Abstract
An ultraviolet-visible laser absorption-scattering technique (UV-LAS) was developed for obtaining the evaporation characteristics of binary-component fuel spray. P-xylene and fluorobenzene were selected as the substitutes for low and high boiling points components, respectively, in a binary fuel spray. The molar absorption coefficients (MAC) of p-xylene and fluorobenzene at 266 nm wavelength were measured with relative uncertainty of 2.8% in a calibration system, which was designed to facilitate control of the mixture temperature and vapor concentration. UV-LAS was applied to a quantitative detection of vapor concentration distributions of each component in a binary-component fuel spray injected into a high-temperature and high-pressure constant volume chamber. A difference in spatial stratification of each component fuel in the spray due to their volatility difference was observed. An uncertainty estimation of UV-LAS for binary-component fuel spray was performed, and the results show that the maximum uncertainty is less than 9.8% in measurement of vapor mass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The evaporation characteristics of multi-component fuel have significant effects on the fuel–air mixing process and the subsequent processes of combustion and harmful emission formations. It is crucial to understand the physics behind the fuel–air mixing process and to validate spray evaporation models for multi-component fuel, therefore, a reliable laser diagnostic is needed to facilitate quantitative measurements on evaporation characteristics of multi-component fuel.
Several laser-based techniques were proposed over the past decade for exploring evaporation characteristics of fuel spray, such as schlieren technique [1, 2], planar laser Rayleigh scattering (PLRS) [3, 4], laser-induced (exciplex) fluorescence (LIF/LIEF) [5,6,7,8,9], ultraviolet/visible laser absorption-scattering (UV-LAS) imaging technique [10, 11]. Few research groups were attempting quantitative investigation on the evaporation characteristics of multi-component spray based on planar laser-induced fluorescence (PLIF). Zhang and Sick [5] developed a two-tracer (acetone and p-xylene) PLIF diagnostic technique and applied it to visualize the in-cylinder distributions of different volatility fuel components in an optical spray guided spark-ignition direct-injection (SIDI) engine. Yoon et al. [6] analyzed the vapor phase distribution and behavior of each component in three-component fuel using PLIF with acetone, TMPD and anthracene as dopants which represent low boiling point, middle boiling point and high boiling point components, respectively. Itani et al. [7] and Kranz [8] investigated the effects of preferential evaporation of multi-component fuels by detection of two aromatic fluorescence tracers (p-difluorobenzene and α-methylnaphthalene) with complementary evaporation characteristics matched to different components of a multi-component fuel.
The PLIF technique worked well in an environment without droplets for the vapor concentration measurement; however, it was not suitable for a drop-laden evaporating spray. To overcome this difficulty, Melton [12] proposed the laser-induced exciplex fluorescence (LIEF) to determine both vapor and liquid concentration distributions of single-component fuel simultaneously. Two independent laser-induced exciplex systems (LIEF) with two pair of tracers: fluorobenzene (FB) and triethylamine (TEA), naphthalene and TMPD representing the light and heavy component of gasoline, respectively, were used for the measurement of liquid and vapor phase distributions of binary-component fuel [9]. Nevertheless, difficulties such as fluorescence quenching by oxygen and cross-talk between monomer (vapor) fluorescence and exciplex (droplets) fluorescence due to an overlap in the emission spectra were encountered for LIEF.
Ultraviolet-visible laser absorption-scattering technique (UV-LAS) [13,14,15,16,17,18,19] is another laser diagnostic technique developed for detecting the distributions of both fuel vapor and droplets mass distributions in high-pressure and high-temperature conditions. UV-LAS is a line-of-sight (LOS) measurement technique different from PLIF which is planar laser imaging technique. An attempt to develop a quantitative diagnostic was made for determining the vapor phase concentration distributions in an evaporating binary-component fuel spray based on UV-LAS imaging technique with p-xylene and α-methylnaphthalene as the two components of the test fuel [9, 10]. The uncertainty in vapor mass of substitute fuel measurement was larger than 38%. In the study of Chen et al. [13], the spray evaporation processes of ethanol–gasoline blends were examined by substitution of gasoline and ethanol with toluene and MEK, respectively, without uncertainty estimation. One of the thorniest issues for this technique is how to accurately calibrate the molar absorption coefficient of test fuel under different temperature. So, this diagnostic was only used as a qualitative or semi-quantitative method.
In this paper, the UV-LAS imaging technique system is established to measure the vapor concentration distributions of binary-component fuel spray in a droplet-laden environment, where p-xylene was selected as the substitute for n-octane and fluorobenzene was selected as the substitute for n-hexane in a binary fuel spray. For realizing the quantitative measurement, the molar absorption coefficients (MAC) of substitute fuels were measured using a calibration chamber, which enables controlling of the mixture temperature and vapor concentration precisely and separately. Finally, the vapor concentration distributions of each component in the spray were obtained and a detailed uncertainty estimation for the MAC calibration experiment and the vapor phase measurement of binary-component fuel spray were conducted.
