Abstract
A novel Lagrangian interpolation-based direct laser absorption spectroscopy (LI-DLAS) technique was presented to suppress noise in infrared gas detection by incorporating Lagrangian interpolation and nonlinear least-square fitting (NLLSF). An LI-DLAS analyzer was reported for methane (CH4) detection using a 1654 nm distributed feedback (DFB) laser, a compact digital signal processor (DSP), and a multi-pass gas cell (MPGC) with a 16 m optical path length. The performance of the developed LI-DLAS CH4 analyzer was evaluated by means of laboratory experiments. Compared with the traditional DLAS-based sensor without Lagrangian interpolation, the detection sensitivity was improved from 6 ppmv to 2 ppmv, and the detection stability was enhanced as the Allan–Werle deviation was dropped from 1.514 to 0.531 ppmv for a 1 s averaging time. Compared with a DLAS analyzer based on LabVIEW platform, the DSP-based CH4 analyzer shows the merits of compact size and low cost with potential filed-deployable applications in industrial monitoring and control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Methane (CH4), the second worldwide greenhouse gas after carbon dioxide (CO2), has a 25 times enhancement compared to the global warming potential of CO2 [1]. Hence, atmospheric CH4 monitoring is significant for the observation and analysis of the climate trends [2,3,4]. Besides, as an inflammable gas, CH4 leakage during the exploration and transportation of natural gas is a vital safety hazard [5, 6]. Therefore, CH4 detection has received considerable attention in industrial process control [7,8,9]. Among the existing CH4 detection techniques, infrared laser absorption spectroscopy has wide industrial and academic applications owing to their high-precision sensing capability and fast response [10, 11].
Tunable diode laser absorption spectroscopy (TDLAS) is a mature technique extensively developed in CH4 detection for high sensitivity, long-term stability, and fast response [12]. There are two well-established methods derived from TDLAS: wavelength modulation spectroscopy (WMS) [13] and direct laser absorption spectroscopy (DLAS) [14]. WMS requires frequent sensor calibration, which makes the sensor structure and signal processing procedure complicated. On the contrary, DLAS has the ability to offer quantitative concentration directly from the relative change in light intensity according to Beer–Lambert law, resulting in a much simplified system structure [15]. To avoid interference from other absorption lines and to improve detection accuracy, DLAS requires a tunable laser source with single-frequency emission and a narrow line width at the target absorption line of a gas molecule. Considering that the fabrication technology of a near-infrared (NIR) semiconductor laser is mature and an NIR laser source can be easily fiber-coupled, a continuous-wave (CW) NIR-distributed feedback (DFB) laser is an attractive choice for the development of DLAS-based gas sensors [16, 17].
However, DLAS possesses the inherent drawbacks of being highly susceptible to the noise across a large bandwidth, especially the low-frequency noise due to its simple data processing procedure [18]. To improve the signal-to-noise ratio (SNR) and detection accuracy, apart from reducing the interference from optical path, some effective data processing algorithms can be adopted [19, 20]. In our previously reported traditional DLAS systems, besides the nonlinear absorption line fitting, a sliding average filtering was performed to suppress the electrical-domain background noise and interference [2, 8]. However, this data processing method averages the measured data and, thus, probably changes the signal’s statistical characteristics.
For noise suppression, we developed an alternative technique by introducing Lagrangian interpolation (LI) [21] into DLAS technique. Considering the fact that the noise point exhibits an even or odd symmetry, a filtering range was set by means of the mathematical model of Beer–Lambert law, and then, the filtered data were recovered by LI. After that, the gas concentration was retrieved from the processed data by the real-time nonlinear least-square fitting of the interpolated signal to an absorption line profile, which were widely used in baseline fitting and absorption line fitting [2, 14]. To meet the needs of mobile and field-deployable gas measurements, a compact gas sensor system should be available. In this work, an LI-DLAS analyzer for CH4 detection was realized based on a compact digital signal processor (DSP) board with low-power consumption. Compared to our reported LabVIEW-based system for DLAS CH4 detection [3, 22], the DSP-based CH4 analyzer has a lower power-budget and realizes miniaturization in electrical system. The LI-DLAS CH4 sensing performance was evaluated by laboratory measurements, which was enhanced in terms of sensitivity and stability compared with a traditional DLAS-based CH4 analyzer without such data processing.
