Abstract
The combination of interband cascade lasers (ICL) with cavity-enhanced absorption spectroscopy (CEAS) offers new perspectives in trace analysis and isotope ratio measurements. ICLs cover a mid-infrared spectral window (3–4 µm), in between those covered by Ga(InAs)Sb diode lasers and quantum cascade lasers (QCL), where strong molecular transitions can be found. While ICLs have lower emission power than QCLs, their thermal dissipation is much closer to that of telecom diode lasers and their current tuning range larger, which are both major advantages for developing compact instruments. We present an OF-CEAS implementation with an ICL at 4.015 µm, in which optical feedback (OF) enables efficient injection into the high-finesse cavity. In this paper, we also discuss a procedure allowing to obtain an accurate measurement of the OF rate. With regard to performance, we obtain a rms noise-equivalent absorption of 7.7 × 10−9 cm−1 for one acquired spectrum (80 ms) with a cavity of finesse 3900, which translates to a normalized figure of merit of 2.2 × 10−9 cm−1/√Hz, allowing for SO2 trace analysis down to ppbv levels with a response time of seconds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
High-sensitivity absorption spectroscopy has a vast domain of applications, mainly due to two means of exploiting its ability to measure weak spectral features: on the one hand, to obtain information on intrinsically weak molecular transitions and on the other hand, to selectively measure the number density of a given molecule in a mixture, down to trace concentrations. The first enables refined studies of forbidden transitions in molecular spectroscopy (e.g., overtone, magnetic dipole, electric quadrupole, and collisionally induced transitions). These studies support the development of theoretical models and computations in molecular physics. They also provide data for molecular spectroscopy databases such as HITRAN [1] or GEISA [2] necessary for the modeling of Earth or planetary atmospheres (e.g., with respect to composition and radiative transfer properties). The second is the basis for laser-based techniques applied to trace gas analysis and isotope ratio determination. In contrast to many of the conventional techniques such as mass spectrometry or gas chromatography, laser-based techniques offer the advantage of, among others, relatively low cost, compactness, robustness, fast response time, high sensitivity, and continuous sample monitoring, often with no need for purification or pre-treatment of the sample. This has led to the widespread application of laser analyzers in industrial processes [3], medical [4] and greenhouse gas monitoring [5], atmospheric pollution, and climate studies in general [6]. In this article, we are interested in sulfur dioxide, a toxic molecule that may be emitted during the combustion of fossil fuels and by several industrial processes. It is thus an atmospheric pollutant affecting industrialized countries: In particular, China is the main producer of atmospheric SO2 today [7]. It is also a major component in volcanic emissions: As an example, it accounts for ~17 % of the total outgassing budget of Mt. Etna [8]. Changes in its concentration can be a useful indicator of increasing volcanic activity [9]. In combination with water, it converts to sulfuric acid and, together with nitric acid from NO2, is a precursor of acid rain.
While multiple-pass absorption cells are still used in high-sensitivity absorption spectroscopy, high-finesse optical cavities used for cavity-enhanced absorption spectroscopy (CEAS) offer several advantages, not only in terms of detection sensitivity, due to longer effective absorption path lengths. For example, the sample volume is much smaller allowing faster response and/or smaller sample flows. In some cases (such as in OF-CEAS), exploiting the resonance mode structure of an optical cavity provides an intrinsically linear frequency scale and well-defined sampling frequencies for the acquired spectra. On the other hand, coupling laser radiation into a high-finesse optical cavity is often difficult, due to the presence of spectrally narrow cavity resonances occurring at precisely defined optical frequencies. A particularly efficient scheme of CEAS relies on optical feedback (OF) from the cavity toward the laser to efficiently inject light into the cavity. An in-depth discussion of OF-CEAS and its application in a range of different fields appeared recently [10].
Recently, distributed-feedback interband cascade lasers (ICLs) operating single frequency close to room temperature started becoming commercially available. These sources, in particular when combined with high-sensitivity CEAS methods, offer new perspectives in applications to the measurement of traces or the analysis of variations of isotope ratios, in the gas phase. In fact, ICLs cover a mid-infrared spectral window (3–4 µm), in between those covered by Ga(InAs)Sb diode lasers and quantum cascade lasers (QCL), where strong molecular transitions can be found. ICLs have lower emission power than QCLs, but their thermal dissipation is also much lower and close to that one of telecom diode lasers. In addition, as we will see below, they feature a significantly wider current tuning coefficient. These are both major advantages for the above-cited applications.
