Abstract
The broadband spectral conversion from near-UV absorption into near-infrared emission around 1 μm is reported in the ZnO–LiYbO2 hybrid phosphor, which is the benefit from the efficient energy transfer from ZnO to the Yb3+ ions that are specifically located at the interfacial diffusion regions between ZnO and LiYbO2, rather than those in LiYbO2 crystals. The Li+-related and Yb3+-related defect energy levels are formed inside the ZnO band gap in the ZnO–LiYbO2 hybrid phosphor; the former act as the quenching centers for the excitons in ZnO and meanwhile the efficient energy donors for Yb3+ ions, and the latter are responsible for the red shift of ZnO visible emission when the excitation energy is lower than E g. The excitation power dependence of Yb3+ emission intensities is measured to investigate the number of photons that are involved in the energy transfer process, which reveals that there are two channels for the sensitizing of Yb3+: One is due to the energy transfer by the recombination of electrons and holes, which is a cooperative energy transfer process, and the other is via the energy feeding from the Li+-related energy levels, which is a phonon-assistant energy transfer process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a green, sustainable, and ubiquitous energy, the sunlight has been widely explored to alleviate the increasingly serious energy crisis since no other energy supply could be as plentiful as 120,000 terawatts provided by the sun [1]. At present, the majority of commercial solar cells are silicon based out of hundreds materials that have been considered, such as oxides, organic molecules, and polymers, due to its advantages of non-toxicity, stability, and abundance. However, it has not yet been an economic one due to the low energy conversion efficiency in the photovoltaic (PV) devices. Due to the mismatch between the solar spectrum and the spectral response of Si, much of the solar energy in UV and IR regions cannot be efficiently utilized. According to Shockley and Queisser’s model, the theoretical efficiency limit for a single-junction solar cell with band gap of 1.1 eV is about 30 % [2]. One of the solutions to improve the PV conversion efficiency is to take full usage of the UV solar photons by “cutting” one incident UV or visible photon into two low-energy photons that can be effectively absorbed by silicon crystal [3–5].
Recently, the rare earth (RE) doped luminescent materials have been explored as spectral converters to improve the PV conversion efficiency of silicon solar cells [4]. It has been reported that the near-infrared quantum cutting in the RE3+–Yb3+ (RE=Pr, Nd, Tb, Ho, Er, and Tm) co-doped luminescent materials may improve the PV conversion efficiency of silicon solar cells by spectral modification [6–13]. In these RE ions co-doped systems, the Yb3+:2F5/2→2F7/2 transition gives off a broadband emission around 1 μm, which corresponds to the maximum spectral response range of the crystal silicon PV devices. However, in these co-doped phosphors, the energy absorption is governed by the 4f–4f parity forbidden transitions of trivalent RE ions, in which only a small portion of the solar spectrum was involved in the spectral conversion process. This is a big drawback for practical application. Effectively harvesting the solar energy in a wide wavelength range is essential for ultimately enhancing the solar cell PV conversion efficiency. One effort has been made is to use RE ions with f–d transitions as the sensitizers for the Yb3+ ions, such as Ce3+ and Eu2+ [14–17]. Meanwhile, the silicon–oxygen-related defects with broadband absorption in the near UV region were also used as the energy donors for the Yb3+ in glassy materials [18, 19].
In this work, the direct band gap semi-conductor of ZnO was selected as the energy sensitizer for Yb3+ ions in the ZnO–LiYbO2 hybrid phosphor, through which the broadband spectral conversion can be achieved due to the efficient energy transfer from ZnO to Yb3+. The structural and spectroscopic measurements were carried out to systematically study the mechanism that is responsible for the energy transfer process, which indicates that there are two channels for the energy feeding of Yb3+ ions. As an environmental friendly phosphor that can effectively harvest the UV–visible solar photons in a broad wavelength region and convert them into the near-infrared emission around 1 μm, the ZnO–LiYbO2 hybrid phosphor can be considered as a promising down conversion layer to enhance the PV conversion efficiency of silicon solar cells.