2 Structure and design of the LI-DLAS CH4 analyzer
2.1 Selection of CH4 absorption line and optical source
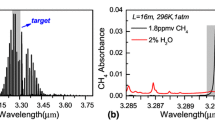
According to the high-resolution transmission (HITRAN) molecular absorption database [23], the CH4 absorption line located at 6046.965 cm−1 is the strongest absorption line in the near-infrared 2ν3 absorption band, as shown in Fig. 1a. Simultaneously, the absorbance of 2% H2O and 100 ppmv CH4 in Fig. 1b indicates that absorption strength of H2O is much less than CH4’s, and could be omitted [24]. Hence, the NIR absorption line at 6046.96 cm−1, whose detailed information is listed in Table 1, was selected as the target line. The DFB laser emitting at a center wavenumber of 6046.95 cm−1 with an output power of 5.5 mW requires an operation temperature of 17 °C and a driving current of 70.0 mA. According to the linear relation between the DFB laser emitting wavenumber and driving current shown in Fig. 1b, the current range was decided to be 55.5–83.0 mA at an operation temperature of 17 °C to scan the CH4 line.
HITRAN-based absorption spectra of a CH4 from 5900 to 6200 cm−1, and b 100 ppmv CH4 and 2% H2O at a pressure of 1 atm, an effective optical path length of 1600 cm and a temperature of 297 K. b DFB laser emission wavenumber as a function of the driver current at a laser operation temperature of 17 °C
2.2 LI-DLAS analyzer structure
The structure of the DSP-based LI-DLAS analyzer for CH4 detection is depicted in Fig. 2, consisting of an optical and an electrical part. In the optical part (Fig. 2a), a CW NIR butterfly packaged DFB laser (Sichuan Bolian Photonics Technology, China) was used to target the CH4 absorption line at 1.654 μm. The laser beam was collimated and coupled to a multi-pass gas cell with an effective optical path length of 16 m (physical size: 290 × 90 × 80 mm3, Liujiu Sensing Technology, Wuhan, China). After passing through the cell, the output laser beam was focused onto an InGaAs photodetector which generated an electrical signal.
As shown in Fig. 2b, the electrical part includes a temperature controller (TED200C, Thorlabs, USA), an integrated laser current driver (LDC202C, Thorlabs, USA), and a DSP-based direct laser absorption signal processor with a dimension size of 10.0 × 6.1 cm2. The used DLAS technique for CH4 detection only requires a saw tooth scan signal. This scan signal was generated by a direct digital synthesizer (DDS) module (model #AD9834). The detector signal output from the InGaAs photodetector was sent to the DSP for data processing. The processed absorption signal was recorded and displayed on a liquid crystal display (LCD) screen and also could be delivered to a laptop via an UART port for post-data analysis. The supply voltage and power consumption of the compact DSP board are ± 12 V and ~ 3.612 W, respectively. In addition, a pressure controller (model 649, MKS Instruments, USA) and a vacuum pump (N838.3 KN.18, KNF, Germany) were used to control and maintain the pressure inside the MPGC.
2.3 Absorption fundamental
The absorption of a target gas specie can be determined by measuring the attenuation of light intensity when light transmits through the gas, as defined by the Beer–Lambert law [25]:
where ν (cm−1) is the laser frequency, \( I\left( v \right) \) and \( I_{ 0} \left( v \right) \) are the transmitted laser light intensity with and without the presence of the target absorbers, respectively, \( P \)(atm) is the gas pressure, c is the mole fraction of the gas, \( S(T) \) (cm−2 atm−1) is the absorption line intensity at temperature T, \( \phi (v) \) (cm) is the line-shape function, and L (cm) is the absorption path length.
A polynomial was used to fit the transmitted laser light intensity I (ν) to generate a baseline Ib (ν) (the detailed fitting method can be seen in Sect. 3.1), which is considered as the background light intensities I0 (ν). Then, the absorbance α (ν) can be expressed by the following equation:
The linear function ϕ (ν) is related to pressure, temperature, composition, and diagnostic wavelength. The line type function ϕ (ν) can take three different form of function: Gaussian, Lorentzian, and Voigt [26, 27].