We report here on the implementation of an interband cascade laser (ICL) in OF-CEAS, including a detailed description of the procedure allowing determination of the OF level needed for optimal performance of the technique. At the end, we demonstrate the application to SO2 trace analysis, down to ppbv (part per billion in volume) concentration levels. With respect to a previous ICL–OF-CEAS proof-of-concept experiment using a cavity of similar finesse [11], the demonstrated minimum detectable absorption coefficient is about ten times better (7.7 × 10−9 vs. 7.1 × 10−8 cm−1) and is achieved with a 25 times shorter acquisition time (80 ms vs. 2 s).
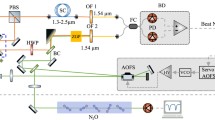
We implement a standard OF-CEAS scheme [12], illustrated in Fig. 1, very similar to the experimental arrangement used in our group with DFB diode lasers [12] and QCLs [13, 14], respectively, in the near-infrared (NIR) and in the mid-infrared (MIR). The V-shaped cavity geometry avoids direct back-reflection from the input cavity mirror, while enabling the return of part of the intracavity field toward the laser when its frequency goes through a cavity resonance. This OF induces a laser frequency locking and spectral narrowing, which results in the apparent broadening of the cavity resonances (modes) since the laser frequency remains locked for some time on each resonance during a frequency scan. The time during which this locking occurs clearly depends on laser tuning speed, but also on the amount of feedback. The latter is adjusted using a variable attenuator (here a polarizer, as shown in Fig. 1). Another important condition to be satisfied is that the OF wave should be in phase with the standing wave inside the laser cavity. This requires sub-wavelength control of the laser–cavity distance, in our case by a PZT-mounted mirror. All these points are detailed elsewhere [10, 13, 14].
OF-CEAS setup: lens L 0 (f′ = 4 mm) focuses the laser beam into the cavity composed of three mirrors (M 0, M 1, and M 2). The beam splitter (BS) is a wedged zinc selenide window. A polarizer is used as a variable attenuator (VA) to control the OF rate. Attenuators (A) avoid signal saturation of the photodiodes (PDref and PDsig) that monitor the laser power incident and transmitted by the cavity. The OF phase is controlled using the mirror mounted on a piezoelectric translator (PZT). P 0, P 1, P 2, and P 3 are the power of, respectively, the beam at the laser output, the beam at the cavity input, the reflection on the beam splitter, and the direct reflection on the coupling mirror, used for the OF rate measurement (see text)
The folded V-shaped cavity is 49 cm long (98-cm effective length), resulting in an FSR of 153 MHz. The cavity mirrors (LohnStar Optics) have been designed for a high reflectivity of 99.997 % at a central wavelength of 4.4 µm. At the laser wavelength near 4.015 µm, the reflectivity is 99.957 %, as deduced from the observed ring-down time of 3.9 µs measured with the cavity at atmospheric pressure. The corresponding finesse is 3900, and, as we confirmed experimentally, this remains unchanged at low pressure due to negligible atmospheric absorption around this wavelength.
As indicated above, the OF rate is adjusted by rotating the linear polarizer axis with respect to the laser polarization. Optimal OF rate for OF-CEAS is obtained when the apparent width of the cavity modes is slightly below their separation (inset in Fig. 2), so that there is negligible dead time between modes during which the laser light would not be injected into the cavity (or worse, when it could excite transverse modes, if no special efforts are dedicated to transverse mode matching). In our ICL setup, this optimum is achieved with a single-pass transmission of 12.5 % (1.6 % over the round trip). However, as we will see below, this does not give the OF coupling rate directly.
