Abstract
The results of a numerical and experimental investigation into the evaporation characteristics of the 3-pentanone–isooctane binary system are presented. The behaviour of the system is shown to be nonideal, exhibiting positive deviations from Raoult's law. Thus, the evaporation characteristics of 3-pentanone–isooctane mixtures are shown to be controlled by the relative mole fractions of the two components contained within the liquid phase. The preferential evaporation of 3-pentanone from mixtures containing low concentrations of 3-pentanone is predicted numerically and is confirmed experimentally. The implications of this result with respect to the use of 3-pentanone as a fluorescence marker for isooctane fuel in engine research are discussed at length. The influence high 3-pentanone concentrations on the atomisation behaviour and the optical depth of 3-pentanone–isooctane mixtures is predicted and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The past decade has seen laser-induced fluorescence (LIF) techniques assume a significant role in combustion and flowfield diagnostics. In the field of internal combustion (IC) engine research, for example, the use of tracer LIF for the determination of in-cylinder vapour-phase fuel distributions prior to ignition is widely viewed as an established experimental technique. Typically, a fluorescent marker compound (seeded within an optically transparent base fuel) is excited in the UV spectral region, and information regarding the local fuel–air equivalence ratio is inferred from the resulting Stokes-shifted fluorescence signal. The success of the technique is, to a large part, dependent on the choice of carrier fuel and seed. Although early literature makes reference to acetaldehyde–isooctane (Arnold et al. 1990), acetone–isooctane (Wolff et al. 1994), and biacetyl–isooctane blends (Baritaud and Heinze 1992), a review of recent work suggests that the 3-pentanone–isooctane binary system has become a de facto standard tracer–fuel combination for spark-ignition (SI) engine research. Qualitative mixture preparation studies in IC engines using 3-pentanone–isooctane fuel blends are reported by, amongst others, Neij et al. (1994), Ossler and Aldén (1997), Richter et al. (1999), Ekenberg and Johansson (2000), Steeper and Stevens (2000), and Gold et al. (2001). Berckmüller et al. (1994), Einecke et al. (2000), and Stojkovic and Sick (2001) claim quantitative measurements of local air–fuel ratio (AFR) determined from 3-pentanone fluorescence intensity. The ratio of Mie scattering intensity to LIF intensity from a 3-pentanone–isooctane fuel mixture has also been used to determine the distribution of Sauter mean diameter (SMD) within an automotive hollow-cone fuel spray (Stojkovic and Sick 2001).
The physical and photophysical properties required of a fluorescent tracer for in-cylinder LIF studies were summarised by Baritaud and Heinze (1992) as follows: the tracer should absorb at an available laser wavelength, show a satisfactory fluorescence quantum yield, be insensitive to the effects of oxygen quenching, demonstrate sufficient Stokes shift, and have similar vaporisation characteristics to the carrier fuel. It is also desirable that the physical properties of the carrier fuel affecting spray and droplet break-up, such as viscosity, density, and surface tension, are not significantly changed by the addition of the fluorescent tracer compound. The photophysical behaviour of 3-pentanone is well documented in the literature (Hansen and Lee 1975; Ghandhi and Felton 1996; Grossmann et al. 1996; Ossler and Aldén 1997), and judged by these criteria alone 3-pentanone would appear to be well suited to use in IC engine research. However, the popular choice of 3-pentanone as a fluorescent marker for isooctane is largely attributable to the widespread, and erroneous, belief that the similarity between the two pure components' boiling points and heats of vaporisation (respectively, 372.4 K and 30.79 kJ/mol for isooctane and 375.0 K and 33.45 kJ/mol for 3-pentanone) are reflected in the evaporation characteristics of the blend. In fact, experimental data exists in the literature to show that the 3-pentanone–isooctane mixture is nonideal (Fuchs et al. 1984), and that the system forms a minimum-boiling azeotrope; that is, the mixture exhibits positive deviations from Raoult's law. With the exception of an earlier study by Le Coz and Baritaud (1996), the significance of such nonideal behaviour in the liquid phase, which is typical of mixtures containing a combination of polar and nonpolar components, has not been investigated in relation to the use of ketone LIF tracers in alkane fuels.
