Abstract
Metal carbonitride is a new type of tunable plasmonic materials and can be tuned by nitrogen and carbon content. In this work, zirconium carbonitride (ZrC\(_{x}\)N\(_{y}\)) thin films are prepared by dual ion beam deposition. The effects of C content and assisting ions on the structure and plasmonic properties of the films are studied. The results show that all the films are in B1-structure. C content increasing can reduce the shielding plasma frequency \(\hbar\) \(\omega\) \(_\textrm{c}\) and the carrier concentration of the film. Appropriate assisting ion beam energy \(E_\textrm{a}\) and current density \(J_\textrm{a}\) can promote the crystallinity of the film. As \(E_\textrm{a}\) and \(J_\textrm{a}\) increases, \(\hbar\) \(\omega\) \(_\textrm{c}\) increases initially and then decreases. The effects of the assisting ions can be attributed to the C content and the C-related defects, which is confirmed by the calculation of electronic states. The calculated density of state of the electrons shows that increasing C-substitute defects can decrease the threshold energy of interband transition, and the interstitial C defects lead to the similar effect. The study shows that metal carbonitride is a more tunable plasmonic material in visible and infrared region, and can also be modulated by the assisting ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, surface plasmon has been used in medical treatment [1, 2], photovoltaic devices [3], energy storage [4], optical fiber [5], sub-wavelength communication devices and surface coating [6, 7], and become one of the hot research areas of nano photonics [8,9,10]. Among plasmonic materials, gold and silver were favored because of their good stability, high negative permittivity, and low loss [11, 12]. However, their poor adjustability, high cost, and low melting point limit the practical application of gold and silver [13,14,15,16,17]. Therefore, it is necessary to find alternative plasmonic materials applied in some fields.
B1-structured transition metal nitrides have been regarded as an attractive plasmonic materials, owing to their excellent thermal stability and tunable optical properties from ultraviolet to infrared light [18]. Among them, TiN is the most widely studied [14, 16, 19, 20], and has been widely used in optical hyperbolic materials [21], perfect absorbers [22], sensor chips [23], and other fields. Other binary nitrides, mainly ZrN [24,25,26,27], have received increasing attention to meet various demands. Additionally, ternary nitride compounds have attracted more and more attention. Advantages that binary nitride could not reach have been obtained [28,29,30,31] in ternary nitride. By doping element and doping content, we can modulated the plasmonic properties of the materials in a wider region. Ternary nitride is usually metal-doped by alloying different metal in nitridization. Most of the nonmetal-doped ternary nitride are metal oxynitride [32,33,34,35]. In fact, carbon-doping is an effective way to get various properties and applications for nitride. Metal carbonitride is an effective coating for surface modification to promote the hardness and resistance to corrosion [36, 37]. Researchers prepared a series of metal carbide and ternary XCN (X = Ti, Zr) to study their properties [38,39,40,41]. About the optical and plasmonic properties of binary metal carbide, the materials are usually in MAX structure [42,43,44]. However, the plasmonic properties of cubic ternary XCN have not been studied extensively.
Most of the ZrCN samples reported so far are prepared by chemical vapor deposition [41], cathode arc deposition [38] and magnetron sputtering [39]. In this work, ZrC\(_{x}\)N\(_{y}\) films are prepared by dual ion beam deposition (DIBD), in which the growing films are bombarded by an assisting ion beam. The energy and current of the assisting ions can be used to affect the structure and characteristics of the deposited films. So DIBD gives more ways to modulate and optimize the properties of films.
In this work, the effect of carbon content and assisting ions on the crystal structural and plasmonic properties of the prepared films is investigated experimentally. The electronic states were studied by first-principle calculation to explain the experimental results. The results show that ZrC\(_{x}\)N\(_{y}\) is a potential tunable and applicable plasmonic materials in visible to infrared region, and DIBD is an effective method to prepare and modulated the plasmonic XCN films.