Top panel shows the OF-CEAS spectrum of laboratory air recorded in 80 ms and its fit at atmospheric pressure (respectively, black dots and red line). The green and blue solid lines give the HITRAN spectrum simulations at the cavity temperature of 296 K for CO2 at 1500 ppmv and N2O at 315 ppbv, respectively. The baseline of the OF-CEAS spectrum (derived from the fit) was subtracted. The inset in the upper-right corner shows the photodetector signals during a small section of the scan monitoring the light transmitted by the cavity (in black) and the incident laser power (in blue). The bottom panel shows the residuals of the spectral fit to the data. The standard deviation of the fit is 7.7 × 10−9 cm−1
The DFB-ICL used here was manufactured by Nanoplus GmbH. It is a single-mode laser with CW emission centered at 4.015 µm (2490 cm−1). Thermal regulation is provided by a Wavelength Electronics temperature controller (model LFI-3551) driving the Peltier cooler located inside the sealed laser case (TO66 header with TEC and thermistor, and anti-reflective coated window). The laser driving current is supplied by a low-noise ILX Lightwave current driver (model LDX-3620), operated on its internal battery. The wavelength can be tuned by temperature (0.4 nm/K) or by injection current (0.15 nm/mA). At an operating temperature of 5 °C, this ICL has an output power of 2.1 mW for a current of 64.8 mA. Compared with QCLs (quantum cascade lasers) that operate in the same spectral region, ICLs have lower operating voltage and threshold current (20 mA here, 20–50 times lower than that of a typical QCL), which makes them as easy to implement as telecom DFB diode lasers. Given the low power dissipation of ICLs, they can be operated at relatively low temperatures, such as 5 °C in our case, with modest thermal dissipation provided by a passive radiator. The slope efficiency (output laser power vs. injection current) is lower than for both diode lasers and QCLs (a factor 10 compared to QCLs), while the current and temperature tuning coefficient are much larger (more than 10 times for the current tuning coefficient [14]). These are both important advantages for applications in molecular spectroscopy, allowing fast acquisition of spectra with a uniform signal level over wider spectral regions.
A periodic current ramp from 50 to 70 mA produces scans over a spectral region of about 2 cm−1, which corresponds to about 400 spectral data points (Fig. 2). Such a modest current ramp procures therefore an observation span much larger than could be obtained with a QCL, even when allowing for a larger current ramp (e.g., with a 80 mA modulation, only 100 cavity modes could be current-scanned in [14]). In addition, the power change associated with the current ramp is smaller, resulting in a more uniform signal-to-noise ratio over an absorption spectrum. Furthermore, the incident laser power change induced by the OF is only 1 % as observed in the PD reference signal (inset in Fig. 2), against more than 10 % observed for a QCL [13].
Real-time spectra are then acquired at a repetition rate of 12 Hz (80 ms per laser frequency scan). A data acquisition card (NI USB-6251) is used for the recording of signals from the two photodiodes PDref and PDsig providing the laser power and the cavity output signal (photodiodes VIGO PVI-2TE-4, operated at room temperature, without thermoelectric cooling). These signals are analyzed in real time using LabVIEW software on a PC. The conversion of the signals to an absorption spectrum is explained in [15] and uses a cavity finesse calibration based on a single ring-down event on the last cavity mode in each scan. Note that the OF-CEAS spectral data points are perfectly equidistant in frequency, with the spacing between adjacent points equal to the free spectral range of the V-shaped cavity. Therefore, the frequency axis of the recorded spectra is intrinsically linear. Experimental data of each acquired OF-CEAS spectrum may be analyzed by a fitting routine in order to obtain trace concentrations, as we previously demonstrated at different occasions (see, e.g., [12, 14, 15]). All absorption lines are then fitted with a Rautian profile, of which we fix the Doppler width to its theoretical value, leaving the Lorentzian width and the Dicke narrowing parameter to be determined in the fitting process.
Before presenting the results on trace analysis, we first consider how to evaluate the feedback coupling rate in an OF-CEAS setup. Given the imperfect mode matching and the various optical losses inside and outside the cavity, this actually requires careful measurement of several experimental parameters. We start from the definition of the effective feedback rate, which is the power feeding back into the laser cavity normalized to the emitted power. Determination of this parameter is particularly interesting for the modeling of the laser–cavity coupled system, which however we will not pursue here. Laser feedback models [16] usually imply a simplification by neglecting the transverse beam characteristics. In reality, we have to consider that imperfect transverse mode matching to the optical cavity implies an effective power loss.