This paper presents the results of a numerical study, performed at University College London (UCL), into the evaporation characteristics of the 3-pentanone–isooctane binary system. The model results are validated against existing literature data and are supported by experimental data from an independent investigation recently completed at Sandia National Laboratories' Combustion Research Facility (see also Han and Steeper 2002a, 2002b). The implications of the presented results with respect to the use of 3-pentanone as a fluorescent marker for isooctane are discussed in detail. It is expected that the findings of this study will be of significant interest to all users of the tracer LIF technique and, in particular, those researchers working in the field of IC engine research.
2 Numerical
2.1 Background
The prediction of fluid-phase equilibrium in multicomponent systems is an established research discipline within the fields of chemical and process engineering. The design of distillation and extraction operations, for example, typically requires quantitative estimates of the partial equilibrium properties of the working fluid mixtures. Although extensive data sets on many different liquid mixtures may be found in the literature, it is clear that the large number of possible mixture compositions and operating conditions preclude the wholly experimental approach to phase-equilibrium data acquisition. Accordingly, vapour–liquid equilibrium (VLE) modelling is an essential (and well-validated) part of chemical process design. Reid et al. (1987) and Sandler (1999) provide excellent introductions to the methods used.
2.2 VLE model
The equilibrium properties of the 3-pentanone–isooctane binary system were calculated according to the gamma–phi method, whereby (assuming an ideal vapour phase) the partial vapour–liquid equilibrium of each component is given by the relationship shown in Eq. (1).
Here, y i is the vapour-phase mole fraction, P is the total pressure of the system, x i is the liquid-phase mole fraction, γ i is the activity coefficient, and P i 0 is the pure component vapour pressure. The relative activity coefficients of the two components, γ 1 and γ 2, were estimated using the Wilson equation, which expresses the activity coefficients in terms of binary mixture parameters.
The binary mixture parameters for the 3-pentanone–isooctane system, Λ 12 and Λ 21, were found from simultaneous solution of Eqs. (3) and (4), where γ i ∞ is the activity coefficient at infinite dilution estimated using the modified separation of cohesive energy density (MOSCED) method of Thomas and Eckert (1984). Note that the value of the polar parameter for 3-pentanone used in the authors' MOSCED calculations differs from that given by Thomas and Eckert. A value of 2.64 (as opposed to Thomas and Eckert's value of 2.77) was found to improve the agreement between the authors' isothermal bubble-point calculations at 333.15 K and the experimental data points of Fuchs et al. (1984) (Fig. 2).
The vapour pressure of each pure component P i 0 for a given temperature T was calculated from the simplified version of the Wagner equation shown in Eq. (5), where A, B, C, and D are the Wagner coefficients of the pure component; T c is the component's critical temperature (K); P c is the component's critical pressure (bar); and θ is given by [1−(T/Tc)].
Under equilibrium conditions, the sum of the vapour phase mole fractions is equal to one. Thus, with P i 0, γ i , and x i known for both 3-pentanone and isooctane the total equilibrium pressure of the system was obtained using Eq. (6)
With the total pressure of the system known, the vapour-phase mole fraction of each component, y i , was calculated using Eq. (1).
2.3 Evaporation characteristics
The VLE model of the 3-pentanone–isooctane system described in Sect. 2.2 was used to investigate the isothermal evaporation characteristics of various liquid 3-pentanone–isooctane blends. The simplified evaporation calculations presented in this work assumed an initial liquid mass corresponding to 1000 mol of liquid 3-pentanone–isooctane mixture at the system temperature. Evaporation was assumed to take place in air, and the total pressure of the vapour phase was assumed to be 100 kPa. Following an initial sensitivity study, performed to confirm the number of numerical steps required to ensure convergence, the calculation was completed in 1000 steps corresponding to the removal of 1 mol of liquid mixture from the system at each stage. The system was assumed to be in equilibrium at all times.
The calculation method for each numerical step was as follows: the partial pressures of 3-pentanone and isooctane at the vapour–liquid interface were calculated by the methods presented in Sect. 2.2. Given the fixed total pressure of the vapour phase, the corresponding partial pressure of air was obtained by subtraction. The molar composition of the vapour phase (composed of air, 3-pentanone, and isooctane) was calculated assuming ideal gas behaviour, and the vapour-phase mole fraction of 3-pentanone and the corresponding mass of the liquid-phase mixture were recorded. Each numerical step was concluded by the recalculation of the liquid-phase mixture composition and mass following the removal of 1 mol of liquid at the predicted equilibrium mole fractions.