2 Measurement principle
2.1 Principle of UV-LAS on binary-component fuel spray
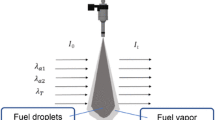
UV-LAS technique is based on light extinction due to absorption and scattering of fuel vapor and droplets in a spray. It consists of two measurements: a visible light measurement to detect the droplets optical thickness and an ultraviolet light measurement to determine the joint optical thickness of vapor and droplets. Figure 1 shows the process of the light extinction due to absorption and scattering of spray (vapor/droplets) at ultraviolet wavelength (absorption wavelength, λa= 266 nm) and visible wavelength (transparent wavelength, λT = 532 nm). The incident light with intensity of I0 will be attenuated and turn into It. The extinction of the absorption wavelength is attributed to the vapor absorption as well as droplets absorption and scattering, while the only reason for the extinction of the transparent light is the scattering from the liquid fuel. According to the Lambert–Beer’s law and the scattering theory of small particles, the following equations are obtained [14, 15]:
where I0 is the intensity of incident light, IT is the intensity of transmitted light, ε (λa) (l cm−1 mol−1) is the molar absorption coefficient (MAC) of fuel vapor at λa, Cv(x) is the vapor concentration (mol m−3) at position x, L is the optical path length (m), \(K_{{\lambda_{\text{a}} }}^{\text{ext}}\), \(K_{{\lambda_{\text{T}} }}^{\text{ext}}\) are the extinction coefficient (m−1) for a cloud of droplets at the two wavelengths.
From Eqs. (1) and (2), the line-of-sight optical thickness (OT) of the vapor at the absorbing wavelength can be derived as follow:
where R is defined in Eq. (4) as the ratio of droplets’ optical thickness at the two wavelengths λa and λT. It has been experimentally and theoretically confirmed that the ratio of optical thickness of droplets in a typical fuel spray, R approaches 1 when the half-detection angles are designed differently for two wavelengths to eliminate the effect of droplets scattering due to wavelength difference [13,14,15,16,17,18,19,20].
From Eq. (3), the line-of-sight averaged fuel vapor concentration \(\overline{{C_{\text{v}} }}\) will be:
The spatial concentration distributions can be reconstructed based on the onion-peeling model if the spray structure can be regarded as axisymmetric [15, 19, 21]. If not, the 2-D concentration distributions in the projected plane along the line-of-sight can still be obtained through Eq. (6),
Moreover, ε (λa) always depends on mixture temperature. It is not easy to obtain the mixture temperature in an evaporating fuel spray. In this study, the mixture temperature was predicted based on a thermal energy conservation called adiabatic mixing model [19, 22]. Spray was assumed to be axisymmetric and the onion-peeling model was adopted to reconstruct the spray. The temperature of mixture (Tmix) can be estimated by the bulk mean temperature of liquid and ambient gas as follows:
where Cv is the concentration of fuel vapor (kg m−3), ρa is the concentration of the ambient gas (kg m−3), cfv is specific heat of fuel vapor [J/(kg K)], Cpa is specific heat of the ambient gas [J/(kg K)], cfl is specific heat of the liquid fuel [J/(kg K)], Ta is the temperature of ambient gas (K), H is the latent heat of fuel evaporation, Tsat is the saturate temperature of the fuel (K) which was estimated as the temperature of bubble point of binary-component fuel, Tf is the liquid fuel temperature. Ambient temperature Ta was set as the initial temperature of the mixture to calculate the initial vapor concentration. Then, the mixture temperature and vapor concentration distributions were obtained by iterative computations based on Eqs. 5, 6, 7 pixel by pixel in the whole spray. More detailed presentation about the temperature calculation model and iterative process can found in Zhang [19] and Senda et al. [22]. The validation of the temperature correction model was conducted by applied the vapor mass fraction and temperature experimental results from Zigan et al. [23]. Temperature profiles which were obtained by Eq. (7) and measurement result from Zigan et al. [23], respectively, are shown in Fig. 2. The similarity of temperature variation profiles of the calculated result using the adiabatic mixing model and the experimental results from Zigan et al. [23] can be verified through comparison in Fig. 2. The maximum difference of temperature between calculation and measurement happened in the center area of spray, which is less than 18 K.
The detailed depictions of the UV-LAS principle and the processing method are presented in the previous publications [13,14,15,16,17,18,19]. If the spray can be assumed axisymmetric, it is believed that by means of the UV-LAS technique, cumulative masses of vapor phase can be quantitatively measured. The flowchart for image processing is shown in Fig. 3.
