Abstract
The Tm3+/Yb3+ co-doped germanate–tellurite glasses with good thermal properties were prepared. Based on the absorption spectra and the Judd–Ofelt theory, the J–O intensity parameters (Ω t ), radiative transition probability (276.78 s− 1), fluorescence lifetime (3.89 ms), absorption and emission cross sections (\({\sigma _{\text{e}}}\) = 1.35 × 10− 20 cm2) were calculated. The ~ 2 µm mid-infrared emission resulting from the 3F4→3H6 transition of Tm3+ sensitized by Yb3+ was observed pumped by 980 nm LD. Besides, the energy transfer mechanism between Yb3+ and Tm3+ was thoroughly discussed. The measured ~ 2 µm emission lifetime of Tm3+/Yb3+ co-doped glass can reach as high as 2.38 ms. The above results showed that Tm3+/Yb3+ co-doping glass could be expected to be a promising material to achieve high efficient ~ 2 µm lasing with a 980 nm LD pumping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the past several years, increasing efforts have been made to develop ~ 2.0 µm fiber lasers driven by their wide applications such as remote sensing, laser surgery, environmental monitoring, and eye-safe light detection and ranging (LIDAR) [1,2,3,4].
Among the rare earth ions, Tm3+ and Ho3+ are well known for the generation of ~ 2.0 µm emission, due to Tm3+:3F4 → 3H6 and Ho3+:5I7→5I8 transitions, respectively. In comparison with Ho3+, Tm3+ is of special interests for its unsurpassed advantages, such as broad emission about 800 nm suitable for tunable lasers [5] and high quantum efficiency beneficial from the cross relaxation energy transfer (3H4 + 3H6 → 3F4 + 3F4) [6]. What is more, the 3F4 level of Tm3+ can be populated using sensitizer ions such as Yb3+ and Er3+ [7, 8]. Yb3+ ion can be used as a sensitizer and can be pumped by a more powerful and relatively inexpensive laser diode (LD) at 980 nm. Fast diffusion among the Yb3+ ions can enhance the efficiency of the nonresonant energy transfer from Yb3+ to Tm3+ (2F5/2 + 3H6 → 2F7/2 + 3H5). Yb3+ has a high absorption cross section and can effectively absorb excitation of commercial 980 nm LD emission, and then transfer energy to Tm3+ (3H5 level) via non-resonant energy transfer process.
To research the luminescence properties of rare earth ions, it is very significant to select glass hosts for researchers. The first successfully Tm3+-doped silica fiber laser was demonstrated in 1988 by Southampton [9]. In 1999, Jackson et al. proposed a theoretical model that describes the ion-pair dynamics relevant to the Tm-doped silica system, which can deduce equations for the steady-state intracavity photon density and for the steady-state population densities of the isolated and paired ions [10]. In 2009, Moulton et al. used a piece of 25-µm-diameter and 0.08 numerical aperture (NA) core thulium-doped silica fiber to derive single mode laser output of the output powers of 300 W and the slope efficiency as high as 64.5% for launched pump power [11]. Various host materials including fluoride [12], tellurite [13], germanate [6] and silicate glasses [14] have been successfully applied to achieve ~ 2.0 µm fiber lasers with different efficiencies. Especially, actively Q-switched and mode-locked Tm3+-doped silicate 2 µm fiber laser have been already achieved in 2012 [14, 15]. The obvious defects of silicate glasses are the low rare earth solubility and high phonon energy. The fluoride glasses have the drawbacks of inferior mechanical and chemical properties. Germanate glasses have been intensively studied as promising candidates in mid-infrared optical applications and high-energy laser system [6, 16, 17]. Particularly, one system, germanate–tellurite (GT) glass, has been practically applied in large infrared (IR) windows, because of the excellent combinations of superior IR transparency, comparatively low phonon energy, high rare earth solubility, and good mechanical characteristics [18]. Tellurite glass has attracted a great deal of interest not only for its relatively lower maximum phonon energy (~ 760 cm−1) among all the oxide glasses, but also for the improvement in chemical and mechanical stability as well as the high refractive index and excellent infrared transmission [19]. Compared with germanate glass (~ 900 cm−1) [19], tellurite glass (~ 760 cm−1) [20] has much lower phonon energy, which is favorable for 2 µm emission and that is helpful to reduce the multi-phonon relaxation rate for Tm3+:3F4 → 3H6 transition. However, tellurite glass has lower glass transition temperature (~ 350 °C) than germanate glass (~ 600 °C). Thus, tellurite glass has poor thermal stability to resist thermal damage at high pumping power. In contrast, the thermal stability, chemical durability and resistance to thermal damage of germanate glass are superior. Therefore, germanate–tellurite glass combines the advantages of both germanate and tellurite glasses, i.e., good thermal stability, chemical durability, lower phonon energy, high rare earth solubility and high transparency in a wide wavelength range [21, 22]. These features render germanate–tellurite glass an ideal host for mid-infrared laser material and have potential applications for cw, modelocked and Q-switched, micro-cavity lasers [23, 24]. To the best of our knowledge, the fluorescence corresponding to the 3F4 → 3H6 transition of the Tm3+ sensitized by Yb3+ has been under-reported in germanate–tellurite glasses [25,26,27].