2.4 Comparison with experiment
The validity of the evaporation calculations described in Sect. 2.3 was tested against experimental measurements of vapour-phase fluorescence intensity from evaporating 3-pentanone–isooctane blends. Comparison between the numerical results and the experimental measurements (described in Sect. 3 below) was made on the basis of the following assumptions and simplifications. The liquid-phase mixture was assumed to be well mixed, and the effects of vapour-phase mass diffusion were assumed to be negligible. Note that the vapour-phase mass diffusion coefficients of 3-pentanone and isooctane in air are of similar magnitude: 12.4×10−6 m2s−1 and 10.0×10−6 m2s−1, respectively, at a pressure of 100 kPa and a temperature of 273.15 K (Berckmüller 1996). The measured fluorescence intensity at constant temperature and pressure was assumed to be proportional to the vapour-phase mole fraction of 3-pentanone. Thus, the predicted vapour-phase 3-pentanone mole fraction during evaporation (normalised by the initial vapour-phase mole fraction of 3-pentanone) was deemed directly comparable with the normalised fluorescence intensities measured by experiment.
3 Experimental
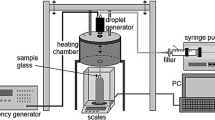
Validation of the VLE model was performed by experimentally measuring the evolving composition of vapour produced by a batch evaporation chamber. The chamber is illustrated in Fig. 1 and is described in more detail elsewhere (Han and Steeper 2002a). Air metered through a sonic orifice at a rate between 0.007 and 0.030 g/s was bubbled through a liquid 3-pentanone–isooctane mixture of known initial composition loaded into the evaporation chamber. Both the air and the liquid were maintained at a controlled temperature using a water-bath heat exchanger. Air saturated with the fuel–tracer mixture emerged as a 4.6-mm-diameter jet from an exit tube that was heated slightly above the chamber temperature to prevent condensation. A 266-nm-wavelength, 0.5-mm-thick sheet from a Nd:YAG laser bisected the jet along its axis, and an 8-bit intensified CCD camera recorded the resulting fluorescence signal from the 3-pentanone vapour.
Multiple LIF images were recorded at regular intervals during the evaporation, while a scale beneath the evaporation chamber monitored the liquid mass remaining. The images were corrected for background noise and for laser energy variation, and an average LIF intensity was extracted from a region corresponding to the potential core of the jet (detailed in Fig. 1). With temperature and pressure held constant during the experiment, the measured LIF intensities were assumed proportional to instantaneous mole fractions of 3-pentanone vapour. The uncertainty in the measurements was dominated by noise introduced by the LIF system. Analysis showed that shot-to-shot variations of laser intensity could not be completely corrected using an energy meter. In addition, the camera intensifier contributed noise to the images. As an estimate of uncertainty, the multiple images recorded at each stage of evaporation showed standard deviations as high as 5% of mean intensity. Uncertainties associated with nonlinearity of the intensifier, incomplete saturation of the air, variation in temperatures, and mass measurement errors were all determined to be negligible in comparison.
4 Results and discussion
The computed isothermal vapour–liquid equilibrium diagram for the 3-pentanone–isooctane system at 333.15 K was compared to the experimental data points of Fuchs et al. (1984) (Fig. 2). The average difference between the measured and calculated bubble-point pressures was found to be less than 1%, with a maximum difference of less than 3%. This good agreement demonstrates the ability of the authors' VLE model to predict activity coefficients for this system over the full range of molar compositions from 100% isooctane to 100% 3-pentanone. As is expected from the literature, the 3-pentanone–isooctane system is shown to be nonideal, with a minimum boiling azeotrope at this temperature at approximately 0.588 mole fraction isooctane and 33.4 kPa.
With respect to the relative compositions of the liquid and vapour phases at this equilibrium condition, Table 1 lists the predicted liquid-phase x 2 and vapour-phase y 2 mole fractions of 3-pentanone for selected initial volume percentages of 3-pentanone in the liquid phase. Note that the initial volume percentages of the liquid phase are calculated at 293.15 K and 101 kPa pressure to reflect the laboratory conditions under which fuel mixtures are typically prepared. It can be seen that the liquid and vapour-phase compositions differ significantly when the 3-pentanone concentration is low. As an example, the difference between the liquid and vapour-phase equilibrium mole fractions of 3-pentanone in a 10%-by-volume blend of 3-pentanone in isooctane (expressed as a percentage of the liquid-phase mole fraction) is close to 47%. This error decreases steadily to zero as the molar concentration of 3-pentanone increases towards the system's azeotropic composition, becoming negative as the azeotropic concentration is exceeded. Accordingly, the vapour-phase composition of the system is only truly analogous to the liquid-phase composition at the azeotrope, i.e. for the precise composition at which the bubble- and dew-point pressures coincide.