2 Experimental details
ZrC\(_{x}\)N\(_{y}\) thin films were prepared by a DIBD system, the schematic diagram of which is shown in Fig. 1. There are two Kaufman ion sources in this system, one of which serves as a sputtering source (\(\phi\) 8 cm) and the other as an assisting source (\(\phi\) 6 cm). The sputtering source is placed horizontally and used to generate sputtering Ar\(^{+}\) beam. The assisting ion source is placed at 45\(^{\circ }\) to the horizontal direction, and used to generate the assisting ion beam. The substrate is fixed to the top of the vacuum chamber and a target is placed below the substrate.
During the deposition, Zr atoms are sputtered from a 99.99% pure Zr target by an beam of argon ions. All films are deposited on \(1\times 1\) \(cm^{2}\) JGS1 SiO\(_{2}\) substrates. The background vacuum pressure is 8\(\times\)10\(^{-4}\) Pa and the working pressure is kept at 4.8\(\times\)10\(^{-2}\) Pa. The sputtering source is injected with a flow of Ar gas of 4 sccm to generate sputtering ions. The energy and current density of the sputtering beam were set to 800 eV and 1.2 mA/cm\(^{2}\). A mixed gas flow of Ar and N\(_{2}\) were supplied into the assisting ion source, in which the flow rate of Ar is 2.5 sccm, and the flow rate of N\(_{2}\) is a variable to get different nitrogen content in films. A flow of CH\(_{4}\) gas was supplied to the chamber to get the carbon doping. The substrate rotates at a constant speed during deposition to ensure the uniformity of the film. The thickness of all the films is 100 (±10) nm, which is detected with a quartz crystal oscillator. All films were deposited at room temperature.
We prepared three groups of samples, the parameters of which are shown in Table 1. To study the effect of carbon doping content, we changed the partial pressure ratio of CH\(_{4}\) r. r was set to 0/0.17/0.33/0.67/1.0, and the total flow rate of CH\(_{ 4}\) and N\(_{2}\) was keep constant of 3 sccm. The effect of assisting ion energy \(E_\textrm{a}\) and current density J\(_\textrm{a}\) were studied with r set at 0.17 in the other two groups. \(E_\textrm{a}\) was different and \(J_\textrm{a}\) was kept stable at 0.3 mA/cm\(^{2}\) in one group. While in the other group, \(J_\textrm{a}\) was different and \(E_\textrm{a}\) was maintained at 300 eV. As a contrast, a thin film was prepared without assisting ion bombardment (\(E_\textrm{a}\) = 0, \(J_\textrm{a}\) = 0) under the same condition.
The elemental composition of the film was determined by an energy X-ray dispersive spectroscopy (EDS) on a scanning electron microscope (SEM, Zeiss, SUPRA55) system under a vacuum of 1.0\(\times\)10\(^{-8}\) Pa. The crystal structure of the films were investigated by X-ray diffraction (XRD) with Cu–K\(\alpha _{1}\) radiation (\(\lambda\) = 0.15406 nm) as the diffraction source. The plasmonic properties of thin films are mainly reflected by the complex permittivity, which was measured by spectroscopic ellipsometry (smart SE 850 DUV) with an incident angle of 70\(^{\circ }\). All measurements were performed at room temperature.
3 Results and discussion
3.1 Effects of carbon doping
Table 2 shows the atomic composition of the ZrC\(_{x}\)N\(_{y}\) film deposited with different CH\(_{4}\) flow ratio r. It can be seen that both the ZrN\(_{x}\) and the ZrC\(_{x}\) film are metal-rich, especially ZrC\(_{x}\) film. The Zr content of the ZrC\(_{x}\)N\(_{y}\) film is lower than that of the binary films. As we expected, C content increases with r increasing. For the film of r=0.66, C content is lower than N content, even the CH\(_{4}\) flow is higher than N\(_{2}\) flow during the deposition, which is because that N atom is easier to combine with Zr atom.