2.4 Line-shape function selection
The DLAS analyzer was operated at an atmospheric pressure (1 atm) and room temperature (296 K). The spectroscopic models with an NIR absorption line at 1.654 μm under this operation condition are shown in Fig. 3 [24]. Since the selected model of the absorption line has a direct effect on filtering parameters, the quantitative measurement of CH4 concentration requires a more refined spectral model. As shown in Fig. 3, a validation of HITRAN 2012 yielded a generally good agreement with the Lorentzian profile fitting in the [6046.0 cm−1, 6048.0 cm−1] range at 296 K and 1 atm. ϕ (ν) can be expressed as a Lorentzian line-shape function, written as follows:
Here, ΔvL is the half width at the half maximum (HWHM), and v0 is the central wavenumber of the absorption line [28].
3 LI-DLAS technique
The traditional DLAS technique used in CH4 detection is shown in Fig. 4a, which requires a baseline fitting (BF) algorithm to get the background signal without a reference channel and a nonlinear least-square fitting (NLLSF) to obtain the CH4 absorbance for concentration determination. The sensitivity of traditional DLAS is limited by low-frequency noise, and therefore, an improved LI-DLAS technique is proposed to improve the measurement precision. As shown in Fig. 4b, the LI-DLAS technique includes three steps: BF based on least-square fitting (LSF), data processing algorithm based on LI, and NLLSF of absorption line profile.
3.1 Background fitting
A periodic triangular signal generated by the DDS module is used to adjust the output wavelength of the DFB laser, which can be expressed as follows:
where Atri and Ttri are the amplitude and the period of the scan signal, respectively. Without gas absorption, the laser optical intensity Ib (t) received by the InGaAs detector is as follows:
where I0 is the laser output optical intensity and m is the light intensity modulation coefficient. According to Eqs. (1) and (5), the electrical signal ur generated and amplified by the photoelectric detector can be expressed as follows:
where K is the amplifying factor and Doe is the photoelectric conversion parameters.
The transmitted laser light intensities in the [6046.0, 6046.5] cm−1 and the [6047.5, 6048] cm−1 region were extracted and fitted by applying a polynomial to generate a spectral baseline, which is considered as the background light intensity. Divide the CH4 absorption band [6046 cm−1, 6048 cm−1] into N subbands with a length of \( h = 1/N \) cm−1 each, and define \( x_{i} = 6046 + ih \)(i = 1,2,…,N). Then, the polynomial expression \( u_{\text{b}} = k_{1} x + k_{2} \) is considered as the mathematical expression of background signal, where k1 and k2 can be calculated according to the following equation:
3.2 Data processing algorithm based on Lagrangian interpolation
Given the systematic and random noise of the DLAS, it is necessary to use a simple and fast algorithm for data processing. Let \( y(x) = 1/\ln (u_{\text{b}} (x)/u(x)) = 1/\alpha (x) \), and the second-order differential equation of \( y^{\prime\prime}(x_{i} ) \) will be the following:
where 2π/(PcLS(T)ΔvL) is a definite value. Then, we set a constant as a threshold for filtering. Replace the derivatives by the second-order finite-difference Si [29]:
Then, Eq. (8) can be re-written as follows:
Define \( \bar{S} \) as the average value of the array {Si}, and Eq. (11) can be obtained as the following form:
Note that \( \bar{S} \) only relates to the edge data. From a long-term monitoring of \( \left| {S_{i} - \bar{S}} \right|_{\text{normal}} \) for the normal data points and \( \left| {S_{i} - \bar{S}} \right|_{\text{abnormal}} \) for the abnormal data points, we can derive a statistical threshold value \( W_{1} \) to distinguish the normal and abnormal data points. That is to say, when a data point satisfies \( \left| {S_{i} - \bar{S}} \right| > W_{1} \), it is abnormal; otherwise, it is normal.