The implication of the presented results is that the evaporation characteristics of the 3-pentanone–isooctane system are controlled by the relative proportions of each component within the liquid mixture. Coevaporation of the 3-pentanone tracer and the isooctane carrier fuel only occurs when the mixture composition corresponds to the system's azeotrope. At a constant temperature of 333.15 K, liquid 3-pentanone–isooctane mixtures containing greater than 0.412 mole fraction 3-pentanone (i.e. to the right of the minimum boiling azeotrope shown in Fig. 2) will, as a result of molecular interactions, exhibit the preferential evaporation of isooctane. Similarly, 3-pentanone–isooctane mixtures containing less than 0.412 mole fraction 3-pentanone at this temperature (i.e. to the left of the azeotrope shown in Fig. 2) will exhibit the preferential evaporation of 3-pentanone. In either case, preferential evaporation of one component or other causes the remaining liquid mixture to become increasingly dilute in that component. In the case of 3-pentanone, the difference between the equilibrium liquid and vapour-phase concentrations of the compound increases greatly as its dilution in the liquid-phase mixture increases (Table 1). Accordingly, the evaporation rate of 3-pentanone from a liquid 3-pentanone–isooctane mixture of a given initial composition is nonlinear.
The effect of initial mixture composition on the vapour-phase mole fraction of 3-pentanone leaving an evaporating 3-pentanone–isooctane mixture at 333.15 K is shown in Fig. 3. This figure also shows the results of experimental measurements of vapour-phase fluorescence intensity for selected mixture compositions. In all cases, the agreement between the numerical and experimental results, which are presented in the form of liquid mass fraction remaining against normalised values of 3-pentanone mole fraction and fluorescence intensity, respectively, is seen to be acceptable. The results clearly demonstrate that 3-pentanone is preferentially evaporated from the mixture at 333.15 K when the initial concentration of 3-pentanone is less than the azeotropic composition, which occurs at approximately 32% (by volume) 3-pentanone at this temperature. For these compositions, the normalised values of vapour-phase 3-pentanone mole fraction and fluorescence are shown to decrease as the evaporation process proceeds, thus indicating that the remaining liquid mixture has become increasingly dilute in 3-pentanone.
The results presented in Fig. 3 confirm the predicted influence of initial mixture composition on the evaporation characteristics of the blend. It can be seen that the rate at which 3-pentanone is evaporated from the liquid phase is notably more pronounced for liquid mixtures with a low initial concentration of 3-pentanone than for mixtures with compositions closer to the composition of the azeotrope. Note also the increasing vapour-phase 3-pentanone mole fraction that is seen during the evaporation of a liquid 3-pentanone–isooctane mixture containing an initial 35% (by volume) 3-pentanone. This increasing signal is indicative of the preferential evaporation of isooctane that is expected to occur when the mixture composition is to the right of the minimum-boiling azeotrope shown in Fig. 2.
The effects of temperature on the predicted vapour-phase mole fraction of 3-pentanone leaving an evaporating liquid mixture of initial composition 20% 3-pentanone and 80% isooctane (by volume) are shown in Fig. 4. For this particular mixture, and for the limited temperature range illustrated, the effects are seen to be minor. The increase in normalised 3-pentanone mole fraction was approximately 2% per 50 K, indicated with increasing temperature at 0.3 liquid mass fraction remaining.
The results illustrated in Fig. 4 are explained by the temperature dependence of the 3-pentanone–isooctane system's azeotropic composition (Fig. 5). The composition of the azeotrope is predicted to be a linear function of temperature, moving from 0.367 mole fraction 3-pentanone at 300.15 K and 8.4 kPa to 0.640 mole fraction 3-pentanone at 500.15 K and 165.3 kPa. Hence, as the temperature of the system is increased, the composition of the azeotrope would move away from that of the fixed initial mixture composition illustrated in Fig. 4. As shown by the results presented in Fig. 3, the rate of preferential evaporation that occurs at a given system temperature is controlled by the proximity of the initial mixture composition to the system's azeotrope at that temperature. Thus, the preferential evaporation of 3-pentanone from a binary mixture initially containing 20% 3-pentanone and 80% isooctane (by volume) would be expected to increase with increasing temperature.