Figure 2 shows the AFM images of the films deposited with different r. It can be seen that as r increases, the film becomes smoother. The roughness (\(R_\textrm{a}\)) of the films is 0.63, 0.62, 0.55, 0.43, and 0.37 nm for the films of r=0, 0.17, 1.33, 0.67, and 1, respectively. Roughness is related to grain size. The grains in the films prepared with higher r are smaller, which is consistent with the following XRD results. The AFM results reflect that N\(_{2}\) is easier to combine and crystalize with Zr atoms than CH\(_{4}\).
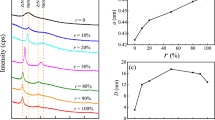
Figure 3a shows the XRD patterns of the films deposited with different r. The analysis of the XRD was based on JCPDS cards (35-0784 and 35-0753). The XRD results show that all the films are in B1-structure. The films have characteristic diffraction peaks of (111) and (200), and (200) peak is much weaker than (111) peak. So the films can be regarded as mainly (111)-oriented. The (111) characteristic diffraction peaks are located between the fcc-ZrC (111) peak and the fcc-ZrN (111) peak. With r increasing, the (111) peak gradually move towards ZrC (111), and the (200) peak shows similar behavior. No diffraction peak of other chemical phase appears, which indicates that in carbon-doped zirconium nitride, C atoms enter or fill the voids in the ZrN lattice, and even the N atoms in the original ZrN lattice will be replaced by C atoms. That is to say, the defects caused by carbon doping mainly exist in the form of various point defects. ZrN and ZrC are both B1-structured, and the radius of N atom and C atom is very close. Therefore, according to Hume-Rothery rule [45], ZrC\(_{x}\)N\(_{y}\) film can easily form a solid solution of cubic ZrN\(_{x}\) and ZrC\(_{x}\), in which the distribution of N atoms and C atoms has a certain uniformity. The final preferred orientation of thin films is the result of growth competition among grains with different alignment, and (111) alignment is obviously dominant in grain growth.
Figure 3b shows the lattice constants a of ZrC\(_{x}\)N\(_{y}\) films calculated by the Bragg equation. It can be seen that the lattice constant of ZrC\(_{x}\)N\(_{y}\) increases with r in the range of 4.66–4.77 Å, which is due to that a Zr–C bond is longer than a Zr–N bond.
The crystallinity of the film can also be reflected by grain size. Based on the full width at half maximum \(\beta\) of the (111) peak, the grain size D was calculated with Scherrer equation:
The D values in Fig. 3c shows that the grain size of the film is 28.7 nm without C doping, but decreases sharply to about 16 nm with r increasing. The grain size of ZrC\(_{x}\) is only about 12.6 nm. In fact, the crystallization of ZrC\(_{x}\) is more difficult than that of ZrN\(_{x}\) in the physical vapor phase deposition owing to the weaker oxidizability of C atoms. C atoms may replace the N atoms, and fill the interstitial space, and so prevent the crystallization and growth of grain.
Permittivities are the basis for the plasma materials. The permittivity of the films were determined by ellipsometry with an incident angle of 70\(^{\circ }\). The complex permittivity \(\varepsilon\)=\(\varepsilon\) \(_{1}\)+\(i\varepsilon\) \(_{2}\) is determined by the measured ellipsometric angles \(\Psi\) and \(\Delta\), and then fitted to Drude–Lorentz model with two Lorentz factors.
Figure 4 shows the complex permittivity of the films prepared at different CH\(_{4}\) flow rate r. Figure 4a describes the real part \(\varepsilon\) \(_{1}\) of the permittivity varying with the wavelength. For ZrC\(_{x}\)N\(_{y}\) films, the curves are positive at short wavelengths and negative at long wavelengths. This means that as the wavelength increases, the film turns from dielectric to metallic. In the range of negative \(\varepsilon\) \(_{1}\) 0, with the same \(\lambda\), the larger r is, the smaller the absolute value of \(\varepsilon\) \(_{1}\) is, and the less metallic the film is. The real part of permittivity of ZrC film (r = 1.0) exhibits a double near zero dielectric property in 200–900 nm, in which, \(\varepsilon\) \(_{1}\) transforms from dielectric to metallic and then to dielectric. The double near zero characteristics of the ZrC thin film possibly originates from the interband and intraband transition of electronic state, as reported in NbN [46], nonstoichiometric defects can also lead to different ENZ behavior.