In this work, we report the Tm3+/Yb3+ co-doped germanate–tellurite glasses for generating emission in the ~ 2.0 µm wavelength band. The spectroscopic properties of Tm3+ and energy transfer mechanism between Tm3+ and Yb3+ ions are discussed in detail. Energy transfer microscopic parameters were calculated based on the absorption spectra. Furthermore, the thermal properties are investigated and Judd–Ofelt intensity parameters were determined to evaluate the radiative properties. In this paper, the lifetime and gain spectra have also been discussed. The results verify that the Tm3+/Yb3+ co-doped germanate–tellurite glasses could be potential materials for mid-infrared lasers.
2 Experiment
2.1 Material synthesis
The sample glasses were prepared by traditional melt-quenching method using high-purity chemicals (99– 99.99+%). The investigated glasses of molar compositions: 80(GeO2 + TeO2) − (19.5 − y)(K2CO3 + Nb2O5 + La2O3) − xYb2O3 − yTm2O3(x = 0.5; y = 0.1, 0.25, 0.4, 0.5 and x = 0; y = 0.1) were placed in a Al2O3 crucible and heated with an SiC-resistance electric furnace at 1200 °C for 25 min. The melts were then poured onto a preheated stainless steel mold, followed by annealing at 530 °C for 5 h to remove the inner stresses. Then, it was allowed to cool slowly to room temperature. The cooled samples had been cut and polished to the size of 20 × 20 × 1.5 mm3 carefully, prepared for optical property measurements.
2.2 Performance measurements
In this paper, the refractive index (1.73) and density (4.27 g/cm3) of 0.25Tm3+/0.5Yb3+ co-doped sample were tested by prism minimum deviation method and Archimedes principle using distilled water as an immersion liquid. The maximum phonon energy of the present germanate–tellurite glass is ~ 785 cm−1. The maximum phonon energy (~ 785 cm−1) is less than that of tellurite glasses (~ 900 cm−1) [6]. The characteristic temperatures of glass transition (Tg), onset crystallization peak (Tx) and top crystallization (Tp) were obtained at 10K/min using the NETZSCH DTA 404 PC differential scanning calorimeter. The fluorescence spectra of the samples in the range of 1500–2400 nm were measured by a liquid nitrogen-cooled PbS detector using a 980 nm laser diode (LD) as an excitation source. The absorption spectra measurements were performed by Perkin Elmer Lambda 900 UV/VIS/NIR double beam spectrophotometer (Waltham, MA) in the range from 300 to 2100 nm. The lifetime decay curves were measured and shown by TDS 3012C type Digital Phosphor Oscilloscope (100 MHz, 1.25GS/S). For different samples, they were tested under the same experimental conditions. In addition, all the test results were carried out at room temperature.
3 Results and discussions
3.1 Thermal stability analysis
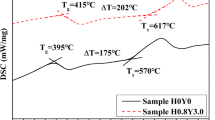
To correctly evaluate the thermal properties of the glasses, as shown in Fig. 1, the DSC measurement of sample glasses were performed. The values of Tg, Tx and Tp are 538 °C, 726 °C and 750 °C, respectively. Tg is a significant factor for laser glasses. The transition temperature (Tg) of the samples (538 °C) is higher than that of bismuth (269 °C) [28], tellurite (354 °C) [29], tellurite(300 °C) [13], germanate (526 °C) [30] and fluoride glasses (332 °C) [31]. The higher Tg is generally considered to possess better thermal properties to resist thermal damage at high pumping intensities. The ∆T (Tx − Tg) can usually be used to evaluate the thermal properties of glasses. A bigger ∆T reveals that the glass possesses an excellent thermal ability against nucleation and crystallization as well. From Table 1, it is obvious that ∆T is 188 °C, which is significantly larger than that of bismuthate (67 °C) [28], tellurite (141 °C) [29], germanate (129 °C) [30] and fluoride glasses (76 °C) [31], revealing that germanate–tellurite glasses could be potential materials for mid-infrared lasers. The above results indicate that germanate–tellurite glasses have better thermal performance. Hence, the prepared germanate–tellurite glasses have good anti-crystallization properties and could be selected as potential laser material.
