasses is in the range of 3.92–3.86 eV for direct and 3.51–3.36 eV for indirect transitions. As a result, it is found that the decrease in optical band gap with increasing Nd3+ concentration may be attributed to the decrease in structural disorder in the glasses as observed from Urbach energy analysis.
Therefore, a structural disorder or Urbach edges, which is related to width of the localized states available in the optical band gap of the 0.1, 0.5, and 1.0 mol% of Nd3+:NaLiCdB glasses was studied for further investigation. The Urbach energy varied inversely to the optical band gap can be expressed by the spectral dependence of absorption coefficient (α(ν)) by the following empirical formula [35]
where α0 is a constant, and ∆E is the Urbach’s energy. For the Nd3+:NaLiCdB glasses, ∆E values were calculated from reciprocal of the straight line slopes plotting against the photon energy (hv) as shown in Fig. 6, and these values are listed in Table 3. Urbach energy values give the number of disorder in solids, and the nature of disorder is different for amorphous and crystalline solids [36, 37]. In amorphous solids, the static atomic structural disorder leads and is due to the presence of defects like loose bonds [36, 37]. Mott and Devis reported the Urbach energy values in the range of 0.045–0.567 eV for amorphous solids [36, 37]. Accordingly, the calculated (Table 3) Urbach energy values 0.187, 0.168, and 0.190 eV are almost in the predicted range and reveal that there is no much disorder in the present NaLiCdBNd glasses.
Near-infrared (NIR) emission and radiative properties
From Fig. 7, the NIR emission spectra of the 0.1, 0.5, and 1.0 mol% Nd3+-doped NaLiCdB glasses were recorded in the range 850–1400 nm with 808 nm laser diode excitation. The peaks of the 4F3/2 → 4I9/2, 11/2, 13/2 transitions are centered at 889, 1060, and 1332 nm, respectively. As shown in Fig. 7, the emission intensity enhances with increase in Nd3+ concentration from 0.1 to 0.5 mol%, then it falls abruptly when the doping concentration reaches 1.0 mol% due to the occurrence of the concentration quenching effect. The intensity of 4F3/2 → 4I11/2 transition (1.06 μm) shows stronger NIR emission than that of the other two emission transitions. Moreover, the shape of the 4F3/2 → 4I9/2 (900 nm) emission shows different spectral profiles as a function of Nd3+ concentration due to the radiation reabsorption [38, 39]. These experiments have been performed on NaLiCdBNd glasses in the present work, so that this zero-line phonon radiation could be reabsorbed on the optical path, resulting in lower experimental branching ratio for 4I9/2 than the remaining 4I11/2, 4I13/2 emission transitions as presented in Table 4.
Radiative properties such as radiative transition probabilities (AT), radiative lifetimes (τR), effective bandwidths (Δλeff), branching ratios (βcal), and (βexp) were estimated using the formulae given in refs. [14, 25, 32,33,34,35,36,37,38,39,40]. The stimulated emission cross-section (σemi) of Nd3+:NaLiCdB glasses can be calculated [32,33,34,35,36,37,38,39,40] by using the equation,
In this equation, λp is the emission peak wavelength, c is the speed of light in vacuum, n is the refractive index of the glass, and Δλeff is the emission effective line width and it can be defined [32,33,34,35,36,37,38,39,40],
here I(λ) is the luminescence intensity, and Ip is the intensity at band maximum. Therefore, Δλeff is determined by dividing the area of the emission band by its average height. As expected, stimulated emission cross-section (σemi) is very sensitive to the increase in Nd3+ concentration, which confirms the presence of non-radiative excitation transfer from interaction between Nd3+ ions. These spectroscopic results for the transition from the 4F3/2 level of different Nd3+-doping NaLiCdB glasses are presented in Table 4. From Table 4, the higher βexp value of 58% for 0.5 mol% glass is desirable property to achieve lower threshold and high laser gain. The σemi value for 4F3/2 → 4I11/2 transition is found to be 3.12, 5.69, and 3.78 × 10−20 cm2 corresponding to the NaLiCdBNd01, NaLiCdBNd05, and NaLiCdBNd10 glasses, respectively. The value of σemi for NaLiCdBNd05 is higher than these of previously reported glass TAKLNP10 (3.0 × 10−20 cm2) [2], TTNW10 (4.53 × 10−20 cm2) [13], NCB (2.465 × 10−20 cm2) [13], BSKNLNd10(5.85 × 10−20 cm2) [14], LSBiB06 (5.12 × 10−20 cm2) [19], SPbKNLFNd10 (4.11 × 10−20 cm2) [34], PZNTBNd05 (3.5 × 10−20 cm2) [40], SFBNd (3.46 × 10−20 cm2) [41], GSBNd (2.1 × 10−20 cm2) [42], LTTNd (4.75 × 10−20 cm2) [43], SBiLNd (2.33 × 10−20 cm2) [29], and ZBSN1(2.42 × 10−20 cm2) [44], and also compared with other spectroscopic results in Table 5.