Up to this point, quantitative measurement of vapor concentration can be realized when single-component fuel is used. For a binary-component fuel spray measurement, however, it is difficult to judge which component causes the extinction of ultraviolet light, if more than one component exist which absorbs ultraviolet light. So, to determine the vapor distributions of each component in a binary-component fuel spray, only one of the components must show absorption, while the other components must keep no or scarce absorption of the UV-wavelength. If not, the fuel components should be artificially changed so. For example, as shown in Fig. 4a, if the objective fuel is composed of n components (C1, C2, C3, …Ci, Cn) with no one absorbing ultraviolet, for investigating the evaporation characteristics of ith component (Ci), a substitute ultraviolet-absorbing component (Csi) can be used to replace Ci. In this way, the vapor distribution of the replaced component can be quantitatively measured by the UV-LAS technique. By substituting different component, the vapor behavior of each component in the spray can be obtained, keeping the physical properties (thus, the atomization characteristics) of the fuel similar to the original.
2.2 Investigation of the substitute fuel
As stated above, for examining the vaporization behavior of each component with different boiling points, the substitute fuels used in UV-LAS measurement must meet the following requirements: (1) having at least one absorption band in ultraviolet light, (2) having similar atomization and evaporation characteristics to the objective fuel.
In this paper, an objective fuel with binary-components was adopted for simulating gasoline fuel, that is, n-hexane and n-octane, to represent the low boiling point (LBP) and high boiling point (HBP) components in gasoline, respectively. The component molar ratio of the binary- component fuel is set to 1:1. To apply UV-LAS to this fuel, p-xylene and fluorobenzene were selected as the substitutes for n-octane and n-hexane, respectively. In specific, for determining the evaporation characteristics of LBP (n-hexane) component, the fuel with fluorobenzene and n-octane (abbr. FB/n-oct) will be used, while for determining the HBP (n-octane) component, the fuel with p-xylene and n-hexane (abbr. p-xy/n-hex) will be used. The schematic of fuel substitution strategy is shown in Fig. 4b. The considerations for these fuel substitutions include:
First, both p-xylene and fluorobenzene have absorption at 266 nm wavelength light [10, 24] and are suitable for UV-LAS imaging technique, and the effect of component substitute on drop size distributions in the spray is predicted to be insignificant, according to the experimental study of Hiroyasu [25] on atomization characteristics. In specific, the drop size distributions in a fuel spray depend mainly on such physical properties as surface tension σ, viscosity μ and density ρ of fuel besides nozzle parameters and operating conditions. The correlation of Sauter mean diameter, D32, to fuel physical properties is derived as:
where, c is the constant which depends on nozzle parameters and operating conditions. Some physical properties of these components are given Table 1. From Eq. (8) and Table 1, D32(fluorobenzene)/D32(n-hexane) = 0.9736, D32(p-xylene)/D32(n-octane) = 0.9998, which means that the components changes have little effect on drop size distributions.
To identify quantitatively the effects of component substitutions on the characteristics of liquid atomization and spray evaporation, two experiments were conducted: the one is the high-speed spray imaging by Mie-scattering at Ta= 300 K, Pa= 0.1 MPa for verifying the spray atomization effect of the component substitute, and the other is the high-speed Schlieren imaging under the evaporating conditions (Ta= 623 K, Pa= 0.45 MPa) for verifying the effect of fuel component substitute on vapor phase penetrations and the spray shapes. The results are given in Fig. 5, showing the spray penetrations and shapes of both the liquid and the vapor phases in comparison between the original fuel spray (n-hex/n-oct) and their substitutes (FB/n-oct and p-xy/n-hex). The spray penetration evaluated in Fig. 5 included the length of pre-spray. The spray cone angle was measured at 5–15 mm from the nozzle tip, which was determined according to SAE standard. Schlieren images in Fig. 5b are obtained by subtracting the spray schlieren image from the background image.
Comparisons of the liquid and the vapor spray penetrations and shapes between objective (n-hex/n-oct) and substitute fuel (p-xy/n-hex and FB/n-oct) sprays. (Injection conditions: Pinj= 3 MPa, Tf= 300 K, ∆tinj= 1.0 ms. tavSOI: time after visible start of injection, high-speed photograph: 640 pixel × 536 pixel, 20 kHz). a Liquid phase, b vapor phase
By the Mie-scattering spray images shown in Fig. 5a, it is observed that the spray shapes of the substitute fuel FB/n-oct and p-xy/n-hex are very close to that of the original fuel, and by the measurement results of spray penetration and spray angle it has been confirmed that the component substitutes have very limited effect on the spray penetration and spray angle. In other words, the component substitute will only cause a variation in spray penetration and cone angle of the liquid phase at a degree not beyond the cycle-to-cycle variation of spray penetration and cone angle of the injections. As to the vapor phase, we got some insightful information from the high-speed Schlieren images at Ta= 623 K, Pa= 0.45 MPa. Substantial differences in spray shape are not observed among the sprays of the original fuel and substitute fuels in Fig. 5b. The quantitative comparisons of the vapor penetration and the spray angles shown in Fig. 5b. The results indicate the deviations of penetration and spray angle due to the fuel substitutions were less than that caused from the cycle-to-cycle variation of the injection. So, the substitutions effect on the spray atomization and evaporation shown in Fig. 5 which indicate the substitution methodology may induce at most 5% variation in penetrations of liquid and vapor phases, which is within a range of the cycle-to-cycle variation of fuel injections.