In the case of a V-shaped cavity, the feedback rate can be expressed as [10]:
In this expression, β corresponds to losses along the propagation to the optical cavity, principally by the attenuator and the beam splitter. This term is squared since the same losses are encountered on the backward path. Then, \(\epsilon_{00}\) represents the fraction of the laser beam coupled into the fundamental transverse cavity mode TEM00. This coefficient is, by definition, the square modulus of the superposition integral of the laser beam transverse profile multiplied by the cavity mode transverse profile [17]. This \(\epsilon_{00}\) term also appears squared because we have to take into account the transverse mode coupling a second time when the cavity TEM00 field propagates all the way back into the laser. Indeed, this field must be projected onto the laser mode, and the resulting superposition integral is the same as before, since this type of integral is invariant with respect to the position of the integration plane along the optical axis [17]. This holds if one can approximate the laser beam as a Gaussian mode. A more general argument that justifies the \(\epsilon_{00}^{2}\) term is the reversibility of a linear optical system: The transmission of an optical system non-including chiral interactions (magneto-optic effects) is the same when the direction of the optical field is reversed. We then apply this principle to the propagation from the laser waveguide to the high-finesse cavity, and back, for a wavelength resonant with a TEM00 mode. We should underline that for our choice of cavity geometry, the fundamental transverse mode is not degenerated with other transverse cavity modes. Finally, \(H_{{{\text{OF}},{\text{Max}}}}\) is the transfer function of the cavity for the backward direction taken at resonance, giving rise to the OF. In the following, we suppose that the cavity is filled with a non-absorbing medium, and that its 3 mirrors have all the same reflectivity (R), transmission (T) and loss (L) coefficients (with R + T + L = 1). Then, according to [10]:
In order to experimentally determine the feedback rate, we measure the power of the reflection from the wedged beam splitter (P 2) and the power of the direct reflection from the cavity input mirror (P 3) (see Fig. 1). Indeed, P 2 allows estimating the total power returning to the laser P ret , since the reflection (R BS) and transmission (T BS) coefficients of the wedged beam-splitter window are easily characterized from independent measurements (considering that the backward propagating beam from the cavity has the same polarization as the forward laser beam). In our case, to ensure the polarization does not change, we remove the polarizer and use only calibrated neutral density filters to obtain the optimal OF rate. We can write:
This measured power divided by the laser power output (P 0 in Fig. 1) accounts for the following product of previously defined factors:
Therefore, to obtain the OF rate, we still require to multiply this expression by \(\epsilon_{00}\) to include the backward injection efficiently to the laser cavity, as mentioned before. We determine this transverse mode coupling factor from a measurement of P 3 at resonance. The cavity transfer function of the direct reflection (from the front surface) of the cavity input mirror, \(H_{\text{DR,Max}}\), is completely determined by the cavity mirror R and T coefficients in a similar manner as \(H_{\text{OF,Max}}\):
However, the measured signal also depends on the transverse mode coupling factor \(\epsilon_{00}\):
where P 1 is the power at the cavity input (Fig. 1). The first term in the latest equation, proportional to \(\epsilon_{00}\), represents the cavity response to the mode-matched laser beam component, while the second term proportional to R is the non-mode-matched fraction of the laser power that is totally reflected (in the same direction) by the input cavity mirror. We neglected the reflection from the back surface of the cavity mirror, justified since the mirror is anti-reflection coated and slightly wedged. We use the fact that, in a perpendicular (transverse) plane, the laser beam can be written as a linear combination of the cavity fundamental mode and a term orthogonal to it (which is a sum over all the higher-order transverse cavity modes, not simultaneously resonant with the TEM00 mode, as noted above). The measurement of P 3 with a photodetector cannot distinguish these two (orthogonal) contributions and provides just their sum.
R and T coefficients are independently determined. R = 99.957(1) % was derived from a measurement of the empty cavity ring-down time, whereas T = 0.042(3) % was determined by comparing the photodiode signal from the laser beam at 4015 nm attenuated by one mirror with a signal of similar amplitude obtained by replacing the mirror by a series of calibrated neutral density filters. Here, R and T values are given with a one-sigma uncertainty specified by the quantity between brackets referring to the corresponding last digit.
According to Eq. (6), from the measurement of P 3 and P 1, and using the values of R and T to estimate \(H_{\text{DR,Max}}\), we estimate the transverse mode coupling factor for our setup: \(\epsilon_{00}\) = 26(2) %. Note that no special effort is made to optimize the mode matching; we simply use a single collimation lens L 0 adjusted to obtain a focal point at the center of the cavity.
As a result, the optimal OF rate needed here for OF-CEAS with an ICL is around 8.2 (1.2) × 10−5, which is close to the value that was estimated for OF-CEAS using a NIR DFB diode laser (\(\kappa\) ∼ 10−4–10−5) [12], but significantly lower than what appears to be needed with a QCL laser (\(\kappa\) ~ 10−3–10−4) [14].