3.2 Absorption spectra and J–O analysis
As shown in Fig. 2, the absorption spectra of 0.25Tm3+, 0.5Yb3+ single-doped and 0.25Tm3+/0.5Yb3+ co-doped glass samples of thickness of 1.5 mm are depicted in the wavelength range of 300–1750 nm. For Tm3+ singly doped sample, in the range of 300 to 1750 nm absorption bands could be discerned, centered at around 468, 684, 791, 1208 and 1713 nm, corresponding to the transitions from the 3H6 ground state to excited levels 1G4, 3F2,3, 3H4, 3H5 and 3F4, respectively, according to the absorption spectrum of Tm3+ single-doped germanate glass. The shapes and the peak positions of each transition in Tm3+ single-doped germanate–tellurite glasses nearly do not change when compared with the Tm3+/Yb3+ co-doped germanate–tellurite glasses and are very similar to those in other Tm3+-doped glasses [31]. The phenomenon could be explained by the fact that the Tm3+ ions are homogeneously incorporated into the glass network without clustering and any changes in the local ligand field [33]. In this paper, the 980 nm LD is used as the pumping source. As well known, the center of the absorption peak of Yb3+ ions at 976 nm is indeed more intense than all the absorption peaks of Tm3+. The radiation wavelength of high-power commercial 980 nm LD can be matched with 976 nm absorption peak of Yb3+ ions, showing that codoping with Yb3+ and Tm3+ is an efficient approach to enhance ~ 1.8 µm emission from Tm3+ [34, 35].
The Judde–Ofelt (J–O) theory [36, 37] plays a significant role in analyzing the spectroscopic properties (radiative lifetimes, intensity parameters Ω t (t = 2, 4, 6), spontaneous emission probabilities and so on) for rare earth ions-doped glasses.
Table 2 compares the Judd–Ofelt parameters of Tm3+ ions in germanate, tellurite and bismuthate glasses. The Ω2 parameter is intensively dependent on the local environment of the rare earth ions. The value of Ω2 (4.45 × 10−20 cm2) of Tm3+ is relatively large and higher than that of silicate (3.70 × 10− 20 cm2) [38] and tellurite (3.30 × 10− 20 cm2) [39] glasses. On the one hand, it is straightly related to the symmetry of rare earth ions with ligand formation. On the other hand, the polarization of the local structure and the covalent nature of the chemical bonds result in the formation of rare earth ions with ligands. A large field strength is mainly attributed to a high polarizability of oxygen at the rare earth site. For the crystal field parameter, the value of Ω2 might also be affected by the asymmetry of the rare earth sites. Ω2 is an important factor to obtain good spectral properties of the host materials. From Table 2, the value of Ω6 is larger than those of silicate glasses (0.60 × 10−20 cm2) [39], but smaller than tellurite (1.28 × 10−20 cm2) [40] and germanate (1.92 × 10−20 cm2) [41] glasses. At the same time, comparison with Ω2, Ω6 does not depend on the environment, but is more dependent on the overlap integrals of the 4f and 5d orbits [42].
From Table 3, a full understanding of the radiation characteristics of the prepared glasses, the radiative lifetimes (τrad), spontaneous transition probabilities (Arad) and fluorescence branching ratios (β) were obtained. The radiative transition probability (Arad) of Tm3+:3F4 → 3H6 transition is calculated to be 276.78 s−1. The higher spontaneous radiative transition probability provides larger opportunity to achieve better laser action, so the prepared glasses could be a promising candidate for mid-infrared laser [43]. It is known from other papers that the radiative transition probability (Arad) of Tm3+ in the sample is higher than that of tellurite and germanate glasses [44, 45]. We can see that the radiative lifetime of 3F4 → 3H6 transition is as long as 3.89 ms, which is longer than that of tellurite (1.64 ms) and germanate (0.859 ms) glasses [44, 45]. The highly efficient ~ 2 µm radiations can be achieved in the prepared glass.