Finally, the LBP component, n-hexane, has similar values of boiling point and vaporization heat with its substitute, fluorobenzene. Likewise, the HBP component, n-octane, and its substitute, p-xylene, share the similar boiling point, and vaporization heat. It is necessary to examine whether the evaporation of the substitute match that of the original components. In the work of Zhang and Sick [5], 5% p-xylene was doped into iso-octane to represent the high boiling point component of gasoline in LIF diagnostics technique. Melton [28] recommended 10% TEA and 0.5% FB in 89.5% n-hexane as test fuel because of the good co-evaporation properties in the LIEF technique. In the work of Düwel et al. [24], 2% FB and 9% DEMA in 89% n-hexane was selected as test fuel due to the good co-evaporation properties in LIEF technique. Zhang and Nishida [11] selected p-xylene (50%) as the substitute of n-octane in gasoline in the viewpoint of activity coefficient in UV-LAS diagnostic technique. In general, p-xylene and FB were chosen frequently as the tracer or substitute to represent the evaporation characteristic of alkane.
Besides, distillation curves (as shown in Fig. 6) of the original fuel (n-hex/n-oct) and the substitute fuel (FB/n-oct and p-xy/n-hex) were calculated based on thermodynamics with commercial software Aspen Plus [29]. The component molar ratio of the binary-component fuel is set to 50%:50% in each combination of binary components. As shown in Fig. 6, at the initial stage of evaporation, a slight difference in distillation curves is observed between the original fuel (n-hex/n-oct) and the substitute fuel (FB/n-oct), but the difference diminished to a very small degree as the fraction increases, e.g., there is less than 5 K temperature difference at the evaporation fractions between 50 and 90% for all the binary-component fuels.
Distillation curves of the original fuel (n-hex/n-oct) and the substitute fuel (FB/n-oct and p-xy/n-hex) in comparison with gasoline [7]
As confirmed in the above discussion, the substitutions have little effect on the spray formation of both liquid and vapor phases. It is believed that fluorobenzene and p-xylene are suitable for the substitutes of LBP/HBP component in binary n-octane/n-hexane fuel in the UV-LAS measurement. Naturally, the substitution may cause deviation in spray atomization and evaporation characteristics to those of the target fuel spray, but the deviation occurs at a limited degree.
3 Experimental setup and conditions
3.1 Experimental setup
The calibration of molar absorption coefficient (MAC) in Eq. 6 is one of the most difficult problems for the quantitative measurement of binary-component concentration distributions using UV-LAS. Accurate control of mixture concentration and production of homogeneous mixture were crucial factors for the calibration precision. To achieve this, a MAC calibration system for different fuels like p-xylene/fluorobenzene, which are in liquid state at normal temperature and pressure, was newly designed [30] and manufactured.
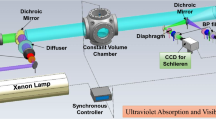
The calibration system consists of a Rectangular Constant Volume Chamber (RCVC), a mass flow controller, a fuel injection system, a heating system, a pressure regulator. Figure 7 shows the setup of the calibration experimental system. The RCVC is a cuboid chamber with the internal dimension of 50 × 50 × 270 mm3. Three quartz windows are mounted on three sides of RCVC: one narrow window was installed at one side for incident laser sheet access; two parallel windows (40 mm × 50 mm) were installed at the other two sides which allow the laser light to pass through when applied to the absorption measurements. As shown in Fig. 7, the carrier gas (nitrogen) was supplied by N2 tanks and controlled by a mass flow controller (Bronkhorst EL-FLOW) with accuracy of ± (0.5% Rd + 0.1% FS). The nitrogen was heated by electrical resistances and introduced into a premix tank, into which the pressurized p-xylene/fluorobenzene (purity > 99.5%, Aladdin) was injected through a GDI fuel injector.
To enhance the fuel evaporation so that the evaporation process of droplets completes before entering RCVC, a flash boiling effect was adopted in this calibration system. The superheated conditions were reached by elevating the fuel temperature around the injector tip through a constant temperature oil bath system. Under superheated circumstances, both atomization and evaporation characteristics of liquid fuel spray would be enhanced [31]. The effect of flash boiling spray on the fuel spray atomization and evaporation characteristics was verified by Mie-scattering measurement in a similar premix-tank equipped with quartz windows (Fig. 8). The results indicate that under the superheated conditions the fuel sprays evaporated completely at 1.5 ms after end of injection (AEOI). Under the non-superheated conditions, however, significant liquid droplets still remained and impinged on the wall of the premix-tank at the same time. Thus, using the strategy of flash boiling spray guaranteed the rapid fuel evaporation so that the fuel was completely vaporized when mixing with N2 in the premix-tank. After fuel-N2 mixing, the gaseous mixture flowed into RCVC through a perforated plate which was designed to guarantee the uniformity of flow, generating a homogenous flow field and concentration distribution within the measurement area in the RCVC. The uniformity of the mixture flow will be discussed in Sect. 5.1. In addition, the controlled temperature and pressure were monitored by thermocouple and pressure transducer with uncertainties of 0.5% and 1% respectively.