The selected data at the ith deposition should be processed to minimize the distortion of the recovered data. An LI function was used to obtain the predictive values. The interpolation data \( L_{2} (x_{i} ) \) of the three data points near \( \left( {x_{i,} y_{i} } \right) \) can be regarded as the ith predictive value, which is written as follows:
Besides, the data estimated by the above method may be far from the true data due to spikes and interference of abrupt noise. Therefore, the interpolation point is modified by adding another judging criteria W2 for preserving more information from the measured data. Then, we have the following:
The corresponding limit can be evaluated as \( W_{2} = {{2\left( {y_{{\text{max}} } - y_{{\text{min}} } } \right)} \mathord{\left/ {\vphantom {{2\left( {y_{{max}}} - y_{{min}} \right)} N}} \right. \kern-0pt} N}. \)
3.3 Nonlinear least-square fitting of absorption line profile
The line-shape function \( \phi (v) \) is determined by the line profile. The data perturbed by random noise can be fixed by means of an NLLSF. A regression function \( f(x) \) represents the fitting formula \( y(x) \) as follows:
where pk is the fitting parameters, and \( \varphi_{k} (x) = x^{k} \). The key issue is to build the profile fit with correct parameters pk. Let P = [p0, p1, p2], and the n-dimensional nonzero vectors \( \varphi_{k} (x_{s} ) = x_{s}^{k} \). Define a matrix A and vectors b, which satisfies the following:
where the elements aij in matrix A becomes \( a_{ij} = {\mathbf{\varphi }}_{i} \, \cdot \,{\mathbf{\varphi }}_{j}^{\text{T}} \) and the elements bj in vectors b is \( b_{i} = {\mathbf{\varphi }}_{i} \, \cdot \,{\mathbf{y}} \). A is a positive definite matrix because aij is equal to aji. For reducing the calculation complexity, A can be decomposed into a lower triangular matrix L and a transpose of L:
The element lij of matrix L becomes the following:
Supposing that \( {\mathbf{LT}} = {\mathbf{b}} \) and \( {\mathbf{L}}^{\text{T}} {\mathbf{P}} = {\mathbf{T}} \), the elements of T can be described as \( t_{k} = \frac{{b_{k} - \sum\limits_{j = 1}^{k - 1} {l_{jk} t_{j} } }}{{l_{kk} }} \). The optimal coefficient is determined as \( p_{k} = \frac{{t_{k} - \sum\limits_{j = 1}^{n} {l_{jk} x_{j} } }}{{l_{kk} }} \). Then, the regression function f(x) can be achieved for the inversion of CH4 concentration.
3.4 LI-DLAS technique evaluation
As shown in Fig. 5, the developed analyzer was used to detect CH4 (1 atm, 297 K, 16 m, 10 ppmv) for evaluating the data processing performance of the LI-DLAS algorithm. The scan signal was a triangular signal with a variation range from ~ 1.2 V to ~ 2.8 V and a frequency of 1 Hz. The first half-period (rising part) of the detector signal was sampled and was processed at the second half-period (downing part). The two wings of the sample signal were extracted and the BF algorithm was used to get the baseline, which is shown in Fig. 5b. Subsequently, the background signal was removed from the absorption signal, resulting in an absorbance signal, as shown in Fig. 5c. In the traditional DLAS sensor, the absorbance curve is obtained directly using NLLSF as depicted in Fig. 5d. The subgraph of Fig. 5d shows that there is a big difference between the fitted and theoretical absorbance curve, which affects the concentration retrieval. The processed data obtained using the LI algorithm to effectively decrease the noise is shown in Fig. 5e. It can be seen from the subgraph in Fig. 5f that the fitted absorption curve obtained almost coincides with the theoretical curve, which confirms the denoising operation of the LI-DLAS algorithm.
Data processing based on the LI-DLAS analyzer for the detection of the CH4 sample with a concentration level of 10 ppmv. a The output signal from the detector with no absorption. b The output signal from the detector and its baseline fitting for a 10 ppmv CH4 sample. c The measured absorbance curve and d the fitting curve of the measured signal using the traditional DLAS, where the subgraph shows the fitted absorbance curve and the theoretical curve. e The LI-processed absorbance signal and f the fitting curve of LI-processed signal, where the subgraph shows the fitted absorbance curve and the theoretical curve
3.5 Detection procedure and software
We compiled a C program for the DSP processor (TMS320F28335) using CCS 6.0 platform. The entire program can be overviewed by a flowchart in Fig. 6. The flowchart illustrates the logic of the developed LI-DLAS algorithm, in comparison with a traditional DLAS method (i.e., the flowchart without the program contained in dashed box).
4 Application of the LI-DLAS analyzer for CH4 sensing
4.1 Absorbance measurements
A multi-component gas mixing system (Environics 4000, Environics, USA) was used to prepare CH4 samples with eight concentration levels of 26, 27, 28, 30, 32, 38, 52, and 74 ppmv by mixing a standard 100 ppmv CH4 sample and pure nitrogen (N2). Each sample was tested for ~ 10 min. The amplitude of the normalized absorbance signal (\( u_{{\text{absorbance}}} (t) = - \ln \left( {u(t)/u_{\text{b}} (t)} \right) \)) for each concentration is plotted in Fig. 7a. The relationship between the measured concentration and the theoretical CH4 concentration is shown in Fig. 7b. The fitting curve indicates that the measured concentration is basically equal to the theoretical concentration.