Luminescence decay rate analysis
Figure 8 displays the decay curves of 4F3/2 level for the NaLiCdB glass doped with 0.1, 0.5, and 1.0 mol% of Nd3+ ions. The decay curves of present glasses exhibit the non-exponential behavior and fitted well to the double exponential function as given by [45],
here I(t), I0 are the emission intensity at time ‘t’ and t = 0, respectively. A1 and A2 are constants, τ1 and τ2 are the short and long lifetimes for the exponential components, respectively. Due to a non-exponential behavior of the luminescence decay, the average decay lifetime (τexp) value can be calculated by the following equation [14, 46],
The τexp of glasses is estimated by the integration of experimentally measured intensity I(t), where the number of photons emitted per unit time. The physical meaning of τexp is the average time during which the emitters (PL centers) remain in the excited state after the end of an excitation pulse [14, 46]. If to apply normalization condition \(\int {I(t){\text{d}}t = 1}\), then I(t) can be considered as probability density of emission. The PL decay times excited at 808 nm are found to be 318, 267, and 100 μs for NaLiCdBNd01, NaLiCdBNd05, and NaLiCdBNd10 glasses, respectively. The decrease in τexp values could ensure the energy transfer through non-radiative decay rates (WNR) around Nd3+ ions [14, 29, 32, 43]. Thus, the non-radiative decay rates (WNR) at Nd3+ ions are also obtained by using the relation [14],
The values of WNR for NaLiCdBNd01, NaLiCdBNd05, and NaLiCdBNd10 glasses are found to be 237, 498, 2805 μs−1, respectively, and listed in Table 4. Also presented the quantum efficiency (η) values in Table 4, which is evaluated from τexp/τR. It can be concluded that the maximum η value of 86% for the optimum NaLiCdBNd05 glass can be considered as good candidate for laser emission at 1.06 μm.
Threshold property-saturation intensity
Finally, it is necessary to introduce the saturation intensity (Is) for the low threshold operation, which depends on the σemi and τexp. The pump input power to reach a low threshold is desirable for cw laser operation. This threshold is directly proportional to the Is. The equation for calculation of the Is can be expressed as [9, 10, 14]
From the above expression, the product (σemi × τexp) is calculated from the higher σemi of NaLiCdBNd05 glass and its τexp. A large product of 151.92 × 10−25 cm2s (σemi × τexp) resulting in low value of the Is (1.23x108 W/m2). This Is value is lower than the other Nd3+-doped glasses such as BBaAlZnNaNd1.5(3.67 × 108 W/m2) [10], SPbKNLFNd10 (1.91 × 108 W/m2) [34], GSBNd (4.63 × 108 W/m2) [42], TZN10 (4.21 × 108 W/m2) [47], PKAlCaFNd10 (2.41 × 108 W/m2) [48], BSKNLNd10 (1.71 × 108 W/m2) [14], BNaAf (2.96 × 108 W/m2) [49], BBONd (3.0 × 108 W/m2) [50], and SBNAC (2.5 × 108 W/m2) [51] listed in Table 5. The optical gain (σemi × τR) calculated to be 175.2 × 10−25 cm2s is an another important parameter for NaLiCdBNd05 glass and compared with other Nd3+-doped glasses in Table 5 and Fig. 9. Therefore, the high gain and low threshold of NaLiCdBNd05 glass is a promising laser material at 1.06 μm for Nd3+:4F3/2 → 4I11/2 transition.