2 Experimental
The ZnO–LiYbO2 hybrid phosphor was synthesized using the solid-state reaction method in a weak reducing atmosphere. The starting materials were high purity ZnO, Yb2O3, and Li2CO3. 1 mol% Yb2O3 and 1 mol% Li2CO3 were mixed first and grounded for 20 minutes, then added to ZnO. The mixtures were ground for 2 hrs, and sintered at 1050 °C for 2.5 hours by putting the crucible filled with raw materials into a bigger graphite crucible. For comparison, pure ZnO powder, ZnO mixed with 1 mol% Yb2O3 or 1 mol% Li2CO3 were sintered at the same conditions. The LiYbO2 crystal was also prepared by sintering an equal molar percent of Li2CO3 and Yb2O3 mixture at 1050 °C for 2.5 hours in air. X-ray diffraction (XRD) profiles were obtained on a Rigaku D/MAX-RA diffractometer using a Cu target. The photoluminescence excitation (PLE) and photoluminescence (PL) measurements were carried out using a RF-5300PC (Shimadzu). The excitation power dependence of emission intensity was measured using a computer controlled measurement system including a xenon lamp (Asahi Spectra Co., Ltd., MAX-302), 350 and 400 nm band pass filters with bandwidth of 10 nm, a monochromator (Nikon, G250), a lock-in amplifier (NF Electronic Instruments LI-570A), and a Si photodiode (Electro-Optical System Inc., S-025-H).
3 Results and discussion
3.1 Structure by XRD measurement
The XRD profiles of the sintered materials are shown in Fig. 1. In the non-doped ZnO, all the diffraction peaks belong to the hexagonal wurtzite ZnO. In the mixture of ZnO and Yb2O3, the diffraction peaks consist of both hexagonal ZnO and cubic Yb2O3, and there is no detectable change in the peak positions for ZnO, which indicates that the Yb3+ ions cannot be doped into the ZnO lattice by the solid-state reaction method. While in the Li2CO3 and Yb2O3 co-mixed ZnO, the new diffraction peaks belonging to LiYbO2 phase were also observed, as shown in Figs. 1(c) and 1(d). According to our previous structural study [20], the LiYbO2 crystals were grown on the ZnO surface with the formation of diffusion interfaces, and thereafter called ZnO–LiYbO2 hybrid phosphor.
3.2 Origin of the intense Yb3+ emission
Figure 2 shows a group of PL spectra for the ZnO–LiYbO2 hybrid phosphor under the excitation of 350–400 nm with a wavelength step of 10 nm. All of the PL spectra consists of a broadband visible emission and an infrared emission around 1 μm. The former is due to the radiative recombination of the electrons from the conduction band edge with deeply trapped holes in the ZnO [21, 22], and the latter is originated from the Yb3+:2F5/2→2F7/2 transition. Interestingly, the peak position of the visible emissions shows a red shift from 510 to 550 nm with the increase of excitation wavelength when the excitation energy is lower than the band gap of the ZnO semiconductor (E g∼3.37 eV). The excitation wavelength dependence of the visible emission peak position is illustrated in the inset of Fig. 2.
The PLE spectra of these visible emissions at 510, 520, 540, and 550 nm, and the Yb3+ infrared emission at 986 nm were measured, as shown in Fig. 3. The PLE spectra for the visible emissions show a similar spectral profile, which is composed of a broad band in the near UV region and a smooth edge around 395 nm. The PLE spectra for Yb3+ infrared emission also show a similar broadband structure in the near UV region, however, a sharp peak at 395 nm. The similarity of the broad excitation band in the near-UV region indicates the energy transition from ZnO to Yb3+, however, the origin of the sharp excitation need to be a further investigation.