5 Implications for IC engine research
With respect to the use of 3-pentanone as a fluorescence marker for isooctane in IC engine research, the tracer LIF technique provides information concerning the distribution of the marker as opposed to the distribution of the carrier fuel. For the distribution of the tracer to be truly representative of the carrier fuel distribution, it is necessary for the tracer–fuel blend to behave as if it were a single-component fuel. Coevaporation of the two components under realistic engine conditions is clearly of utmost importance. Law (1982) suggested two regimes by which a multicomponent droplet might vaporise. During rapid vaporisation, the rate of surface regression is one or two orders of magnitude greater than the rate of liquid-phase mass diffusion. Thus, the core composition of the droplet is essentially frozen and all of the components within the droplet gasify at similar rates. This is known as 'diffusion-limited' vaporisation. On the other hand, during slow vaporisation and/or in the presence of internal circulation, the rate of liquid-phase mass transport to the droplet surface becomes significant. In this case, fractional distillation occurs and the effects of nonideal mixture behaviour are important.
At first glance, it is tempting to dismiss the fractional distillation of multicomponent fuels as a low-temperature effect whose potential impact on IC engine research would be restricted to fuel spray experiments performed under ambient laboratory conditions. However, a substantial body of literature exists to suggest that fuel evaporation in both port-fuel-injected (PFI) and direct-injected (DI) SI engines occurs primarily in the fractional distillation regime (Skippon and Norton 1998; Krämer et al. 1998; Davy et al. 2000; Tong et al. 2001). On reflection, this is to be expected. In PFI engines, a typical fuel spray may be in the region of 200–300 μm SMD and the majority of the injected fuel is, by design, evaporated from liquid fuel films deposited on the back of the intake valve and the intake manifold walls. Wall film evaporation is a comparatively slow process and the effects of nonideal fuel–tracer behaviour are therefore significant. In the case of the DI gasoline engine, the injected fuel sprays are considerably finer than their PFI cousins, often having a SMD of 25 μm or less. Although the reduced droplet sizes within the spray enhance the evaporation process, individual droplets typically possess high relative velocity with respect to the surrounding charge. Under these circumstances, liquid-phase mass transport to the droplet surface is enhanced and the effects of fractional distillation and nonideal fuel–tracer behaviour cannot be discounted. Moreover, not all of the injected fuel is evaporated in droplet form. The wetting of in-cylinder surfaces by both intentional and unintentional fuel spray impaction is an established feature of practical DI gasoline engine operation. Evaporation of the resulting in-cylinder fuel films is expected to be in the fractional distillation regime. Finally in the extreme case of flash boiling DI gasoline sprays such as those reported by Davy et al. (2000), it should be noted that not all of the spray is vaporised in the immediate vicinity of the injector (Zuo et al. 2002). In fact, flashing DI gasoline sprays typically form dense clouds of finely atomised liquid fuel that evaporate relatively slowly because of the effects of local charge cooling and adverse concentration gradients (Davy and Williams 1999). Accordingly, the effects of fractional distillation and nonideal fuel–tracer behaviour must be considered nonnegligible for these sprays.
The results shown in Figs. 2 and 3 of this work indicate that coevaporation of 3-pentanone and isooctane can be achieved at 333.15 K by the use of an azeotropic mixture corresponding to a composition of 0.412 mole fraction 3-pentanone (equivalent to 32% 3-pentanone by volume, mixed at 293.15 K and 101 kPa pressure). However, the use of such high concentrations of 3-pentanone in tracer LIF experiments leads to problems with laser attenuation. Applying Beer's law and using absorption coefficients for 3-pentanone derived from Hansen and Lee (1975), it is possible to derive transmission curves for a range of 3-pentanone–isooctane fuel compositions as they are isentropically compressed, as in an engine (Fig. 6). The results indicate that laser transmission though air–fuel mixtures containing high number densities of 3-pentanone would be markedly reduced. This potential problem of optical depth is exacerbated by the results shown in Fig. 5. The increased concentrations of 3-pentanone that are necessary in the initial liquid tracer–fuel blend to make coevaporation possible at higher operating temperatures also serve to increase laser attenuation within the medium. Although computational methods exist to correct for attenuation effects, see Hertz and Aldén (1987) and Sick and Stojkovic (2001) for examples, the dynamic range and signal-to-noise ratio (SNR) of heavily attenuated regions within fluorescence images is often poor.