4.2 Detection sensitivity
To show the superiority of the algorithm proposed in Sect. 3, we carried out an experiment with and without the algorithm. The minimum concentration change that can be distinguished by the analyzer is defined as detection sensitivity. The gas cell was first filled with pure N2, and then, a standard 100 ppmv CH4 sample was injected. To observe the detection performance, we obtained 60 absorbance peak readings for each gas sample and the detected amplitudes are shown in Fig. 8. The measurement results without data processing algorithm are shown in Fig. 8a. The variation ranges of the detected amplitude under 27.5, 27, 28, 30, and 32 ppmv are overlapped. In addition, there are some abnormal concentration data dots during measurement. This can be attributed to the absorption line fitting error because of the abnormal data point in the absorption signal. After removing these abnormal data points using LI, Fig. 8b shows reduced overlap in the detected amplitude and no abnormal concentration data dots. The minimum detected amplitude at 28 ppmv is larger than the maximum detected amplitude under 26.5 ppmv. It means that, under certain concentration, the detection sensitivity using a traditional method was about 6 ppmv (Fig. 8a), while the detection sensitivity is decided to be about 2 ppmv using the developed algorithm (Fig. 8b).
4.3 Allan variance and detection stability
To examine the long-term stability of the developed LI-DLAS analyzer, long-term measurement of a 4 ppmv CH4 sample was carried out for ~ 2 h. The measured concentration results without using the algorithm and using the algorithm were recorded and are exhibited in Figs. 9a and 10a, respectively. A histogram plot was used to observe the distribution of the measured concentration, as shown in the insets of Figs. 9b and 10b, respectively. The Allan–Werle deviation plots obtained from the time-series measurement result in Figs. 9a and 10a are depicted in Figs. 9b and 10b, respectively, under the two cases. As shown in Fig. 9b, in the traditional DLAS, the distribution of the measured CH4 concentration fits to a normal distribution. When the averaging time is < 109 s, the Allan deviation is proportional to 1/sqrt(τ), implying that the sensor was mainly dominated by White-Gaussian noise. However, the Allan deviation derived using the algorithm does not follow a 1/sqrt(τ) dependence, which indicates that the Gaussian white noise was suppressed and the LI-DLAS gas analyzer was mainly dominated by sensor drift. The Allan deviation of the traditional DLAS analyzer is ~ 1.514 ppmv for a 1 s averaging time. While at the same averaging time, the Allan deviation of the proposed LI-DLAS system is ~ 0.531 ppmv. Therefore, the detection stability was enhanced through the use of the LI-DLAS technique with an observation time of 1 s. With increasing the averaging time (e.g., up to > 10 s), the Allan deviation of the traditional DLAS system drops to the same level as that of the LI-DLAS system, and thus, the traditional DLAS system reveals a similar stability but at the expense of a longer measurement time than the LI-DLAS system.
5 Conclusions
To suppress the electrical-domain background noise, a novel LI-DLAS analyzer for CH4 detection was proposed by incorporating an LI data processing algorithm, a nonlinear least-square fitting (NLLSF) algorithm, and a 1654 nm distributed feedback (DFB) laser. Comparative experimental results of the traditional DLAS sensor and the developed LI-DLAS sensor prove the superiority of the LI-DLAS technique. Using the developed algorithm for Gaussian white noise suppression, the detection sensitivity was decreased from 6 to 2 ppmv, and the Allan deviation was decreased from 1.514 to 0.531 ppmv for a 1 s averaging time. Compared with the traditional DLAS analyzer based on LabVIEW platform, the DSP-based CH4 sensor shows the merits of compact size and low cost with potential filed applications in industrial monitoring and control. Meanwhile, the demonstrated LI-DLAS sensor architecture is also applicable in other infrared gas sensing.