In order to get clear on the mechanism that is responsible for the strong Yb3+ infrared emission and the red shift of ZnO visible emission in the ZnO–LiYbO2 hybrid phosphor, the PLE and PL spectra were also measured in the non-doped, 1 mol% Yb2O3 single-mixed and 1 mol% Li2CO3 single-doped ZnO, respectively. The results are given in Fig. 4. The PLE spectrum of the visible emission in the non-doped ZnO consist of a broad band in the near-UV region and a sharp peak just below E g, which are corresponding to the intrinsic absorption and excitonic absorption of ZnO, respectively [23, 24]. In the Yb2O3 single-mixed ZnO, only the visible emission from ZnO can be detected and the PLE spectrum of the visible emission shows similar spectral profile with that of the non-doped ZnO. In contrast, in the Li2CO3 single-doped ZnO sample, the sharp excitation band for the visible emission due to the excitonic absorption is annihilated. The quenching of the sharp excitation band for ZnO visible emission has also occurred in the ZnO–LiYbO2 hybrid phosphor where the Li+ ions are included; however, which is dominated in the Yb3+ excitation spectra.
As we know, the introduction of alien ions into a crystal lattice produces defects. In both the Li2CO3 single-doped ZnO and the ZnO–LiYbO2 hybrid phosphor, the Li+ ions may occupy substitutional and interstitial positions in the ZnO host, and thus the Li+-related defect energy levels are formed in the band gap. The chemical defect formation due to Li+-doping mainly occurs as follows [25–27]:
Based on the observations in Figs. 2–4, the Li+-related defect energy levels may act as the quenching centers for the excitons in ZnO and meanwhile the energy donors for Yb3+ ions. It should be noticed that under the sub-band excitation of ZnO at 380 nm (∼3.26 eV), the emissions at 625, 653 and 758 nm specially appeared in the Li2CO3 single-doped ZnO, as shown in Fig. 4(c), which can be attributed to the radiative emissions from the Li+-related defects. In the ZnO–LiYbO2 hybrid phosphor, these Li+-related defect emissions are completely quenched, as shown in Fig. 2, indicating the Li+-related defect energy levels are very efficient energy donors for Yb3+ ions. Meanwhile, by comparing the PLE spectra for the Li+-related defect emissions and Yb3+ emission in Fig. 5, we can observe that the Yb3+ PLE spectrum envelops both of the excitation bands for the Li+-related defect emissions; this is another evidence for the energy transfer from the Li+-related defect energy levels to Yb3+ ions.
Moreover, we suggest that the strong infrared emission in the ZnO–LiYbO2 hybrid phosphor is due to the efficient energy transfer from ZnO to the small amount of Yb3+ ions that are incorporated in to ZnO lattice along with the Li+ ions at the interfacial diffusion regions, rather than the Yb3+ ions in the LiYbO2 crystals. This suggestion can be proved by comparing Yb3+ PL spectra in the ZnO–LiYbO2 hybrid phosphor and the pure LiYbO2 crystals under the direct excitation of Yb3+:2F5/2 energy level. As shown in Fig. 6, under the excitation of 937 nm LD, the ZnO–LiYbO2 hybrid phosphor shows intense Yb3+ infrared emission with the same spectral profile as indirectly excited ZnO with near-UV light (as shown in Fig. 2). In contrast, the LiYbO2 crystal shows much weaker Yb3+ emission with different spectral profile. Because the structure of Yb3+ PL spectrum is sensitively related to the local crystal field, the comparison in Fig. 6 indicates that the intense infrared emission in the ZnO–LiYbO2 hybrid phosphors is originated from the Yb3+ ions that are located at the interfacial diffusion region.
3.3 Shift of the ZnO visible emission peak position
Considering the incorporation of Yb3+ ions into ZnO lattice at the interfacial diffusion region, the Yb3+-related defect energy levels may be formed close to the conduction band edge, which can shallowly trap electrons. Comparing the electrons from the conduction band, the radiative recombination of the electrons from the Yb3+-related defect energy levels with the deeply trapped holes gives off lower-energy emission. As a result, when the excitation energy is lower than E g, the lower the excitation energy, the higher probability of the electron to be trapped by the Yb3+-related defect energy levels, therefore, the more the low-energy emission contributes to the overall visible emission. This leads to the red shift of the visible emission with the increase of excitation wavelength. While when the excitation energy is higher than E g, the electrons can be excited deep into the conduction band, so that the visible emission is due to the radiative recombination of the electrons relaxed to the conduction band edge with the deeply trapped holes, which gives off higher-energy emission with constant value.