The issues of laser attenuation and correction aside, the physical properties of tracer–fuel mixtures composed of high concentrations of 3-pentanone in isooctane must be considered with respect to fuel spray and droplet behaviour. The liquid densities of pure isooctane and a 3-pentanone–isooctane mixture containing 0.364 mole fraction 3-pentanone (corresponding to the system azeotrope at 298.15 K) were estimated at 298.15 K and 101 kPa using the Hankinson–Brobst–Thompson (HBT) techniques detailed by Reid et al. (1987). The liquid viscosity of the mixture at the same temperature and pressure was estimated using a modified Grunberg and Nissan correlation (Isdale et al. 1985). The surface tension of the mixture was estimated using the Macleod–Sugden correlation with the parachors for isooctane and 3-pentanone regressed from the experimental data of Jasper (1972). Values of viscosity and surface tension for pure isooctane at 298.15 K were taken from the literature. The results of this exercise (Table 2) show the 3-pentanone–isooctane mixture to have a significantly lower viscosity than pure isooctane at the same temperature and pressure. A 19% decrease is predicted. At the same time, an 11% increase in surface tension with respect to the surface tension of pure isooctane is estimated, and the mixture is predicted to have a 5% greater density than the pure isooctane fuel.
The effects of liquid fuel properties on the performance of pintle-type port fuel injectors were examined in some detail by Williams and Beckwith (1994). A 20% reduction in viscosity was noted to produce a 10% reduction in mean droplet diameters for the fuel injector examined. In agreement with Lefebvre (1989), the effects of liquid density and surface tension on atomisation and break-up were found to be of lesser importance. With respect to the 3-pentanone–isooctane mixture examined in this present exercise (0.364 mole fraction 3-pentanone), the predicted increase in viscosity is large enough to imply a significant effect on atomisation behaviour.
6 Conclusions
Liquid mixtures of 3-pentanone and isooctane are shown to behave in a nonideal manner; thus, the evaporation characteristics of the system are controlled by the relative quantities of each component within the blend and not, as is commonly believed, by the relative volatilities of the two pure components. Accordingly, the molar composition of the vapour phase is only analogous to the composition of the liquid phase at the system's azeotrope, and only for this precise liquid composition will coevaporation of the two components occur. The preferential evaporation of 3-pentanone that occurs when the composition of the mixture contains less than the correct azeotropic mole fraction of 3-pentanone is clearly demonstrated (experimentally and numerically) in this study. This result highlights an often-neglected source of uncertainty in experimental studies where the coevaporation of 3-pentanone and isooctane is assumed on the basis of similar boiling points.
Although the coevaporation of 3-pentanone and isooctane can be arranged by operating at the system's azeotrope, the azeotropic mixture composition is shown (between the limits of 300 and 500 K) to be a linear function of temperature. At 300 K, the azeotrope is predicted to be at 0.367 mole fraction 3-pentanone, and at 500 K the azeotrope is predicted at 0.640 mole fraction 3-pentanone. Calculations indicate that the use of such high concentrations of 3-pentanone would cause significant attenuation of the laser energy over a pathlength equivalent to the diameter of a typical IC engine cylinder. Furthermore, the physical properties of the 3-pentanone–isooctane mixtures containing high concentrations of 3-pentanone are predicted to differ sufficiently from those of pure isooctane so as to affect atomisation performance.
The results presented in this study demonstrate the ability of VLE modelling techniques to predict the vapour-phase composition originating from nonideal liquid mixtures during evaporation. Although the present study has focussed on the 3-pentanone–isooctane binary system, VLE modelling techniques are readily extendible to multicomponent mixtures (ideal and nonideal), and to mixtures containing compounds drawn from other chemical groups. They may also be extended to include the effects of oxygen dissolved in the liquid phase and hence the effects of pressure on fuel film fluorescence (for example, Lin and Sick 2002). From this, the authors conclude that VLE modelling techniques may be usefully applied to the development of improved LIF tracers for use in evaporating systems. The authors' investigations into new or improved fluorescence tracers for use in IC engine research are expected to form the basis of a future publication.