Usually the frequency where permittivity crosses zero (\(\varepsilon\) \(_{1}\)=0) is used to define the screened plasma frequency, under which the total valence electron is partly shielded[47, 48]. Figure 4b shows the corresponding energy value \(\hbar {\omega }_{c}\) of the ZrC\(_{x}\)N\(_{y}\) when \(\varepsilon\) \(_{1}\)=0. Obviously, as r increases, \(\hbar {\omega }_\textrm{c}\) decreases. The behavior of \(\omega _\textrm{c}\) directly reflects the change of carrier concentration n, because \(\omega _\textrm{c}\) is directly proportional to plasma frequency \(\omega _\textrm{ps}\), and \(\omega\) \(_{ps}\) is directly proportional to the square root of carrier concentration n, as the following relation[47, 48]
Based on the results and the formula, it can be concluded that the carrier concentration n decreases with r, which is consistent with the previous discussion that \(\hbar {\omega }_\textrm{c}\) decreases with the increase of r at a fixed wavelength.
The free electrons in the conduction band of metallic Zr atom can be absorbed by the valence band of non-metallic atoms. The valence band of carbon atoms can absorb more free electrons than nitrogen atoms. So the increasing of doping C atoms reduces the carrier concentration and the \(\omega\) \(_\textrm{c}\) value. Additionally, the defects and grain boundary can also capture free electrons and reduce the carrier concentration, which make the films less metallic.
Figure 4c describes the imaginary part \(\varepsilon\) \(_{2}\) of the permittivity. Since \(\varepsilon\) \(_{2}\) is considered to be related to optical loss, it can be seen from the figure that in the near infrared region, a higher r will reduce optical loss. To further investigate the properties of the film, the energy loss of the film is calculated by the following formula [49]:
Figure 4d shows the energy loss spectra of films deposited with different r. It can be seen that the energy loss of the film decreases with C content increasing, which indicates that appropriate C doping of ZrN can effectively reduce the energy loss. With the increase of r, the peak energy loss moves slightly toward the long wavelength direction. The energy loss peak of pure ZrC is located in the ultraviolet region near 230 nm. The behavior of energy loss may be related to the interband transition.
3.2 Effects of assisting ions
Figure 5 shows the EDS results of the films as a function of assisting ion energy \(E_\textrm{a}\) and current density \(J_\textrm{a}\). It can be seen that \(E_\textrm{a}\) and \(J_\textrm{a}\) have obvious effect on the content, especially the carbon atom content of the films. For \(E_\textrm{a}\) of 0–300 eV, Zr content of the films is about 50\(\%\). However, for the \(E_\textrm{a}\) above 300 eV, the Zr content decreases with \(E_\textrm{a}\) increasing. This result can be attributed to the increase of implantation of N and C atoms at higher \(E_\textrm{a}\). Possibly, the optimal \(E_\textrm{a}\) for the combination and crystallization of ZrN is near 300 eV. So as \(E_\textrm{a}\) increases, N content increases initially and then decreases, and consistently, C content decreases initially and then increases. The effect of \(J_\textrm{a}\) on the content of the films in similar to, but much less than that of \(E_\textrm{a}\).