Conclusion
In this investigation, the Nd3+:alkalicadmino borate glasses were prepared by the suddenly melt-quenching technique and studied their optical properties via absorption, NIR emission spectra, and decay curves. The amorphous and structural behavior of undoped NaLiCdB glass were characterized through XRD and Raman. The decrease in optical band gap energy for direct (3.92–3.86 eV) and indirect (3.51–3.36 eV) transitions leads to structural disorder of the Nd3+:NaLiCdB glasses. The PL decay times excited at 808 nm were found to be 318, 267, and 100 μs for NaLiCdBNd01, NaLiCdBNd05, and NaLiCdBNd10 glasses. The experimental NIR spectra show highest emission at 1.06 μm (4F3/2 → 4I11/2) with favorable spectroscopic parameters such as higher stimulated emission cross-section (5.69 × 10−20 cm2), quantum efficiency (87%), and long bandwidth (18.1 × 10−26 cm3). Interestingly, large optical gain (175.2 × 10−25 cm2s) and low threshold (1.23 × 108 W/m2) have been obtained for NaLiCdBNd05 glass. Results demonstrate that the NaLiCdBNd05 glass might be a promising laser material at 1.06 μm.
References
K. Jha, M. Jayasimhadri, Spectroscopic investigation on thermally stable Dy3+ doped zinc phosphate glasses for white light emitting diodes. J. Alloys Compd. 688, 833–840 (2016)
C.B. Annapurna, S. Mahamuda, M. Venkateswarlu, K. Swapna, A.S. Rao, G. Vijaya Prakash, Dy3+ ions doped single and mixed alkali fluoro tungsten tellurite glasses for LASER and white LED applications. Opt. Mater. 62, 569–577 (2016)
N. Deopa, A.S. Rao, S. Mahamuda, M. Gupta, M. Jayasimhadri, D. Haranath, G. Vijaya Prakash, Spectroscopic studies of Pr3+ doped lithium lead alumino borate glasses for visible reddish orange luminescent device applications. J. Alloys Compd. 708, 911–921 (2017)
I.Z. Hager, R. El-Mallawany, A. Bulou, Luminescence spectra and optical properties of TeO2–WO3–Li2O. Physica B 406, 972–980 (2011)
G. Neelima, K. Venkata Krishnaiah, N. Ravi, K. Suresh, K. Tyagarajan, T. Jayachandra Prasad, Investigation of optical and spectroscopic properties of neodymium doped oxyfluoro-titania-phosphate glasses for laser applications. Scr. Mater. 162, 246–250 (2019)
K. Linganna, R. Narro-Garcia, H. Desirena, E. De la Rosae, Ch. Basavapoornima, V. Venkatramu, C.K. Jayasankar, Effect of P2O5 addition on structural and luminescence properties of Nd3+-doped tellurite glasses. J. Alloys Compd. 684, 322–327 (2016)
C. Madhukar Reddy, N. Vijaya, B. Deva Prasad Raju, NIR fluorescence studies of Nd3+ ions doped sodium fluoroborate glasses for 1.06 μm laser applications. Spectrochim. Acta A 115, 297–304 (2013)
B. Karthikeyan, R. Philip, S. Mohan, Optical and nonlinear optical properties of Nd3+ doped heavy metal borate glasses. Opt. Commun. 246, 153–162 (2015)
N. Deopa, A.S. Rao, M. Gupta, G. Vijaya Prakash, Spectroscopic investigations of Nd3+ doped lithium lead Alumino borate glasses for 1.06 μm laser applications. Opt. Mater. 75, 127–134 (2018)