Abbreviations
- A :
-
empirical constant
- B :
-
empirical constant
- C :
-
empirical constant
- D :
-
empirical constant
- P :
-
total pressure
- P c :
-
critical pressure
- P i 0 :
-
vapour pressure of component i
- T :
-
absolute temperature
- T c :
-
critical temperature
- x i :
-
liquid-phase mole fraction of component i
- y i :
-
vapour-phase mole fraction of component i
- γ i :
-
activity coefficient of component i
- γ i ∞ :
-
activity coefficient of component i at infinite dilution
- η :
-
liquid viscosity
- θ :
-
1−(T/T c), see Eq. (5)
- ρ :
-
liquid density
- σ :
-
surface tension
- Λ ij :
-
empirical constant
- 1:
-
isooctane
- 2:
-
3-pentanone
References
Arnold A, Becker H, Suntz R, Monkhouse P, Wolfrum J, Maly R, Pfister W (1990) Flame-front imaging in an internal-combustion engine simulator by laser-induced fluorescence of acetaldehyde. Opt Lett 15:831–833
Baritaud T, Heinze T (1992) Gasoline distribution measurements with PLIF in a SI engine. SAE technical paper 922355
Berckmüller M (1996) A study of mixture formation in a lean burn research engine using laser fluorescence imaging. Dissertation, School of Mechanical Engineering, Cranfield University, UK
Berckmüller M, Tait N, Locket R, Greenhalgh D, Ishii K, Urata Y, Umiyama H, Yoshida K (1994) In-cylinder crank-angle-resolved imaging of fuel concentration in a firing spark-ignition engine using planar laser-induced fluorescence. In: Twenty-fifth symposium (international) on combustion. The Combustion Institute, Pittsburgh, pp 151–156
Davy M, Williams P (1999) The effects of flash boiling on mixture formation in a firing direct-injection spark-ignition (DISI) engine. In: Spicher U (ed) Proc of international congress on Direkteinspritzung im Ottomotor II (gasoline direct injection engines). Expert, Renningen-Malmsheim, ISBN 3–8169-1822-0, pp 154–170
Davy M, Williams P, Anderson R (2000) Effects of fuel composition in a firing direct-injection spark-ignition (DISI) engine: an experimental study using Mie scattering and planar laser-induced fluorescence (PLIF) techniques. In: SAE special publication SP-1547. Society of Automotive Engineers, Warrendale, pp 67–82 (also published as SAE technical paper 2000-01-1904)
Einecke S, Schulz C, Sick V (2000) Measurement of temperature, fuel concentration and equivalence ratio fields using tracer LIF in IC engine combustion. Appl Phys B 71:717–723
Ekenberg M, Johansson B (2000) Fuel distribution in an air assist direct injected spark ignition engine with central injection and spark plug measured with laser induced fluorescence. In: SAE special publication SP-1547. Society of Automotive Engineers, Warrendale, pp 1–8 (also published as SAE technical paper 2000-01-1898)
Fuchs R, Krenzer L, Gaube J (1984) Excess properties of binary mixtures composed of a polar component and an alkane. Ber Bunsenges Phys Chem 88:642–649
Ghandhi J, Felton P (1996) On the fluorescent behaviour of ketones at high temperatures. Exp Fluids 21:143–144
Gold M, Stokes J, Morgan R, Heikal M, Begg S, De Sercey G (2001) Air–fuel mixing in a homogeneous charge DI gasoline engine. In: SAE special publication SP-1584. Society of Automotive Engineers, Warrendale, pp 123–131 (also published as SAE technical paper 2001-01-0968)
Grossmann F, Monkhouse P, Ridder M, Sick V, Wolfrum J (1996) Temperature and pressure dependencies of the laser-induced fluorescence of gas-phase acetone and 3-pentanone. Appl Phys B 62:249–253
Han D, Steeper R (2002a) Examination of iso-octane/ketone mixtures for quantitative lif measurements in a DISI engine. In: SAE special publication SP-1693. Society of Automotive Engineers, Warrendale, pp 173–184 (also published as SAE technical paper 2002–01–0837)
Han D, Steeper R (2002b) An LIF equivalence ratio imaging technique for multicomponent fuels in an IC engine. In: Twenty-ninth symposium (international) on combustion. The Combustion Institute, Pittsburgh
Hansen D, Lee E (1975) Radiative and nonradiative transitions in the first excited singlet state of symmetrical methyl-substituted acetones. J Chem Phys 62:183–189
Hertz H, Aldén M (1987) Calibration of imaging laser-induced fluorescence measurements in highly absorbing flames. Appl Phys B 42:97–102