Figure 6a shows the XRD patterns of films deposited under different \(E_\textrm{a}\), in which we can see that all the films are fcc-structured. All the films show a preferred (111) orientation and some films show a weak (200) orientation. Obviously, the experimental environment is beneficial to the growth of (111) orientation of grain. The positions of characteristic peaks show that a solid solution of ZrN and ZrC formed. We can see that as \(E_\textrm{a}\) increases, the diffraction peak becomes stronger, but is weakened when \(E_{a}\) increases to 500 eV and 700 eV, which indicates that too high \(E_\textrm{a}\) is not conducive to crystal crystallization. Figure 6b shows the XRD patterns of the films deposited under different \(J_\textrm{a}\). We can see the films still exist as a solid solution of (111)-oriented fcc-ZrN and fcc-ZrC. With \(J_\textrm{a}\) increasing, the diffraction peaks gradually increases, but do not change much when \(J_\textrm{a}\) reach 0.5 mA/cm\(^{2}\). Obviously, \(E_\textrm{a}\) affects the crystallinity much more significantly than \(J_\textrm{a}\) does.
The alignment selection effect of the ions is mainly related to the preferential resputtering. The resputtering yield of assisting ions on the (111) plane is the lowest. Since (111) orientation has a higher surface energy [25]. The crystallinity enhancement can be attributed mainly to assisting ions promoting the mobility of adatoms. The energy and momentum of the assisting ions were transported to adatoms through collision, and the surface mobility of the adatoms is enhanced consequently. But the assisting ions with energy above 500 eV can cause more defects and depress the crystallinity. To some extent, with the increasing \(E_\textrm{a}\) and \(J_\textrm{a}\), annealing effect happened on the crystal. The effect of \(J_\textrm{a}\) on crystal is more similar to annealing and can promote the crystallinity. However, the effect of increasing \(E_\textrm{a}\) is mainly a kinetic process. High \(E_\textrm{a}\) with low \(J_\textrm{a}\) can cause significant damage to crystal through resputtering.
Figure 7a shows the real permittivity \(\varepsilon\) \(_{1}\) of ZrC\(_{x}\)N\(_{y}\) film prepared under different assisting ions. Panel (a) and (b) shows the results of \(\varepsilon\) \(_{1}\) as a function of \(E_{a}\) and \(J_\textrm{a}\) respectively. The results show the film turns from dielectric to metallic as wavelength increases. The screened plasma energy \(\hbar\) \(\omega\) \(_\textrm{c}\) values of the films are shown in Fig. 8. It can be seen that as \(E_\textrm{a}\) or \(J_\textrm{a}\) increases, \(\hbar\) \(\omega\) \(_\textrm{c}\) increases first and then decreases. Obviously, the effect of \(E_\textrm{a}\) on \(\hbar\) \(\omega\) \(_\textrm{c}\) is much more significant than that of \(J_\textrm{a}\).
Figure 9 depicts the imaginary part \(\varepsilon\) \(_{2}\) of ZrC\(_{x}\)N\(_{y}\) thin films prepared with different \(E_\textrm{a}\) and \(J_{a}\). Obviously, \(\varepsilon\) \(_{2}\) first decreases and then increases with the increase of wavelength. The inset depicts the details near the lowest point of \(\varepsilon\) \(_{2}\). It can be seen that with the increase of \(E_\textrm{a}\) or \(J_\textrm{a}\), the minimum of \(\varepsilon\) \(_{2}\) exhibits a slight red shift.
The variation of permittivity vs. \(E_\textrm{a}\) and \(J_\textrm{a}\) may be contributed to the element content and defects. It can be concluded that the experimental laws of \(\hbar\) \(\omega\) \(_\textrm{c}\) in Fig. 8 is related to those of C content in Fig. 5. As \(E_\textrm{a}\) or \(J_\textrm{a}\) increase, the \(\hbar\) \(\omega\) \(_\textrm{c}\) and carbon content initially increase and then decrease, but C content initially decreases and then increases. Furthermore, \(\hbar\) \(\omega\) \(_\textrm{c}\) reaches its maximum at 300 eV and 0.4 mA/cm\(^{2}\) at which C content arrives at its minimum. The influence of \(E_{a}\) is more significant than that of \(J_\textrm{a}\), because the C content fluctuates more drastically with \(E_\textrm{a}\) than with \(J_\textrm{a}\). As discussed above, C-doping promotes valence band and makes the films less metallic, which can also be shown in the calculation of the electronic states in the following section.