Next, we consider some tests to assess the performance of our setup in view of applications in trace gas analysis. Figure 2 displays the OF-CEAS spectrum at 1 bar of ambient air mixed with human exhaled air, needed to enhance the CO2 concentration (by a factor of roughly 4), and allows the visualization of the weak atmospheric absorption lines present in this spectral window. The experimentally determined N2O concentration is ~315 ppbv, and thus representative of the atmospheric background (exhaled levels of N2O are typically very low [18]). The laser temperature is stabilized close to 5 °C; the 50–70 mA current ramp tunes the laser from 2489.6 to 2491.7 cm−1 as deduced from comparison of the recorded spectra with HITRAN simulations [1]. These are also shown in Fig. 2, with N2O and CO2 concentrations adjusted to match the experimental spectra. Given the low intensity of the absorption lines, the RMS noise of the residuals from the fit of this spectrum, shown in the bottom panel of Fig. 2, provides the baseline noise level of 7.7 × 10−9 cm−1 for a single 80-ms spectral scan.
As a second test, absorption lines of pure CO2 are recorded for different pressures inside the cavity (from 58 mbar to 200 mbar) as shown in Fig. 3. The maximum pressure is limited by the fact that at pressures above 200 mbar, cavity transmission becomes too small at the line centers and results in missing data points, as the data analysis software is no longer able to determine with sufficient precision the center and maximum transmission of the corresponding cavity modes. As shown in Fig. 3, the current ramp is set to a range 30 to 70 mA in order to span an even larger number of cavity modes (800), corresponding to a spectral window from 2489.3 to 2493.3 cm−1. According to HITRAN simulations, the stronger absorption lines observed here belong to the 16O13C18O isotopologue (natural abundance of ~44 ppm). The bottom graph of Fig. 3 illustrates the excellent linearity with pressure of both the Lorentzian half-width at half-maximum (HWHM) and the line area (obtained with the fitting procedure previously mentioned). This demonstrates that the absorption measurements are linear in the probed dynamic range (3 decades from the noise level up to about 10−5 cm−1), and also that there are no effects of saturation of the analyzed molecular transitions by the intracavity optical power. The line intensities and pressure broadening coefficients result in excellent agreement with the HITRAN simulation; the relative error between measurement and simulations is smaller than 1 % (comparison performed on the 2490.5 cm−1 line). We note that the laser scan repetition rate was reduced to 5 Hz to maintain a constant transit time per cavity mode (here 250 µs), which in OF-CEAS should be much larger than the cavity response time (the ring-down time, here 3.9 µs).
Top OF-CEAS spectra recorded in 200 ms showing line width broadening of absorption lines of the 16O13C18O isotopologue for different pressures inside the cavity (from 58 to 202 mbar). The baseline of the spectra is subtracted. The bottom graph represents the dependence on pressure of the Lorentzian width (HWHM) and the line area of the 2490.5 cm−1 line
Finally, we recorded the spectrum of SO2 at low concentration in the wavelength range of the laser, where this molecule possesses strong absorption lines (but still 2 orders of magnitude weaker than for the fundamental band at 7.5 µm). For our test, a small flow of 100 ppmv of SO2 in N2 at a total pressure of 95 mbar was pumped through the cavity. This mixture was supplied by PRAXAIR with a specified SO2 concentration accuracy of 2 %. The laser is tuned over 800 cavity modes as before. Combining 3 single-scan spectra recorded at laser temperatures of −1.7, 9.9 and 19.6 °C results in the spectrum of Fig. 4, spanning a full 9 cm−1 spectral range. When comparing with a HITRAN simulation, we have to use, in order to obtain the best match, a concentration of 95 ppmv, which is compatible with the cumulate errors of the gas mixture and the accuracy of the SO2 line intensities in HITRAN which is between 2 and 5 % for this spectral region.