Isdale J, MacGillivray J, Cartwright G (1985) Prediction of viscosity of organic liquid mixtures by a group contribution method. Nat Eng Lab Rept, Glasgow, Scotland
Jasper J (1972) Surface tension of pure liquid compounds. J Phys Chem Ref Data 1:841
Krämer H, Einecke S, Schultz C, Sick V, Nattrass S, Kitching J (1998) Simultaneous mapping of the distribution of different fuel volatility classes using tracer-LIF and NIR-tomography in an IC engine. SAE technical paper 982467
Law C (1982) Recent advances in droplet vaporisation and combustion. Prog Energy Combust Sci 8:171–201
Le Coz J, Baritaud T (1996) Application of laser induced fluorescence for measuring the thickness of evaporating gasoline liquid films. In: Adrian R et al. (eds) Developments in laser techniques and applications to fluid mechanics, proceedings of the seventh international symposium, Lisbon, Portugal, 11–14 July 1994. Springer, Berlin Heidelberg New York, ISBN 3-540-60236-4, pp 115–131
Lefebvre A (1989) Atomization and sprays. Taylor & Francis, Levittown, PA, pp 11–14
Lin MT, Sick V (2002) Mixture evaporative characteristics predictions for LIF measurements using PSRK (predictive Soave–Redlich–Kwong) equation of state. SAE technical paper 2002-01-2750
Neij H, Johansson B, Aldén M (1994) Development and demonstration of 2D-LIF for studies of mixture preparation in SI engines. Combust Flame 99:449–457
Ossler F, Aldén M (1997) Measurements of picosecond laser induced fluorescence from gas-phase 3-pentanone and acetone: Implications to combustion diagnostics. Appl Phys B 64:493–502
Pádua A, Farleira J, Calado J, Wakeham W (1996) Density and viscosity measurements of 2,2,4-trimethylpentane (isooctane) from 198 K to 348 K and up to 100 Mpa. J Chem Eng Data 41:1488–1494
Reid R, Prausnitz J, Poling B (1987) Fluid phase equilibria in multicomponent systems. In: The properties of gases & liquids, 4th edn. McGraw-Hill, New York, pp 241–387
Richter M, Axelsson B, Aldén M, Carlsson LO, Dahlberg M, Nisbet J, Simonsen H (1999) Investigation of the fuel distribution and in-cylinder flow field in a stratified charge engine using laser techniques and comparison with CFD-modelling. SAE technical paper 1999-01-3540
Sandler S (1999) Chemical and engineering thermodynamics, 3rd edn. Wiley, New York
Sick V, Stojkovic B (2001) Attenuation effects on imaging diagnostics of hollow-cone sprays. Appl Opt 40:2435–2442
Skippon S, Norton D (1998) The effects of gasoline volatility on mass and composition of the inlet port wall film in port injected SI engines. SAE technical paper 982517
Steeper R, Stevens E (2000) Characterization of combustion, piston temperature, fuel sprays, and fuel–air mixing in a DISI optical engine. In: SAE special publication SP-1562. Society of Automotive Engineers, Warrendale, pp 235–244 (also published as SAE technical paper 2000-01-2900)
Stojkovic S, Sick V (2001) Evolution and impingement of an automotive fuel spray investigated with simultaneous Mie/LIF techniques. Appl Phys B 73:75–83
Thomas E, Eckert C (1984) Prediction of limiting activity coefficients by a modified separation of cohesive energy density model and UNIFAC. Ind Eng Chem Process Des Dev 23:194–209
Tong K, Quay B, Zello J, Santavicca D (2001) Fuel volatility effects on mixture preparation and performance in a GDI engine during cold start. SAE technical paper 2001-01-3650
Williams P, Beckwith P (1994) Correlation between the liquid fuel properties density, viscosity and surface tension and the drop sizes produced by an SI engine pintle-type port fuel injector. In: SAE special publication SP-1054. Society of Automotive Engineers, Warrendale, pp 41–55 (also published as SAE technical paper 941864)
Wolff D, Beushausen V, Schlüter H, Andresen P, Hentschel W, Manz P, Arndt S (1994) Quantitative 2D-mixture fraction imaging inside an internal combustion engine using acetone-fluorescence. In: Proc international symposium COMODIA 94, Yokohama, pp 445–451
Zuo B, Gomes A, Rutland C (2002) Modeling superheated fuel sprays and vaporization. Int J Eng Res 1:321–336
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davy, M., Williams, P., Han, D. et al. Evaporation characteristics of the 3-pentanone–isooctane binary system. Exp Fluids 35, 92–99 (2003). https://doi.org/10.1007/s00348-003-0639-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00348-003-0639-4