G. Lakshminarayana, K.M. Kaky, S.O. Baki, A. Lira, A.N. Meza-Rocha, C. Falcony, U. Caldino, I.V. Kityk, A. Mendez-Blas, A.F. Abas, M.T. Alresheedi, M.A. Mahdi, Nd3+-doped heavy metal oxide based multicomponent borate glasses for 1.06 μm solid-state NIR laser and O-band optical amplification applications. Opt. Mater. 78, 142–159 (2018)
Y.C. Ratnakaram, A. Viswanadha Reddy, Electronic spectra and optical band gap studies in neodymium chlorophosphate glasses. J. Non-Cryst. Solids 277, 142–154 (2000)
H. Fares, I. Jlassi, S. Hraiech, H. Elhouichet, M. Ferid, Radiative parameters of Nd3+ doped titanium and tungsten modified tellurite glasses for 1.06 μm laser materials. J. Quant. Spectrosc. Radiat. Transf. 147, 224–232 (2014)
S. Mohan, K.S. Thind, Investigation of luminescence and spectroscopic properties of Nd3+ ions in cadmium alkali borate glasses. Opt. Mater. 57, 134–139 (2016)
M. Deepa, R. Doddoji, C.S. Dwaraka Viswanath, A.V. Chandrasekhar, Optical and NIR Luminescence spectral studies: Nd3+-doped borosilicate glasses. J. Lumin. 213, 191–196 (2019)
S. Sindhu, S. Sanghi, S. Rani, A. Agarwal, V.P. Seth, Modification of structural and electrical conductivity of cadmium borate glasses in the presence of V2O5. Mater. Chem. Phys. 107, 236–243 (2008)
A.K. Yadav, P. Singh, A review of structure of oxide glasses by Raman spectroscopy. RSC Adv. 5, 67583–67609 (2015)
R. Vijaya Kumar, P. Gayathri Pavani, B. Ramesh, M. Shareefuddin, K. Siva-Kumar, Structural studies of xLi2O-(40–x)Bi2O3–20CdO–40B2O3 glasses. Opt. Mater. 35, 2267–2274 (2013)
C. Rubalcava-Cornejo, M.E. Zayas, S.J. Castillo, J.J. Palafox, R. Lozada-Morales, R. Palomino-Merino, J.M. Rincón, Structural properties of rare earth doped ZnO–CdO–TeO2 system. Phys. Chem. Glasses: Eur. J. Glass Sci. Technol. B 50, 19–22 (2009)
M. Parandamaiah, S. Venkatramana Reddy, A.V. Chandrasekhar, Investigation of Nd3+ doped lithium sodium bismuth borate glasses for NIR applications. IJARSET 03, 2720–2729 (2016)
B. Bhatia, S.L. Meena, V. Parihar, M. Poonia, Optical basicity and polarizability of Nd3+ doped bismuth borate glasses. J. Njgc. 5, 44–52 (2015)
M.K. Halimah, W.H. Chiew, H.A.A. Sidek, W.M. Daud, Z.A. Wahab, A.M. Khamirul, S.M. Iskandar, Optical properties of litium borate glass (Li2O)x (B2O3)1–x. Sains Malays. 43, 899–902 (2014)
M. Deepa, R. Doddoji, A.V. Chandrasekhar, Structural and optical properties of Sm3+-doped borate glasses for luminescent applications. Opt. Quantum Electron. 51, 395 (2019)
V.B. Sreedhar, R. Doddoji, K.K. Kumar, V.R. Minnam-Reddy, A study of NIR emission and associated spectroscopic properties of Nd3+:P2O5 + K2O + Al2O3 + ZnF2 glasses for 1.06 μm laser applications. J. Non-Cryst. Solids (2020). https://doi.org/10.1016/j.jnoncrysol.2020.120521
N. Elkhoshkhany, S.Y. Marzouk, N. Moataz, S.H. Kandil, Structural and optical properties of TeO2–Li2O–ZnO–Nb2O5–Er2O3 glass system. J. Non-Cryst. Solids 500, 289–301 (2018)
K. Upendra Kumar, P. Babu, K.H. Jang, H.J. Seo, C.K. Jayasankar, A.S. Joshi, Spectroscopic and 1.06 μm laser properties of Nd3+ doped K–Sr–Al phosphate and fluorophosphate glasses. J. Alloys Compd. 458, 509–516 (2008)