3.4 Two channels for the sensitizing of Yb3+ in the ZnO–LiYbO2 hybrid phosphors
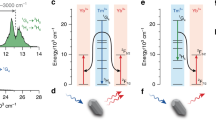
In order to investigate the number of photons that are involved in the energy transfer process, the excitation power dependence of Yb3+ emission intensity was studied under the excitation of intrinsic absorption of 350 nm and excitonic absorption at 400 nm, respectively, as shown in Fig. 7. The relationship between the emission intensity I and the excitation power P can be described as I∝P n, which means the fitted slopes in Log–Log scale indicates the number of photons that are involved in the energy transfer process, and the n=1 for one phonon process and n=0.5 for quantum cutting process [18, 28–30]. It can be observed from Fig. 7 that under the excitation of 350 and 400 nm the slope for excitation power versus Yb3+ emission intensity in Log–Log scale has a value of 0.59 and 0.89, respectively. We therefore suggest that there may be two channels for the energy sensitizing of Yb3+ ions, as schematically shown in Fig. 8. One channel is due to the energy transfer by the recombination of electrons and holes in ZnO, and the other is via the energy transfer from the Li+-related defect energy levels. When the electrons were excited into the conduction band or the Yb3+-related defect energy levels through intrinsic absorption, the recombination of electrons with deeply trapped holes will sensitize two Yb3+ ions simultaneously, which is a cooperative energy transfer process that leads to the “cutting” of one UV photon into two infrared photons. This suggestion is also reasonable from the energy point of view, as the recombination energy is around 2.3 eV, which is twice the energy of Yb3+:2F5/2 excited state. While when the excitation energy is low, the excitons will be created in the ZnO host. The exciton annihilation energy trapped at the Li+-related energy levels can excite one Yb3+, this is a phonon-assistant energy transfer process.
4 Conclusions
The ZnO–LiYbO2 hybrid phosphor was synthesized by the solid state reaction in a weak reducing atmosphere, in which the broadband spectral conversion from near-UV to near-infrared can be achieved due to the efficient energy transfer from ZnO to those Yb3+ ions that are located at the interfacial diffusion region. Due to the introduction of Li+ ions and small amount of Yb3+ ions, the Li+-related and Yb3+-related defect energy levels were formed in the ZnO band gap. The former act as the quenching centers for excitons and meanwhile the efficient energy donors for the Yb3+ ions, while the latter are responsible for the red shift of ZnO visible emission when the excitation energy is lower than E g. The excitation power dependence of emission intensity reveals that there are two mechanisms that are responsible for the energy transfer from ZnO to Yb3+: the cooperative energy transfer process and the phonon assistant energy transfer process. This research revealed a new method to realize the broadband spectral conversion by selecting the direct band gap semi-conductor of ZnO as the energy sensitizer for Yb3+ ions, which may benefit the improvement of silicon solar cell PV conversion by spectral modification.
References
O. Morton, Nature 443, 19 (2006)
W. Shockley, H.J. Queisser, J. Appl. Phys. 32, 510 (1961)