The optical arrangement of MAC calibration experiment system is shown in Fig. 7. The light beam of the fourth harmonic of a Nd:YAG laser (90 mJ 266 nm, diameter = 6 mm) was expanded into 100 mm of diameter and then directed in the test area. When passing though the test area, the light beam was attenuated due to vapor absorption. A group of collimating lenses with a diameter of 100 mm focus the beam into the ICCD camera (PI, PIMAX2).
The evaporation characteristics measurement of binary-component fuel spray was performed in a scavenging spherical constant volume chamber (SCVC) system (Fig. 9). The SCVC system can be operated between 0.01 ~ 6 MPa of the ambient pressure and 298 ~ 900 K of the temperature. The chamber features four quartz windows of 100 mm in diameter with different directions including 90°, 120°, 150° and 180° to provide light accesses for different applications. In this work, only two windows with angular direction of 180° were used for accesses of incident ultraviolet/visible laser light and detection of the attenuation due to the absorption/scattering. Owing to the continuous scavenging features of SCVC, the ambient environment can be refreshed on time when the spray was injected at interval of 10 s. Based on the principle of UV-LAS technique, an imaging system was produced as shown in Fig. 9. A coaxial laser beam consisting of the second harmonic (532 nm) and the fourth harmonic (266 nm) of a Nd:YAG laser was separated into two, an ultraviolet beam (266 nm) and a visible beam (532 nm), by a dichroic mirror. The two beams were then expanded to a diameter of 100 mm, respectively. Specific diffuser was installed before beam expander in Fig. 9 to eliminate the effect of beam steering [32]. After being attenuated due to absorption and/or scattering of the fuel vapor and droplets, the beams were re-separated into two, a visible one and an ultraviolet one. Then, a pair of collimating lens focused the two beams into the respective ICCD (with a 266 nm bandpass filter) and CCD (with a 532 nm bandpass filter). Pinholes before the cameras were used to change half-detection angles so as to eliminate the differences of droplets scattering duo to different wavelengths. The Nd:YAG laser, ICCD/CCD and fuel injection timing are controlled by the synchronizer.
3.2 Experimental conditions
The experimental conditions of MAC calibration and binary-component fuel spray measurement are listed in Table 2. The temperature of the MAC calibration experiment was set at different values between 473 and 573 K at pressure of 0.45 MPa which approached to the typical environment in cylinder during fuel injection process for a turbo-charged gasoline engine. A swirl injector was adopted in this study.
4 Results and discussion
4.1 Results of MAC calibration
MAC calibration measurements were performed according to the conditions in Table 2. The image process route of the MAC calibration is schematically shown in Fig. 10. The background intensity image I0 and the absorption intensity image It were obtained by averaging the respective ten images with correction of the dark current and diminishing the effects of laser fluctuation and noise. Then, the MAC at λa can be calculated according to the Lambert–Beer’s law.
where I0 is the intensity of incident light, It is the intensity of transmitted light, Cv is the fuel vapor concentration (mol m−3) inside the RCVC, L is the optical path length (m).
The correlations of absorbance and vapor concentration (C = Cv × L, mol cm−l) of p-xylene and fluorobenzene at λa= 266 nm are summarized in Fig. 11. The error bars in Fig. 11 were the standard deviation indicating the uncertainty at multiple (ten times) measurements, and so is true for Fig. 14. Note that the absorbance is linearly proportional to the concentration with small standard variations at different temperature for all the cases. It means that the Lambert–Beer’s law is applicable in these conditions. From the slopes of the correlations in Fig. 11, the MAC at different temperature are obtained and shown in Fig. 12. The values decrease as the temperature increases, which is similar in trend to the measurements of p-xylene by Zhang [10], whose value of MAC was about 148 l/mol cm under Ta= 500 K, Pa= 1.5 MPa In the work of Düwel et al. [24], the MAC of FB was about 650 l/mol cm under Ta= 473 K, Pa= 0.1 MPa, which was larger than the result in Fig. 12 (Pa= 0.45 MPa). The inconsistencies could be result from the differences of ambient pressure Pa.