References
W. Ren, W.Z. Jiang, F.K. Tittel, Single-QCL-based absorption sensor for simultaneous trace-gas detection of CH4 and N2O. Appl. Phys. B Lasers Opt. 117(1), 245–251 (2014)
C.T. Zheng, W.L. Ye, N.P. Sanchez, C.G. Li, L. Dong, Y.D. Wang, R.J. Griffin, F.K. Tittel, Development and field deployment of a mid-infrared methane sensor without pressure control using interband cascade laser absorption spectroscopy. Sens. Actuat. B Chem. 244, 365–372 (2017)
W.L. Ye, C.G. Li, C.T. Zheng, N.P. Sanchez, A.K. Gluszek, A.J. Hudzikowski, L. Dong, R.J. Griffin, F.K. Tittel, Mid-infrared dual-gas sensor for simultaneous detection of methane and ethane using a single continuous-wave interband cascade laser. Opt. Express 24(15), 16973–16985 (2016)
E.S.F. Berman, M. Fladeland, J. Liem, R. Kolyer, M. Gupta, Greenhouse gas analyzer for measurements of carbon dioxide, methane, and water vapor aboard an unmanned aerial vehicle. Sens. Actuat. B Chem. 169, 128–135 (2012)
W.W. Ding, L.Q. Sun, L.Y. Yi, E.Y. Zhang, ‘Baseline-offset’ scheme for a methane remote sensor based on wavelength modulation spectroscopy. Meas. Sci. Technol. 27(8), 085202 (2016)
A. Groth, C. Maurer, M. Reiser, M. Kranert, Determination of methane emission rates on a biogas plant using data from laser absorption spectrometry. Biores. Technol. 178, 359–361 (2015)
N.P. Sanchez, C.T. Zheng, W.L. Ye, B. Czader, D.S. Cohan, F.K. Tittel, R.J. Griffin, Exploratory study of atmospheric methane enhancements derived from natural gas use in the Houston urban area. Atmos. Environ. 176, 261–273 (2018)
M. Dong, C.T. Zheng, S.Z. Miao, Y. Zhang, Q.L. Du, Y.D. Wang, F.K. Tittel, Development and measurements of a mid-infrared multi-gas sensor system for CO, CO2 and CH4 detection. Sensors 17(10), 2221 (2017)
A.R. Brandt, G.A. Heath, E.A. Kort, F. O’Sullivan, G. Petron, S.M. Jordaan, P. Tans, J. Wilcox, A.M. Gopstein, D. Arent, S. Wofsy, N.J. Brown, R. Bradley, G.D. Stucky, D. Eardley, R. Harriss, Energy and environment. Methane leaks from North American natural gas systems. Science 343(6172), 733–735 (2014)
R.A. Alvarez, S.W. Pacala, J.J. Winebrake, W.L. Chameides, S.P. Hamburg, Greater focus needed on methane leakage from natural gas infrastructure. Proc. Natl. Acad. Sci. 109(17), 6435–6440 (2012)
J. Jiang, G. M. Ma, H. T. Song, C. R. Li, Y. T. Luo, and H. B. Wang, Highly sensitive detection of methane based on tunable diode laser absorption spectrum. IEEE Conf. Int. Instrum. Measur. Technol. 26, 104–108 (2016)
J.S. Li, B.L. Yu, H. Fischer, Wavelet transform based on the optimal wavelet pairs for tunable diode laser absorption spectroscopy signal processing. Appl. Spectrosc. 69(4), 496–506 (2015)
J.A. Silver, Frequency-modulation absorption spectroscopy for trace species detection: theoretical and experimental comparison among methods. Appl. Opt. 31(6), 707–717 (1992)
L. Dong, Y.J. Yu, C.G. Li, S. So, F.K. Tittel, Ppb-level formaldehyde detection using a CW room-temperature interband cascade laser and a miniature dense pattern multi-pass gas cell. Opt. Express 23(15), 19821–19830 (2015)
H.I. Schiff, D.R. Hastie, G.I. Mackay, T. Iguchi, B.A. Ridley, Tunable diode laser systems for measuring trace gases in tropospheric air. Environ. Sci. Technol. 17(8), 352A–364A (1983)
Q. X. He, C. T. Zheng, H. F. Liu, Y. D. Wang, and F. K. Tittel, A near-infrared gas sensor system based on tunable laser absorption spectroscopy and its application in CH4/C2H2 detection. Proc. SPIE 10111, 1011135-1–1011135-7 (2017)