OF-CEAS spectrum of SO2 compared to a HITRAN simulation at the experimental conditions (respectively, black dots and blue line). The baseline of the OF-CEAS spectrum has been subtracted to match the HITRAN simulation (95 ppmv of SO2 at a total pressure of 95 mbar). The inset in the upper-right shows an enlarged portion of the spectrum
In Fig. 5, we present two Allan-Werle standard deviation plots [19] for the SO2 mixing ratio: one obtained from short series of spectra recorded at 95 mbar for a flow of the 100 ppmv SO2 sample and the other for a flow of dry nitrogen. This last case allows to deduce a detection limit of about 6 ppbv of SO2 over 10 s averaging. The apparatus drift affects the effectiveness of averaging over longer time scales. On the other hand, it is clear that in the 100 ppmv case, the minimum detectable variations of the mixing ratio are larger due to fluctuations and noise that are at the 0.2 % level (0.2 over 100 ppmv) for one acquired spectrum, then drop only to 50 ppbv levels and display a rapid drift after more than 4 s averaging. However, given the complexity of the SO2 spectrum, we applied a line fit over a small clump of relatively isolated absorption lines around 2491.5 cm−1 taking up only 45 of the 750 spectral data points available in the spectral scan. This implies that we could still reduce the laser scan to cover only this region at a ~16 times faster repetition rate, or alternatively, that we could fit the whole acquired spectrum (quite a daunting task). In both cases, we expect that the additional data averaging improves the detection limit by a factor 4, leading to a SO2 detection limit close to 1 ppbv over 10 s averaging. This figure can be easily pushed below 1 ppbv by increasing the cavity finesse (to 10,000 as in [14]) or by averaging over longer times after implementing temperature and pressure stabilization of the instrument.
Allan-Werle variance plots showing the deviation of SO2 concentration for the 100 ppmv sample (black dots) and for dry N2 (white dots) with the integration time. The dashed blue line indicates a white noise behavior proportional to the integration time (1/√t). See text for more details and discussion
In conclusion, we confirm that ICLs are well behaved in the presence of spectrally filtered OF to achieve optimal performance in conjunction with the OF-CEAS technique. The fit residuals of an ambient air spectrum demonstrated a noise-equivalent absorption sensitivity (NEAS) of 2.2 × 10−9 cm−1 Hz−0.5, about 45 times better than obtained in a previous proof-of-principle experiment combining OF-CEAS with an ICL, with similar cavity finesse [11]. Given the ease of use, and particularly the low heat dissipation requirements of these lasers compared to QCLs, this represents an important progress for trace analysis applications in the mid-IR range. In addition, a complete spectral coverage is commercially available with wavelength-selectable ICLs operating near room temperature being proposed from 3 µm up to about 6 µm, thus nicely filling the spectral gap between conventional Ga(InAs)Sb-based diode lasers and QCLs. Finally, we show that ppbv-level sensitivity can be obtained in this spectral region for the selective measurement of sulfur dioxide, while previous applications to the detection of this molecule exploited its 30 times stronger absorption band around 7.4 µm [20].
References