3.3 Emission spectra and cross section
In the past studies [46], the energy transfer process between Yb3+ and Tm3+ is recognized by researchers and the efficiency of the process is very high. As shown in Fig. 3a, with the increase of Tm3+ concentration, the Tm3+:~ 1.8 µm emission is also enhanced. The fluorescence spectra of Tm3+/Yb3+ co-doped germanate–tellurite glass pumped by 980 nm LD is displayed. From Fig. 3b, it can be found that there is no fluorescence when Tm3+ is singly doped in germanate–tellurite glasses. With the assistance of Yb3+ ions, the ~ 1.8 µm emission peak of Tm3+/Yb3+ co-doped germanate–tellurite glasses is excited by 980 nm LD. This confirms the lack of Tm3+ absorption band near 976 nm and the existence of energy transfer from Yb3+ to Tm3+. In addition, with the increase of Tm3+ ion concentration, the fluorescence intensities of Tm3+/Yb3+ co-doped glass samples increase initially and then rapidly decrease. When the concentration of Tm3+ is 0.25 mol%, the intensity of fluorescence emission reaches the maximum in these four sets of glass samples. So, the optimum concentration of Tm3+ is 0.25 mol%. The reason is that the energy transfer probability between Yb3+:2F5/2 and Tm3+:3H5 ions is enhanced. Subsequently, this emission intensity decreases gradually with the increase in Tm3+ concentration because of concentration quenching [47]. Therefore, the optimum concentration ratio between Tm3+ ions and Yb3+ ions is 1:2, and the results show that the Tm3+/Yb3+ co-doped germanate–tellurite glasses can be potential materials for mid-infrared lasers.
Furthermore, emission cross section is an important parameter to estimate the possibility for achieving laser action. According to the above measured absorption spectra, the absorption cross section can be expressed by the Beer–Lambert equation:
where N is the density of rare earth ion (ions/cm3), l is the thickness of the polished sample glass, OD(λ) is the optical density and the value of the optical density is equal to the spectra intensity.
where λ is the wavelength, Arad is the spontaneous transition probability, I(λ) is the emission intensity and c and n are the light speed in vacuum and refractive index, respectively.
As shown in figure Fig. 4a, the values of emission and absorption cross sections of Yb3+ are 0.87 × 10−20 and 0.59 × 10−20 cm2 in the sample glasses, respectively. From Fig. 4b, the values of emission and absorption cross sections of Tm3+ are 1.35 × 10−20 and 0.56 × 10−20 cm2 at 1.8 µm, respectively. We can see that the emission cross section (1.35 × 10−20 cm2) of Tm3+ is larger than those of silicate (0.2 × 10−20 cm2) [48], tellurite (0.86 × 10−20 cm2) [44], tellurite (0.96 × 10−20 cm2) [13] and bismuthate glasses (0.71 × 10−20 cm2) [49]. For a laser material, in general, the high stimulated emission cross section is helpful to increase the gain. A larger gain results in better amplification behavior.
The process of energy transfer between Tm3+ and Yb3+ ions is presented in Fig. 5. As shown in Fig. 5, the efficient energy transfer between Tm3+and Yb3+ can be obtained through the transfer of phonons, and a main energy transfer process in the Tm3+/Yb3+ co-doped germanate–tellurite glass has been shown. The Yb3+ is pumped by 980 nm LD and excited from the ground state (2F7/2) to the upper level (2F5/2), following an energy transfer process from Yb3+:2F5/2 to Tm3+:3H5 (ET: Yb3+:2F5/2 + TmH6 → Yb3+:2F7/2+Tm3+:3H5). The process is assisted by phonons. The Tm3+ ion in the 3H5 level relaxes very quickly to the 3F4 level, and then particle is transferred from the 3F4 level to the 3H6 level. Yb3+ ion as a sensitizer can greatly improve upconversion efficiency through energy transfer (ET), which is due to strong absorption of the Yb3+ ion around 980 nm when Tm3+/Yb3+ co-doped glasses are excited by a 980 nm laser. The simplified energy level diagram exhibits distinct upconversion centered at 476, 650 and 689 nm, which are assigned to the 1G4 → 3H6, 1G4→3F4 and 3F2,3 → 3H6 transitions of Tm3+ ions, respectively.
The microscopic parameter based on the evaluation of the overlap integral between the absorption and emission cross sections can be obtained using the following equation:
\({\text{g}}_{{{\text{low}}}}^{{\text{D}}}\) and \({\text{g}}_{{{\text{up}}}}^{{\text{D}}}\) are the degeneracies of the respective lower and upper levels of the donor, respectively. \(\bar {n}={1 \mathord{\left/ {\vphantom {1 {\left( {{e^{{{\hbar {w_0}} \mathord{\left/ {\vphantom {{\hbar {w_0}} {KT}}} \right. \kern-0pt} {KT}}}} - 1} \right)}}} \right. \kern-0pt} {\left( {{e^{{{\hbar {w_0}} \mathord{\left/ {\vphantom {{\hbar {w_0}} {KT}}} \right. \kern-0pt} {KT}}}} - 1} \right)}}\) is the average occupancy of the phonon mode at T. S0 is Huang–Rhys factor.