4.2 Evaporation characterization by measurement of binary-component fuel spray
Fuel spray vapor concentration distributions of pure fuel, i.e., p-xylene or fluorobenzene, and the binary components fuels, i.e., p-xy/n-hex or FB/n-oct fuel, were attained by UV-LAS technique. Figure 13 shows a sequence of vapor mass distributions of LBP and HBP in binary-component sprays in comparison to the pure fuels. Besides, the raw images at two wavelengths (UV and Vis) were listed together with the vapor mass distributions images (average of three shots). The fuel spray maintained a symmetrical structure at 1 ms after visible start of injection (avSOI), but became somewhat non-axisymmetric 2 ms later. The spray liquid phase is relatively dilute and multiple scattering plays a minor role at 2 ms avSOI and that later. It is obvious that p-xylene (HBP component) vapor phase in p-xy/n-hex binary-component spray was concentrated more near the spray central axis than that in the pure p-xylene spray, while there appeared little difference in vapor distributions of fluorobenzene (LBP component) between the spray of pure fluorobenzene fuel and FB/n-oct binary-component fuel. In other words, a noticeable difference in characteristics of vapor spatial distributions was observed between the pure spray and the binary-component spray, indicating the significant effect of the component volatility on the spatial distribution of each component. More HBP vapor existed in the region in the spray far field. For the binary-component spray under the ambient conditions in this paper, LBP components evaporated quickly and the temperature decrease. HBP component evaporated a litter slower and continue to penetrate. When HBP component evaporated completely, vapor mass of HBP component distributed farther from injector than LBP component. Similar experimental results which is due to the preferential evaporation were also observed by Yoon et al. [6] and Itani et al. [7] where more LBP components remains in the central area. This feature of spatial stratification for different components found in this work is significant not only for studies on evaporation of binary-component fuel spray, but also for the evaporation model calibration, investigation on stability of ignition and primary flame kernel formation processes and so on.
By integrating the concentration distributions, the cumulative vapor mass of each component in pure fuel spray or the binary fuel sprays were obtained and shown in Fig. 14. The vapor mass increases with time and eventually approaches to a constant value when the fuel was evaporated completely. The injection quantity of one cycle was marked in dashed line in the figures. The difference between the stable value of vapor mass obtained by UV-LAS and total injection fuel mass is also useful to estimate the accuracy of UV-LAS when used in pure and binary fuel vapor measurement.
Variation of fuel vapor mass vs. tavSOI at different injection durations, a p-xylene in pure p-xylene fuel, b p-xylene in p-xy/n-hex binary fuel, c fluorobenzene in pure fluorobenzene fuel, d fluorobenzene in FB/n-oct binary fuel. (The experimental conditions: Ta= 623 K, Pa= 0.45 MPa, Pinj= 3 MPa, Tf= 323 K)
5 Uncertainty estimation
Uncertainty estimations of measurements were performed to verify the reliability of the quantitative measurement technique especially for spray model development and validation. Accuracy of both the MAC calibration and pure/binary fuel spray measurement was estimated.
5.1 MAC measurement
The MAC measurements were performed based on the Lambert–Beer’s law in calibration cell shown in Fig. 7, and MAC can be written as:
where A is the absorbance, V is the volume of intake flow, Mf is the mass of fuel injected into the cell. As indicated by Eq. 8, four measured parameters are induced in the calculation of the MAC. Base on theory of error propagation, the errors from each parameter are combined to give the total error on the MAC:
where \(\sigma\) is standard deviation, and subscript symbols represent the parameter. The relative error of the absorbance \((\sigma_{\text{A}} )\) was obtained from occurrence frequency functions (see Fig. 15) in a region of 100 × 200 pixels interest (ROI) as shown in Fig. 10 [23, 33]. This includes the uncertainty of homogeneity of p-xylene/fluorobenzene and nitrogen mixture in RCVC, thermocouple, pressure transducer, shot noise of the camera and laser power fluctuations. The maximum error of the absorbance calibration data is 3.4% for the signal measurement and it is only 2.5% with ten images averaged as shown in Fig. 15. The optical path length L was accurately measured with approximately 0.5% relative error. The volume (V) of intake flow was controlled by a mass flow controller (Bronkhorst EL-FLOW) with 0.7% relative error. The fuel quantity (Mf) injected into the cell by a GDI injector was measured with a max. relative error of 1.0%. Therefore, the averaged relative error of MAC can be estimated as:
5.2 Spray measurement
The contrast of spray signal to the background noise (CSB) has significant effect on the uncertainty of vapor mass measurement. The averaged intensities of red rectangles in and outside spray areas of OTvapor image in Fig. 16 were written as IS and IB, respectively, and CSB was defined as the ratio of IS and IB:
For a typical measurement case shown in Fig. 16, CSB = 274. This means the signal of is strong enough and the uncertainty could be lower than 0.4% (1/274) when extracting the spray signal from the background.
To verify the vapor measurement accuracy when applied UV-LAS to a binary-component fuel spray, a series of measurements with various injection quantity were conducted. The vapor mass of a completely vaporized fuel spray obtained by UV-LAS was compared to the fuel quantity injected. To capture the moment when the liquid fuel has been completely vaporized, the laser shot timings were set adequately late after the end of injection and injection quantity was set adequately small with short injection duration (0.4–1.4 ms). The droplets of fuel spray were considered completely vaporized when mass of vapor ceased to increase with time as shown in Fig. 14, and no liquid phase could be detected by the visible images. It is noteworthy that the injection quantities in mass of p-xylene, fluorobenzene, p-xy/n-hex, FB/n-oct were different at the same injection conditions because of their differences in density and viscosity.