M. Jayasimhadri, L. Rama Moorthy, R.V.S.S.N. Ravikumar, An investigation of the optical properties of Nd3+ ions in alkali telluro fluorophosphate glasses. Opt. Mater. 29, 1321–1326 (2007)
R. BalaKrishnaiah, P. Babu, C.K. Jayasankar, A.S. Joshi, A. Speghini, M. Bettinelli, Optical and luminescence of Nd3+ ions in K–Ba–Al–Phosphate and fluorophosphate glasses. J. Phys.: Condens. Matter 18, 165–179 (2006)
M.V. Sasi Kumar, D. Rajesh, A. Balakrishna, Y.C. Ratnakaram, Thermal and optical properties of Nd3+ doped lead zinc borate glasses-influence of alkali metal ions. Physica B 415, 67–71 (2013)
C. Tian, X. Chen, Y. Shuibao, Concentration dependence of spectroscopic properties and energy transfer analysis in Nd3+ doped bismuth silicate glasses. Solid State Sci. 48, 171–176 (2015)
M. Sobczyk, Temperature-dependent luminescence and temperature-stimulated NIR-to-VIS up-conversion in Nd3+-doped La2O3–Na2O–ZnO–TeO2 glasses. J. Quant. Spectrosc. Radiat. Transf. 119, 128–136 (2013)
C.N. Raju, C.A. Reddy, S. Sailaja, H.J. Seo, B.S. Reddy, Judd-Ofelt theory: optical absorption and NIR emission spectral studies of Nd3+: CdO–Bi2O3–B2O3 glasses for laser applications. Mater. Sci. 47, 772–778 (2012)
D. Ramachari, L. Rama Moorthy, C.K. Jayasankar, Optical absorption and emission properties of Nd3+-doped oxyfluorosilicate glasses for solid state lasers. Infrared Phys. Tehnol. 67, 555–559 (2014)
K.H. Mahmoud, Spectroscopic and radiative properties study of Nd3+ doped cadmium-phosphate glasses. Physica B 405, 4746–4749 (2010)
P. Manasa, D. Ramachari, J. Kaewkhao, P. Meejitpaisan, E. Kaewnuam, A.S. Joshi, C.K. Jayasankar, Studies of radiative and mechanical properties of Nd3+ -doped lead fluorosilicate glass for broadband amplification in a chirped pulse amplification based high power laser system. J. Lumin. 188, 558–566 (2017)
K. Mariselvam, R. Arun kumar, K. Suresh, Optical properties of Nd3+-doped barium lithium fluoroborate glasses for near-infrared (NIR) emission. Physica B 534, 68–75 (2018)
E.A. Davis, N.F. Mott, Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 22, 903–927 (1970)
N.F. Mott, E.A. Davis, Electronic Process in Non-crystalline Materials, 2nd edn. (Clarendom Press, Oxford, 1979)
I. Iparraguirrre, J. Azkargorta, R. Balda, K. Venkata Krishnaiah, C.K. Jayasankar, M. Al-Saleh, J. Fernandez, Spontaneous and stimulated emission spectroscopy of a Nd3+-doped phosphate glass under wavelength selective pumping. Opt. Express 19, 19440–19453 (2011)
R. Chahal, F. Starecki, J.-L. Doualan, P. Nemec, A. Trapananti, C. Prestipino, G. Tricot, C. Boussard-Pledel, K. Michel, A. Braud, P. Camy, J.-L. Adam, B. Bureau, V. Nazabal, Nd3+:Ga–Ge–Sb–S–glasses and fibers for luminescence in mid-IR:synthesis, structural characterization and rare earth spectroscopy. Opt. Mater. Express 8, 1650–1671 (2018)
B. Sudhakar Reddy, H.-Y. Hwang, Y.-D. Jho, B.S. Ham, S. Sailaja, C. Madhukar Reddy, B. Vengala Rao, S.J. Dhoble, Optical properties of Nd3+-doped and Er3+–Yb3+ codoped borotellurite glass for use in NIR laser and fiber amplifiers. Ceram. Int. 41, 3684–3692 (2015)