Table 4 lists the energy transfer microscopic parameters of Tm3+/Yb3+ co-doped germanate–tellurite glass. It is clearly shown that effective energy transfer takes place between the Tm3+ and Yb3+ ions. As shown in Table 4, the energy transfer coefficient between Yb3+:2F5/2 level and Tm3+:3H5 level can be as high as 1.3826 × 10−40 cm6/s. It is worth mentioning that the above energy transfer process is non-resonant and mainly assisted by one phonon (39.076%), two phonons (27.785%) and three phonons (1.696%). This circumstance mainly results from low phonon energy of germanate–tellurite glass. Besides, with increasing Tm3+ concentration, the energy transfer process can hardly happen from Yb3+:2F5/2 to Tm3+:3H5. As shown in Fig. 6, the decay curves can confirm this process. Hence, it is very important for mid-infrared materials to select suitable Tm3+ concentration.
3.4 Fluorescence lifetimes and gain coefficient
As shown in Fig. 6, the 1.8 µm lifetime (2.38 ms) of Tm3+/Yb3+ co-doped germanate–tellurite glass sample is obtained, and is significantly larger than those of silicate (1.42 ms) [50] and germanate (0.36 ms) glasses [51]. The lifetime in Tm3+/Yb3+ co-doped is longer than that in Tm3+ single-doped germanate–tellurite glass. It indicates that there is an effective energy transfer between Tm3+ and Yb3+. We can see that the lifetime of 3F4 level decreases gradually with increase in Tm3+ concentration. The reduced lifetime is not beneficial for the upconversion process. The decreased upconversion process contributes to population aggregation of Tm3+:3F4 and might enhance Tm3+:3H5 → 3F4. Here, the stronger upconversion process is due in part to the excited state absorption (ESA), while the excited state absorption process reduces the 3F4 level number of particles, which is detrimental to the 1.8 µm lifetime. Therefore, the decrease in the conversion process is beneficial to the 1.8 µm lifetime of Tm3+. In addition, the relatively long lifetime generally enhances the luminous efficiency of glasses and it generally reduces the laser oscillation threshold. Hence, the emission lifetime (2.38 ms) indicates that Tm3+/Yb3+ co-doped germanate–tellurite glass is a promising material for ~ 2.0 µm fiber laser.
Besides, to further evaluate the performance of the samples, the fluorescence quantum efficiency was estimated from the lifetime values by the following equation.
where τ is the measured fluorescence lifetime of the sample (0.5Tm3+/0.1Yb3+) and τrad is the theoretical lifetime of the sample (0.5Tm3+/0.1Yb3+). The measured 3F4 lifetime (2.38 ms) for Tm3+ is shorter than the calculated lifetime (3.89 ms), which is due to non-radiative quenching. It can be found that the fluorescence quantum efficiency is as high as 61.18%, which is higher than that of silicate glass (13.0%) [52]. Therefore, Tm3+/Yb3+ co-doped germanate–tellurite glass is a more promising material for improving the Tm3+ 2.0 µm fiber laser performance.
Another important parameter to evaluate the gain performances of the prepared sample quantitatively is gain coefficient. To calculate the gain ability of Tm3+/Yb3+ co-doped germanate–tellurite glasses, the gain coefficient G(λ,P) of Tm3+/Yb3+ co-doped germanate–tellurite glasses is calculated. The room temperature gain coefficient (G(λ,P)) can be simply denoted as
where N is the doping concentration of the rare earth ion and P is the population inversion.
Figure 7 shows the calculated gain coefficients of Tm3+ and Yb3+ transitions as the function of wavelength. The gain coefficient results are calculated by setting P ranging from 0 to 1 at an interval of 0.1. We can see that the gain coefficient increases as the value of P continues to increase. As shown in Fig. 7, the maximum gain coefficients of Tm3+ reach 3.02 cm−1; furthermore, the concentrations of Tm3+ and Yb3+ are 0.1 and 0.5 mol%, respectively. Besides when P is more than 0.4, the value G(λ,P) of the Tm3+:3F4 → 3H6 transition becomes a positive number. In addition when P is less than 0.4, it becomes a negative number. The above results show that Tm3+/Yb3+ co-doped GeO2–TeO2–K2CO3–Nb2O5–La2O3 glass has high gain and low pumping threshold for the laser and is an outstanding gain host material for ~ 2 µm mid-infrared laser.