The results of the cumulative vapor mass measured by UV-LAS when the fuel spray was completely vaporized are illustrated versus the amount of fuel injected in Fig. 17. From the aspect of the total evaporated mass measurement, the uncertainty of UV-LAS was less than 6.94% for the pure single-component fuel sprays and 9.85% for the binary-component fuel sprays. As shown in Fig. 17, the cumulative vapor mass measured by UV-LAS was always larger than the fuel mass of injected. This may result from the following aspects:
Comparison of cumulative vapor mass measured by UV-LAS to the quantity of fuel injected, a p-xylene in pure p-xylene fuel, b p-xylene in p-xy/hex binary fuel, c fluorobenzene in pure fluorobenzene fuel, d fluorobenzene in FB/oct binary fuel. (Experimental conditions: Pinj= 3 MPa, Ta= 623 K, Pa= 0.45 MPa)
-
1.
Temperature drop inside spray due to fuel evaporation. As shown in Fig. 12, MAC of p-xylene and fluorobenzene will increase with decreasing temperature. So, the vapor concentration from Eqs. (5, 6) will be larger as the mixture temperature was lower than the ambient temperature. There is no doubt that the measurement of mixture temperature could be useful to improve the accuracy of vapor concentration measurement, and it has been attempted to add one more wavelength for the measurement of mixture temperature in a pure component fuel axisymmetric spray [33], but it will cause the system more complicated.
-
2.
Negligible absorption of impurity and non-absorbing components (n-hexane/n-octane). The absorption of ultraviolet light by some hydrocarbons is not easy to ignore and will cause a certain degree of uncertainty in measurement of binary-component spray [10, 18]. For example, MAC of n-octane is approximately 0.7% of that of p-xylene with same concentration referring to Zhang [10]. This factor could also be considered as the important reason that the measurement uncertainty of binary-component was larger than the uncertainty of pure component.
-
3.
The assumption of axisymmetric spray shape. Spray is a high-speed turbulent phenomenon containing atomization, evaporation and mixing. The assumption of axisymmetric spray shape will cause uncertainty in the vapor mass measurement. However, the uncertainty due to assumption of axisymmetry can be diminished by averaging multi-shots.
The above-mentioned uncertainties were obtained when spray droplets have been vaporized completely which means that only vapor phase exists. However, it is believed that the uncertainty increases in some degree when applying to an evaporation spray with liquid droplets due to droplet scattering and absorption. Therefore, further investigation is needed to evaluate the effect of droplets measurement accuracy in the future work.
6 Conclusions
UV-LAS imaging technique system was developed for the quantitative measurement of vapor mass distributions of each component in a binary-component fuel spray. The molar absorption coefficients of p-xylene and fluorobenzene at wavelength of 266 nm were obtained in a specially designed calibration system. Evaporation of each component in pure/binary component fuel sprays were characterized at a scavenging high-temperature and high-pressure constant volume chamber. The main conclusions are drawn as follows.
-
1.
The UV-LAS imaging technique was proved to be an effective method to obtain quantitative evaporation characteristics of each component in binary-component fuel sprays when using component substitute strategies and the well-calibrated molar absorption coefficients (MAC) data.
-
2.
Vapor concentration distributions of any component in a binary-component fuel spray can be quantitatively detected by use of UV-LAS technique. The prerequisite is the component to be detected must be a UV-absorption one, and the other components must a weak or non-UV-absorption one. The substitutions have effect on the spray formation of both liquid and vapor phases, but the deviation will be limited to a small degree: 5% for the spray penetrations under the injection condition (Pinj= 3 MPa, Tf= 323 K) and the ambient conditions (Pa= 0.1/0.45 MPa, Ta= 300/623 K) in this study. Besides, the distillation curves of original fuel (n-hex/n-oct) and substitute fuel (FB/n-oct and p-xy/n-hex) are close to each other, with only difference of 3 ~ 5 K in temperature for a distillation portion over 40%. It is believed that fluorobenzene and p-xylene are suitable for the substitutes of LBP/HBP component in binary n-octane/n-hexane fuel in the UV-LAS measurement.
-
3.
The molar absorption coefficients (MAC) of p-xylene and fluorobenzene at 266 nm wavelength were obtained with relative uncertainty of 2.83%.
-
4.
The measurement uncertainty of the total evaporated mass was less than 6.94% in pure component and 9.85% in binary-component fuel measurement. The measurement accuracy was improved significantly compared to 38% measurement uncertainty in the previous work, because of more accurate MAC calibration and higher CSB.
-
5.
Spatial stratification of binary-component fuel spray due to volatility differences was observed, which is useful for evaporation model validation of binary-component fuel spray.