B. Li, C.T. Zheng, Q.X. He, W.L. Ye, Y. Zhang, J.Q. Pan, Y.D. Wang, Development and measurement of a near-infrared CH4, detection system using 1.654 μm wavelength-modulated diode laser and open reflective gas sensing probe. Sens Actuat B Chem 225, 188–198 (2016)
F.A. Blum, K.W. Nill, P.L. Kelley, A.R. Calawa, T.C. Harman, Tunable infrared laser spectroscopy of atmospheric water vapor. Science 177(4050), 694–695 (1972)
J.S. Li, B.L. Yu, W.X. Zhao, W.D. Chen, A review of signal enhancement and noise reduction techniques for tunable diode laser absorption spectroscopy. Appl. Spectrosc. Rev. 49(8), 666–691 (2014)
J.S. Li, H. Deng, P.F. Li, B.L. Yu, Real-time infrared gas detection based on an adaptive Savitzky-Golay algorithm. Appl. Phys. B Lasers Opt. 120(2), 207–216 (2015)
F. Zhang, J.X. Hang, S.B. Wang, Time-delay compensation method of FOG based on Lagrange interpolation. J. Chin. Inertial Technol. 25(5), 676–680 (2017)
C.T. Zheng, W.L. Ye, N.P. Sanchez, A.K. Gluszek, A.J. Hudzikowski, C.G. Li, L. Dong, R.J. Griffin, F.K. Tittel, Infrared dual-gas CH4/C2H6 sensor using two continuous-wave interband cascade lasers. IEEE Photon. Technol. Lett. 28(21), 2351–2354 (2016)
L.S. Rothman, I.E. Gordon, Y. Babikov, A. Barbe, D.C. Benner, P.F. Bernath, M. Birk, L. Bizzocchi, V. Boudon, L.R. Brown, A. Campargue, K. Chance, E.A. Cohen, L.H. Coudert, V.M. Devi, B.J. Drouin, A. Fayt, J.M. Flaud, R.R. Gamache, J.J. Harrison, J.M. Hartmann, C. Hill, J.T. Hodges, D. Jacquemart, A. Jolly, J. Lamouroux, R.J. Le Roy, G. Li, D.A. Long, O.M. Lyulin, C.J. Mackie, S.T. Massie, S. Mikhailenko, H.S.P. Muller, O.V. Naumenko, A.V. Nikitin, J. Orphal, V. Perevalov, A. Perrin, E.R. Polovtseva, C. Richard, M.A.H. Smith, E. Starikova, K. Sung, S. Tashkun, J. Tennyson, G.C. Toon, V.G. Tyuterev, G. Wagner, The Hitran 2012 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transfer 130, 4–50 (2013)
The HITRAN Database. https://www.cfa.harvard.edu/hitran/. Accessed 6 Aug 2018
R.K. Hanson, R.M. Spearrin, C.S. Goldenstein, Spectroscopy and optical diagnostics for gases (Springer, Berlin, 2016)
C. Claveau, A. Henry, D. Hurtmans, A. Valentin, Narrowing and broadening parameters of H2O lines perturbed by He, Ne, Ar, Kr and nitrogen in the spectral range 1850–2140 cm−1. J. Quant. Spectrosc. Radiat. Transfer 68(3), 273–298 (2001)
A. Valentin, Ch. Claveau, A.D. Bykov, N.N. Lavrentieva, V.N. Saveliev, L.N. Sinitsa, The water-vapor ν2 band lineshift coefficients induced by nitrogen pressure. J. Mol. Spectrosc. 198(2), 218–229 (1999)
D.E. Heard, Analytical techniques for atmospheric measurement (Blackwell Publishing, Oxford, 2006)
G.C. Xiong, Adaptive Filter. Geophys. Geochem. Explor. 22(2), 147–153 (2000)
Acknowledgements
The National Key R&D Program of China (No. 2017YFB0405300), National Natural Science Foundation of China (Nos. 61775079, 61627823), Science and Technology Development Program of Jilin Province, China (Nos. 20180201046GX, 20190101016JH), Industrial Innovation Program of Jilin Province, China (No. 2017C027), and the National Science Foundation (NSF) ERC MIRTHE award and Robert Welch Foundation (No. C0586) are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, D., Zhou, Y., Song, F. et al. A Lagrangian interpolation-assisted direct laser absorption spectrum analyzer based on digital signal processor for methane detection. Appl. Phys. B 125, 76 (2019). https://doi.org/10.1007/s00340-019-7184-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-019-7184-1