4 Conclusions
In a word, efficient ~ 1.8 µm emissions have been achieved in Tm3+/Yb3+ co-doped germanate–tellurite glasses. The energy transfer process between Yb3+ and Tm3+ has been investigated in detail. The thermal stability, absorption and emission cross sections, and lifetimes have been analyzed. The results indicated that Tm3+ in the prepared glasses had not only superior thermo-mechanical stability (Tg = 538 °C, ΔT = 188 °C), but also large emission cross section (1.35 × 10− 20cm2). The measured lifetime of Tm3+/Yb3+ co-doped germanate–tellurite glass is as high as 2.38 ms. It also possesses superior gain performance for Tm3+:3F4 → 3H6 and Yb3+:2F5/2 → 2F7/2 transitions. All of the results indicate that the prepared germanate–tellurite glass is a promising candidate for ~ 2.0 µm mid-infrared laser materials applications.
References
G.X. Chen, Q.Y. Zhang, G.F. Yang, Z.H. Jiang, Mid-infrared emission characteristic and energy transfer of Ho3+-doped tellurite glass sensitized by Tm3+. J.Fluoresc. 17(3), 301–307 (2007)
F. Amzajerdian, J. Geng, Q. Wang, T. Luo, Single-frequency narrow-linewidth Tm-doped fiber laser using silicate glass fiber. Opt. Lett. 34(22), 3493–3495 (2009)
Y.H. Tsang, A. El-Sherif, T.A. King, Broadb and amplified spontaneous emission fibre source near 2.0 µm using resonant in-band pumping. J. Mod. Opt. 52(1), 109–118 (2005)
W.J. Zhang, Q.Y. Zhang, Q.J. Chen, Q. Qian, Z.M. Yang, J.R. Qiu, P. Huang, Y.S. Wang, Enhanced 2.0 µm emission and gain coefficient of transparent glass ceramic containing BaF2: Ho3+,Tm3+ nanocrystals. Opt. Express 17(23), 20952–20958 (2009)
S. Agger, J.H. Povlsen, P. Varming, Single-frequency thulium-doped distributed-feedback fiber laser. Opt. Lett. 29(13), 1503–1505 (2004)
J.F. Wu, Z.D. Yao, J. Zong, S.B. Jiang, Highly efficient high-power thulium-doped germanate glass fiber laser. Opt. Lett. 32(6), 638–640 (2007)
S.F. León-Luis, J. Abreu-Afonso, J. Peña-Martínez, J. Méndez-Ramos, A.C. Yanes, J. del-Castillob, V.D. Rodrígueza, Up-conversion and colour tuneability in Yb3+–Er3+–Tm3+ co-doped transparent nano-glass-ceramics. J. Alloys Compd. 479(1), 557–560 (2009)
D. Zhou, Z. Song, G. Chi, J. Qiu, NIR broadband luminescence and energy transfer in Er3+–Tm3+co-doped tellurite glasses. J. Alloys Compd. 48(1), 881–884 (2009)
D.C. Hanna, I.M. Jauncey, R.M. Percival, I.R. Perry, R.G. Smart, P.J. Suni, J.E. Townsend, A.C. Tropper, Continuous-wave oscillation of a monomode thulium-doped fiber Laser. Electron. Lett. 24(19), 1222–1223 (1988)
S.D. Jackson, T.A. King, Dynamics of the output of heavily Tm-doped double-clad silica fiber lasers. Opt. Lett 16(12), 2178–2189 (1999)
P.F. Moulton, Tm-Doped Fiber Lasers: Fundamentals and Power Scaling. J. Sel Topics Quantum Electron 15(1), 85–92 (2009)
R.M. Percival, D. Szebesta, S.T. Davey, Highly efficient and tunable operation of two colour Tm-doped fluoride fibre laser. Electron. Lett. 28(7), 671–673 (1992)
K.F. Li, G. Zhang, L.L. Hu, Watt-level ~ 2.0 µm laser output in Tm3+ doped tungsten-tellurite glass double-cladding fiber. Opt. Lett. 35(24), 4136–4138 (2010)
Q. Wang, J. Geng, T. Luo, S. Jiang, Mode-locked 2.0 µm laser with highly thulium-doped silicate fiber. Opt.Lett. 34(23), 3616–3618 (2009)
E. Michael, K. Christelle, S. Jacek, J. Stuart, D.M. Gwanael, E. Marc, Actively Q-switched and mode-locked Tm3+-doped silicate 2 µm fiber laser for supercontinuum generation in fluoride fiber. Opt. Lett. 37(4), 512–515 (2012)