The results in this paper have shown promising features of UV-LAS in quantitative measurement of binary-component fuel spray. This UV-LAS technique is expected to become a useful tool for determining thermodynamic behaviors of two- or multi-component fuel spray in a wide range of temperature and components molar ratio like those in Ref. [34].
References
W.C. Taber, A.K. Agrawal, J.A. Bittle, Appl. Opt. 56(30), 8385–8393 (2017)
J.V. Pastor, J.M. García-Oliver, J.M. Pastor, L.D. Zapata, SAE Technical Paper 2007-24-0026 (2007)
A. Adam, P. Leick, G. Bittlinger, C. Schulz, Exp. Fluids 47(3), 439–449 (2009)
M. Blessinger, J. Manin, S.A. Skeen, M. Meijer, S. Parrish, L.M. Pickett, Int. J. Engine Res. 16(2), 238–252 (2015)
R. Zhang, V. Sick, SAE Technical Papers 2007-01-1826 (2007)
J.K. Yoon, K.J. Myong, J. Senda, H. Fujimoto, J. Mech. Sci. Technol. 23(9), 2565–2573 (2009)
L.M. Itani, G. Bruneaux, A.D. Lella, C. Schulz, Proc. Combust. Inst. 35(3), 2915–2922 (2015)
P. Kranz, S.A. Kaiser, Proc. Combust. Inst. 37(2), 1365–1372 (2018)
P.L. Kelly-Zion, J.P. Styron, C.F. Lee, R.P. Lucht, J.E. Peters, R.A. White, Proc. Combust. Inst. 27(2), 2111–2117 (1998)
Y. Zhang, K. Nishida, Combust. Sci. Technol. 179(5), 863–881 (2007)
Y. Zhang, Y. Kotani, A. Yoshida, K. Nishida, SAE Technical Papers 2007-01-1892 (2007)
L.A. Melton, Appl. Opt. 22, 2224–2226 (1983)
R. Chen, K. Nishida, B. Shi, Fuel 242, 425–437 (2019)
M. Suzuki, K. Nishida, H. Hiroyasu, SAE Technical Papers 930863 (1993)
Y. Zhang, T. Yoshizaki, K. Nishida, Appl. Opt. 39(33), 6221–6229 (2000)
Y. Zhang, S. Li, W. Qi, K. Nishida, Int. J. Engine Res. 18(8), 776–784 (2016)
Y. Zhang, T. Yoshizaki, K. Nishida, T. Toshizaki, SAE Technical Papers 2001-01-1294 (2001)
J.V. Pastor, J.M. García-Oliver, J.J. López, C. Micó, Fuel 179, 258–266 (2016)
Y. Zhang, Dissertation, Hiroshima University (2001)
C.F. Bohren, D.R. Huffman, Absorption and scattering of light by small particles (Wiley, New York, 1983)
D.C. Hammond, Appl. Opt. 20(3), 493–499 (1981)
J. Senda, T. Kanda, M. Kobayashi, H. Fujimoto, SAE Technical Papers 970796 (1997)
L. Zigan, J. Trost, A. Leipertz, Appl. Opti. 55(6), 145 (2016)
I. Düwel, W. Koban, F.P. Zimmermann, T. Dreier, Appl. Phys. B 97(4), 909 (2009)
H. Hiroyasu, M. Arai, M. Tabata, SAE Technical Papers 890464 (1989)
R.C. Reid, J.M. Pravsnitz, T.K. Sherwood, Properties of Gases and Fluids, 3rd edn. (McGraw-Hill Inc, New York, 1985)
L. Zigan, I. Schmitz, A. Flügel, M. Wensing, A. Leipertz, Fuel 90(1), 348–363 (2011)
L.A. Melton, Appl. Spectrosc. 47(6), 782–786 (1993)
Aspen Plus, https://www.aspentech.com/products/engineering/aspen-plus/
Y. Zhang, S. Li, B. Lin, Patent No. ZL201410223630.5 (2016)
S. Li, Y. Zhang, W. Qi, Exp. Therm. Fluid Sci. 98, 472–479 (2018)
F.R. Westlye, K. Penney, A. Ivarsson, L.M. Pickett, J. Manin, S.A. Skeen, Appl. Opt. 56(17), 5028 (2017)
W. Qi, Y. Zhang, Exp. Fluids 59(4), 70 (2018)
M. Davy, P. Williams, D. Han, R. Steeper, Exp. Fluids 35(1), 92–99 (2003)
Acknowledgements
The research was sponsored by National Natural Science Foundation of China (Grant No. 91741130) and Intergovernmental international cooperation in science and technology innovation (Grant No. 2016YFE0127500).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, W., Zhang, Y. Quantitative measurement of binary-component fuel vapor distributions via laser absorption and scattering imaging. Appl. Phys. B 125, 127 (2019). https://doi.org/10.1007/s00340-019-7237-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-019-7237-5