D. Umamaheswari, B.C. Jamalaiah, T. Sasikala, G.V. Lokeswara Reddy, L. Rama Moorthy, Investigation on 1.07 μm laser emission in Nd3+-doped sodium fluoroborate glasses. J. Rare Earths 30, 413–417 (2012)
T. Xue, L. Zhang, J. Hu, M. Liao, L. Hu, Thermal and spectroscopic properties of Nd3+-doped novel fluorogallate. Opt. Mater. 47, 24–29 (2015)
M. Venkateswarlu, K. Sk Mahamuda, M.V.V.K.S.Prasad Swapna, A. Srinivasa Rao, A. Mohan Babu, G. Suman Shakya, V. Prakash, Spectroscopic studies of Nd3+-doped lead tungsten tellurite glasses for the NIR emission at 1062 nm. Opt. Mater. 39, 8–15 (2015)
L. Pal, A. Agarwal, S. Sanghi, M.P. Aggarwal, S. Bhardwaj, Fluroscence and radiative properties of Nd3+ ions doped zinc bismuth silicate glasses. J. Alloys Compd. 587, 332–338 (2014)
T. Fuji, K. Kodaira, O. Kawauchi, N. Tanaka, H. Yamashitha, M. Anpo, Photochromic behavior in the fluorescence spectra of 9-anthrol encapsulated in Si–Al glasses prepared by the Sol–Gel method. J. Phys. Chem. B 101, 10631–10637 (1997)
M. Greben, P. Khoroshyy, I. Sychugov, J. Valenta, Non-exponential decay kinetics: correct assessment and description illustrated by slow luminescence of Si nanostructures. Appl. Spectrosc. Rev. (2018). https://doi.org/10.1080/05704928.2018.1517263
K.U. Kumar, V.A. Prathyusha, P. Babu, C.K. Jayasankar, A.S. Joshi, A. Speghini, M. Bettinelli, Fluorescence properties of Nd3+-doped tellurite glasses. Spectrochim. Acta A 67, 702–708 (2007)
S.N. Rasool, T. Sasikala, A.M. Babu, L.R. Moorthy, C.K. Jayasankar, Optical spectroscopy, 1.06 μm emission properties of Nd3+-doped phosphate based glasses. Spectrochim. Acta A 180, 193–197 (2017)
R.T. Karunakaran, K. Marimuthu, S. Arumugam, S. Surendra Babu, S.F. Leon-Luis, C.K. Jayasankar, Structural, optical absorption and luminescence properties of Nd3+ ions in NaO–NaF borate glasses. Opt. Mater. 32, 1035–1041 (2010)
G.V. Vazquez, G.H. Munoz, I. Camarillo, C. Falcony, U. Caldino, A. Lira, Spectroscopic analysis of a novel Nd3+ activated barium borate glass for broadband laser amplification. Opt. Mater. 46, 97–103 (2015)
Q. Yanbo, D. Ning, P. Mingying, Y. Liiyun, C. Danping, Q. Jianrong, Z. Congshan, T. Akai, Spectroscopic properties of Nd3+-doped high silica glass prepared by sintering porous glass. J. Rare Earths 24, 765–770 (2006)
Acknowledgement
One of the authors Mrs. M. Deepa would like to thank Dr. R. V. S. S. N. Ravikumar Department of Physics, Acharya Nagarjuna University, for his valuable suggestions, support, and providing laboratory facility to carry out this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deepa, M., Doddoji, R. & Chandrasekhar, A.V. NIR emission from Nd3+-doped Na2O + Li2O + CdO + B2O3 glasses: a study of structural, physical and radiative properties. J Opt 50, 278–288 (2021). https://doi.org/10.1007/s12596-021-00680-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12596-021-00680-4