C. Strumpel, M. Mccann, G. Beaucarne, V. Arkhipov, A. Slaoui, V. Svrcek, C.D. Canizo, I. Tobias, Sol. Energy Mater. Sol. Cells 91, 238 (2007)
B.M. van der Ende, L. Arats, A. Meijerink, Phys. Chem. Chem. Phys. 11, 11081 (2009)
W. Shockley, H.J. Queisser, J. Appl. Phys. 32, 510 (1961)
P. Vergeer, T.J.H. Vlugt, M.H.F. Kox, M.I. Den Hertog, J.P.J.M. Van der Eerden, A. Meijerink, Phys. Rev. B 71, 0114119 (2005)
S. Ye, B. Zhu, J.X. Chen, J. Luo, J.R. Qiu, Appl. Phys. Lett. 92, 141112 (2008)
S. Ye, B. Zhu, J. Luo, J.X. Chen, G. Lakshminarayana, J.R. Qiu, Opt. Express 16, 8989 (2008)
Q.Y. Zhang, G.F. Yang, Z.H. Jiang, Appl. Phys. Lett. 91, 051903 (2007)
X.F. Liu, Y.B. Qiao, G.P. Dong, S. Ye, B. Zhu, G. Lakshminarayana, D.P. Chen, J.R. Qiu, Opt. Lett. 33, 2858 (2008)
L. Aarts, B.M. van der Ende, A. Meijerink, J. Appl. Phys. 106, 023522 (2009)
D.Q. Chen, Y.L. Yu, H. Lin, P. Huang, Z.F. Shan, Y.S. Wang, Opt. Lett. 35, 220 (2010)
H. Lin, D.Q. Chen, Y.L. Yu, A.P. Yang, Y.S. Wang, Opt. Lett. 36, 876 (2011)
J.J. Zhou, Y.X. Zhuang, S. Ye, Y. Teng, G. Lin, B. Zhu, J.H. Xie, J. R Qiu, Appl. Phys. Lett. 95, 141101 (2009)
J.J. Zhou, Y. Teng, S. Ye, Y.X. Zhuang, J.R. Qiu, Chem. Phys. Lett. 486, 116 (2010)
J. Ueda, S. Tanabe, J. Appl. Phys. 106, 043101 (2009)
D.Q. Chen, Y.S. Wang, Y.L. Yu, P. Huang, F.Y. Weng, J. Appl. Phys. 104, 116105 (2008)
S. Ye, B. Zhu, Y. Teng, G. Lakshminarayana, X.P. Fan, J.R. Qiu, J. Appl. Phys. 105, 063508 (2009)
S. Ye, B. Zhu, J. Luo, Y. Teng, J.X. Chen, G. Lakshminarayana, G.D. Qian, J.R. Qiu, Appl. Phys. Lett. 93, 181110 (2008)
S. Ye, N. Jiang, F. He, X.F. Liu, B. Zhu, Y. Teng, J.R. Qiu, Opt. Express 18, 639 (2010)
A. Van Dijken, E.A. Meulenkamp, D. Vanmaekelbergh, A. Meijerink, J. Lumin. 90, 123 (2000)
A. Van Dijken, E.A. Meulenkamp, D. Vanmaekelbergh, A. Meijerink, J. Lumin. 87–89, 454 (2000)
S. Shionoya, W.M. Yen, Phosphor Handbook (CRC Press, Boca Raton, 1998)
J.F. Muth, R.M. Kolbas, A.K. Sharma, S. Oktyabrsky, J. Narayan, J. Appl. Phys. 85, 7884 (1999)
M.G. Wardle, J.P. Goss, P.R. Briddon, Phys. Rev. B 71, 155205 (2005)
S. Majumdar, P. Banerji, Superlattices Microstruct. 45, 583 (2009)
A. Yavuz Oral, Z. Banu Bahsi, M. Hasan Aslan, Appl. Surf. Sci. 253, 4593 (2007)
W. Strek, A. Bednarkiewicz, P.J. Deren, J. Lumin. 92, 229 (2001)
J.J. Zhou, Y. Teng, X.F. Liu, S. Ye, X.Q. Xu, Z.J. Ma, J.R. Qiu, Opt. Express 18, 21663 (2010)
Y. Teng, J.J. Zhou, X.F. Liu, S. Ye, J.R. Qiu, Opt. Express 18, 9671 (2010)
Acknowledgements
This work was financially supported by the grant-in-aid program for young researchers of Kyoto-University Venture Business Laboratory, the JST PRESTO “Wavelength Conversion Material by Quantum Cutting toward Highly Efficient Photovoltaic Generation”, the National Nature Science Foundation of China Nos. 50802083 and 51072133, and the Fundamental Research Funds for the Central Universities (No. 2011KJ018). One of the authors (S.Y.) would like to sincerely thank Professor Jacques Lucas from University of Rennes for all the valuable discussions and suggestions. The work at Arizona State University is supported by DOE award DE-FG52-09NA29451.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, S., Tanabe, S., Jiang, N. et al. Broadband spectral conversion due to cooperative and phonon-assistant energy transfer from ZnO to Yb3+ . Appl. Phys. B 108, 553–558 (2012). https://doi.org/10.1007/s00340-012-4971-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-4971-3