N.P. Barnes, B.M. Walsh, D.J. Reichle, R.J. DeYoung, S.B. Jiang, Tm: germanate fiber laser: tuning and Q-switching. Appl. Phys. B. 89(2–3), 299–304 (2007)
F. Fusari, A.A. Lagatsky, G. Jose, S. Calvez, A. Jha, M.D. Dawson, J.A. Gupta, W. Sibbett, C.T. Brown, Femtosecond mode-locked Tm3+ and Tm3+–Ho3+ doped 2.0 µm glass Lasers. Opt. Express 18(21), 22090–22098 (2011)
R. Xu, M. Wang, Y. Tian, L. Hu, J. Zhang, 2.05 µm emission properties and energy transfer mechanism of germanate glass doped with Ho3+, Tm3+, and Er3+. J. Appl. Phys. 109(5), 285 (2011)
Y.Y. Guo., M. Li, Y. Tian, R.R. Xu, L.L. Hu, J.J. Zhang, Enhanced 2.7 µm emission and energy transfer mechanism of Nd3+/Er3+ co-doped sodium tellurite glasses. J. Appl. Phys. 110(1), 013512–013516 (2011)
M.D. Di, L.F. Santos, A. C. Marques. Vibrational spectra and structure of alkali germanate glasses. J. Non Cryst. Solids 293, 394–401 (2001)
G. Monteiro, L.F. Santos, J. Pereira, R.M. Almeida, Optical and spectroscopic properties of germanotellurite glasses. J. Non Cryst. Solids 357(14), 2695–2701 (2011)
M. Jayasimhadri, E.J. Cho, K.W. Jang, H.S. Lee, S.I. Kim, Spectroscopic properties and Judd–Ofelt analysis of Sm3+ doped lead-germanate–tellurite glasses. J. Phys. D Appl. Phys. 41(17), 175101–175104 (2008)
C. Danie, J.K. Terence, A.K. Do-Kyeong, L. Jongmin, Q-switched operation of a 2.7 µm cladding-pumped Er3+/Pr3+ codoped ZBLAN fiber laser. Opt. Commun. 236, 379–385 (2004)
Q. Wang, J. Geng, T. Luo, S. Jiang, Mode-locked 2 µm laser with highly thulium-doped silicate fiber. Opt. Lett. 34(23), 3616–3619 (2009)
H. Lin, Y.Y. Zhang, E.Y.B. Pun, Fluorescence investigation of Ho3+ in Yb3+ sensitized mixed-alkali bismuth gallate glasses. Spectrochim. Acta. Part A. 71(4), 1547–1550 (2008)
L. Huang, S. Shen, A. Jha, Near infrared spectroscopic investigation of Tm3+–Yb3+ co-doped tellurite glasses. J. Non Cryst. Solids 345–346(20), 349–353 (2004)
D. Di Martino, L.F. Santos, A.C. Marques, R.M. Almeida, Vibrational spectra and structure of alkali germanate glasses. J. Non Cryst. Solids 293–295(1), 394–401 (2001)
E.R. Shaaban, M. Shapaan, Y.B. Saddeek, Structural and thermal stability criteria of Bi2O3–B2O3 glasse. J. Phys. Condens. Matter 20(20), 155108 (2008)
D.D. Chen, Q.Y. Zhang, G.F. Yang, Z.H. Jiang, Thermal stability and spectroscopic properties of Er3+doped niobium tellurite glasses for broadband amplifiers. Mater. Chem. Phys. 90(1), 78–82 (2005)
T.Wei,F. Chen, Y. Tian, S. Xu, 1.53 µm emission properties in Er3+ doped Y2O3 and Nb2O5 modified germanate glasses for an optical amplifier. J. Lumin. 154(154), 41–45 (2014)
Y. Tian, R. Xu, L. Hu, J. Zhang, 2.7 µm fluorescence radiative dynamics and energy transfer between Er3+ and Tm3+ ions in fluoride glass under 800 nm and 980 nm excitation. J Quant Spectrosc Radiat Transf 113(1), 87–95 (2012)
A.A. El-Maaref, K.H.S. Shaaban, M. Abdelawwad, Y.B. Saddeek, Optical characterizations and Judd–Ofelt analysis of Dy3+ doped borosilicate glasses. Opt. Mater. 72, 169–176 (2017)
G.C. Ram, T. Narendrudu, S. Suresh, D. Krishna Rao, Investigation of luminescence and laser transition of Dy3+ ion in P2O5 PbO Bi2O3 R2O3 (R = Al, Ga, In) glasses. Opt. Mater. 66, 189–196 (2017)
F. Huang, Y. Ma, W. Li, X. Liu, L. Hu, D. Chen, 2.7 µm emission of high thermally and chemically durable glasses based on AlF3. Sci.Rep. 4, 3607 (2014)
B.R. Judd, Optical absorption intensities of rare-earth ions. Phys. Rev. 127(3), 750–761 (1962)
G.S. Ofelt, Tensities of crystal spectra of rare-earth ions., J. Chem. Phys. 37, 511–520 (1962)