L.S. Rothman, I.E. Gordon, Y. Babikov, A. Barbe, D. Chris Benner, P.F. Bernath, M. Birk, L. Bizzocchi, V. Boudon, L.R. Brown, A. Campargue, K. Chance, E.A. Cohen, L.H. Coudert, V.M. Devi, B.J. Drouin, A. Fayt, J.M. Flaud, R.R. Gamache, J.J. Harrison, J.M. Hartmann, C. Hill, J.T. Hodges, D. Jacquemart, A. Jolly, J. Lamouroux, R.J. Le Roy, G. Li, D.A. Long, O.M. Lyulin, C.J. Mackie, S.T. Massie, S. Mikhailenko, H.S.P. Müller, O.V. Naumenko, A.V. Nikitin, J. Orphal, V. Perevalov, A. Perrin, E.R. Polovtseva, C. Richard, M.A.H. Smith, E. Starikova, K. Sung, S. Tashkun, J. Tennyson, G.C. Toon, V.G. Tyuterev, G. Wagner, J. Quant. Spectrosc. Radiat. Transf. 130, 4 (2013)
N. Jacquinet-Husson, L. Crepeau, R. Armante, C. Boutammine, A. Chédin, N.A. Scott, C. Crevoisier, V. Capelle, C. Boone, N. Poulet-Crovisier, A. Barbe, A. Campargue, D. Chris Benner, Y. Benilan, B. Bézard, V. Boudon, L.R. Brown, L.H. Coudert, A. Coustenis, V. Dana, V.M. Devi, S. Fally, A. Fayt, J.M. Flaud, A. Goldman, M. Herman, G.J. Harris, D. Jacquemart, A. Jolly, I. Kleiner, A. Kleinböhl, F. Kwabia-Tchana, N. Lavrentieva, N. Lacome, L.H. Xu, O.M. Lyulin, J.Y. Mandin, A. Maki, S. Mikhailenko, C.E. Miller, T. Mishina, N. Moazzen-Ahmadi, H.S.P. Müller, A. Nikitin, J. Orphal, V. Perevalov, A. Perrin, D.T. Petkie, A. Predoi-Cross, C.P. Rinsland, J.J. Remedios, M. Rotger, M.A.H. Smith, K. Sung, S. Tashkun, J. Tennyson, R.A. Toth, A.C. Vandaele, J. Vander, Auwera. J. Quant. Spectrosc. Radiat. Transf. 112, 2395 (2011)
P.W. Werle, in Laser in Environmental and Life Sciences: Modern Analytical Methods, ed. by P. Hering, J.P. Lay, S. Stry (Springer, Berlin, 2004), pp. 223–243
K. Wörle, F. Seichter, A. Wilk, C. Armacost, T. Day, M. Godejohann, U. Wachter, J. Vogt, P. Radermacher, B. Mizaikoff, Anal. Chem. 85, 2697 (2013)
X. Cui, C. Lengignon, W. Tao, W. Zhao, G. Wysocki, E. Fertein, C. Coeur, A. Cassez, L. Croize, W. Chen, Y. Wang, W. Zhang, X. Gao, W. Liu, Y. Zhang, F. Dong, J. Quant. Spectrosc. Radiat. Transf. 113, 1300 (2012)
J.S. Li, W. Chen, H. Fischer, Appl. Spectrosc. Rev. 48, 523 (2013)
T. He, Z. Yang, T. Liu, Y. Shen, X. Fu, X. Qian, Y. Zhang, Y. Wang, Z. Xu, S. Zhu, C. Mao, G. Xu, J. Tang, Sci. Rep. 6, 22485 (2016)
A. Aiuppa, G. Giudice, S. Gurrieri, M. Liuzzo, M. Burton, T. Caltabiano, A.J.S. McGonigle, G. Salerno, H. Shinohara, M. Valenza, Geophys. Res. Lett. 35, 2004 (2008)
C. Oppenheimer, B. Scaillet, R.S. Martin, Rev. Mineral. Geochem. 73, 363 (2011)
J. Morville, D. Romanini, E. Kerstel, Sasdasas, in Cavity-Enhanced Spectroscopy and Sensing, ed. by G. Gagliardi, H.-P. Loock (Springer, Berlin, 2014), pp. 163–209
K.M. Manfred, G.A.D. Ritchie, N. Lang, J. Röpcke, J.H. van Helden, Appl. Phys. Lett. 106, 221106 (2015)
J. Morville, S. Kassi, M. Chenevier, D. Romanini, Appl. Phys. B 80, 1027 (2005)
G. Maisons, P.G. Carbajo, M. Carras, D. Romanini, Opt. Lett. 35, 3607 (2011)
P. Gorrotxategi-Carbajo, E. Fasci, I. Ventrillard, M. Carras, G. Maisons, D. Romanini, Appl. Phys. B 110, 309 (2013)
E.R.T. Kerstel, R.Q. Iannone, M. Chenevier, S. Kassi, H.-J. Jost, D. Romanini, Appl. Phys. B 85, 397 (2006)
P. Laurent, A. Clairon, C. Breant, IEEE J. Quantum Electron. 25, 1131 (1989)
D. Romanini, Appl. Phys. B Lasers Opt. 115, 517 (2014)
T. Mitsu, N. Kato, K. Shimaoka, M. Miyamura, Sci. Total Environ. 208, 133 (1997)
P. Werle, R. Miicke, F. Slemr, Appl. Phys. B 57, 131 (1993)
J.P. Waclawek, R. Lewicki, H. Moser, M. Brandstetter, F.K. Tittel, and B. Lendl, Appl. Phys. B 117, 113 (2014)
Acknowledgments
We acknowledge financing by the French Agence Nationale de la Recherche (Breath-Diag project: ANR-15-CE18-0006-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Richard, L., Ventrillard, I., Chau, G. et al. Optical-feedback cavity-enhanced absorption spectroscopy with an interband cascade laser: application to SO2 trace analysis. Appl. Phys. B 122, 247 (2016). https://doi.org/10.1007/s00340-016-6502-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-016-6502-0