H.Sun,L. Zhang, L. Wen, M. Liao, J. Zhang, Effect of PbCl2 addition on structure, OH– content, and upconversion luminescence in Yb3+/Er3+ co-doped germanate glasses. Appl.Phys. B. 80(7), 881–888 (2005)
R. Cao, M. Cai, Y. Lu, Y. Tian, F. Huang, S. Xu, J. Zhang, Ho3+/Yb3+ codoped silicate glasses for 2 µm emission performances. Appl. Opt. 55(8), 2065–2070 (2016)
S. Xu, Z. Yang, S. Dai, G. Wang, L. Hu, Z. Jiang, Upconversion fluorescence spectroscopy of Er3+doped lead oxyfluoride germanate glass. Mater. Lett. 58(6), 1026–1029 (2004)
R. Xu, Y. Tian, L. Hu, J. Zhang, Broadband 2 µm emission and energy-transfer properties of thulium-doped oxyfluoride germanate glass fiber. Appl. Phys. B. 104(4), 839–844 (2011)
K. Li, H. Fan, G. Zhang, G. Bai, S. Fan, J. Zhang, L. Hu, Broadband near-infrared emission in Er3+–Tm3+ co-doped bismuthate glasses. J. Alloys Compd. 509(6), 3070–3073 (2011)
E.R. Shaaban, M. Shapaan, Y.B. Saddeek, Structural and thermal stability criteria of Bi2O3–B2O3 glasses. J. Phys. Condens. Matter 20(20), 155108 (2008)
K. Dieckmann, Laser Spectroscopy, Vol. 1 World Scientific Publishing Co Pte Ltd., Singapore, (2008) p. 10
J. Yuan, W.C. .Wang, D.D. Chen, M.Y. Peng, Q.Y. Zhang, Z.H. Jiang, Enhanced 1.8 µm emission in Yb3+/Tm3+codoped tungsten tellurite glasses for a diode-pump 2.0 µm laser. J. Non Cryst. Solids 402(17), 223–230 (2014)
C. Zhu, X. Zhao, Z. Wang, H. Lin, Upconversion photon quantification in Tm3+/Yb3+dopedaluminum germanate glasses for waveguide-typedirradiation light sources. Optik 127(23), 11544–11552 (2016)
G. Bai, Y. Guo, Y. Tian, L. Hu, J. Zhang, Light emission at 2.0 µm from Ho–Tm–Yb doped silicate glasses. Opt. Mater. 33(8), 1316–1319 (2011)
Y. Guo, Y. Ma, F. Huang, Y. Peng, L. Zhang, J. Zhang, 2.7 µm emission properties of Er3+ doped tungsten–tellurite glass sensitized by Yb3+ ions.Spectrochim. Acta.A. 111(111), 150–153 (2013)
G. Bai, Y. Guo, Y. Tian, L. Hu, J. Zhang, Light emission at 2.0 µm from Ho3+–Tm3+–Yb3+ doped silicate glasses. Opt. Mater. 33(8), 1316–1319 (2011)
K. Li, S. Fan, L. Zhang, Q. Zhang, J. Zhang, L. Hu, Spectroscopic properties of the 1.8 µm emission of Tm3+/Yb3+ codoped TeO2–ZnO–Bi2O3 glasses with efficient energy transfer. J. Non Cryst. Solids 357(11–13), 2417–2420 (2011)
R. Cao, Y. Lu, Y. Tian, F. Huang, Y. Guo, S. Xu, J. Zhang, Mid-infrared luminescence and energy transfer of Tm3+ in silicate glasses by codoping with Yb3+ ions, Opt. Laser Technol. 94, 106–111 (2017)
Y. Tian, R. Xu, L. Hu, J. Zhang, Efficient 2 µm emission and energy transfer mechanism of Ho3+ doped barium gallium germanate glass sensitized by Tm3+ ions. Appl. Phys. B. 108(3), 597–602 (2012)
X.Q. Liu, M. Li, X. Wang, F.F. Huang, L.L. Hu, D. P. Chen. ~ 2 µm luminescence properties and nonradiative processes of Tm3+ in silicate glass. J. Lumin. 150, 40–45 (2014)
Acknowledgements
This research was financially supported by the Chinese National Natural Science Foundation (no. 61775205, 61605192 and 51502022).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the topical collection “Mid-infrared and THz Laser Sources and Applications” guest edited by Wei Ren, Paolo De Natale and Gerard Wysocki.
Rights and permissions
About this article
Cite this article
Dou, A., Shen, L., Wang, N. et al. Investigation of Tm3+/Yb3+ co-doped germanate–tellurite glasses for efficient 2 µm mid-infrared laser materials. Appl. Phys. B 124, 86 (2018). https://doi.org/10.1007/s00340-018-6957-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-018-6957-2