4 Electronic states
The dielectric properties of the films are related to their electronic structure. To study the mechanism of carbon doping and assisting ions affecting on the dielectric properties of ZrC\(_{x}\)N\(_{y}\) films, we calculated the electronic density of states (DOS) of ZrC\(_{x}\)N\(_{y}\) by first-principles calculations. The calculations were performed using the Perdew-Burke-Ernzerhof function under generalized gradient approximation (GGA-PBE) based on the CASTEP module in Materials Studio. The cutoff energy of the plane wave was set as 310 eV, and the SCF was set as 5\(\times 10\) \(^{-7}\) eV/ atom.
We first studied the electronic DOS of ZrC\(_{x}\)N\(_{y}\) with different C content in which only substitute doping exist. The calculation was based on crystal cells shown in Fig. 10. The lattice constants and atomic positions of ZrC\(_{x}\)N\(_{1-x}\) structure were optimized to make the lattice system reach the lowest energy to ensure accurate and effective calculation results.
As x in the ZrC\(_{x}\)N\(_{1-x}\) crystal cell gradually increases from 0 to 1, the calculated lattice constants range from 4.62 to 4.70 Å, which confirms the XRD results in Fig. 3 (4.66–4.77 Å). The calculated DOS are shown in Fig. 11. The Fermi level in the figure is set as zero. It can be seen that in the band structure of ZrN, there are three bundles of bands: N-2s bands around – 15 eV, hybridized bands of N-2p and Zr-3p regarded as valence bands, and the conductive Zr-4d bands across fermi surface, which is the main contribution of the metallic properties. It can be seen that the peak of DOS below Fermi level moves toward high energy as C content increases.
Plasmon resonance excitation is mainly due to the interaction between interband and intraband transitions [50]. The bands around the Fermi level in Fig. 11 are responsible for the metallicity (negative \(\varepsilon _{1}\)) in the long wavelength range. In this region, the partial coupling of the 2p states contributes little to absorption. At higher energy, interband transition from nonmetal 2p to Zr 4d will create a dielectric screening, which makes \(\varepsilon _{1}\) turn from negative to positive at \(\omega _\textrm{c}\) [50]. Above \(\omega _\textrm{c}\), the interband transition will make \(\varepsilon _{1}\) a small positive value [50].
The electron transitions can be indicated by the characteristic energy levels \(E_{0}\), \(E_{1}\) and \(E_{2}\) marked in Fig. 11 to a certain extent. At \(E_{0}\), the total DOS reaches its minimum, and the hybridized 2p bands begins to dominate the DOS. The values of \(E_{0}\) are approximatively equal to the \(\hbar \omega _{c}\) values in Fig. 4. So \(E_{0}\) can be regarded as the threshold energy of the dielectric contribution to the optical response. Obviously, \(E_{0}\) value decreases as C content increases, which is similar to the experimental laws discussed above. For ZrC, \(E_{0}\) is near fermi surface, which maybe leads to the dual epsilon-near-zero properties shown in Fig. 4. The characteristic energy \(E_{1}\) and \(E_{2}\) indicates the transition from the local maximum and the global maximum of the DOS of 2p bands to the fermi surface. Possibly, \(E_{1}\) and \(E_{2}\) are related to the local maximum of positive \(\varepsilon _{1}\) and \(\varepsilon _{2}\) in the short wavelength region (see Figs. 4, 7, and 9).
Besides the substitute doping, we calculated the DOS of the interstitial-C-doped ZrN. The calculation is based on the crystal cell of interstitial ZrNC\(_{0.25}\), and ZrN\(_{0.75}\)C\(_{0.5}\) with mixed (substitute and interstitial) doping, as shown in Fig. 12. The calculated corresponding DOS of ZrC\(_{x}\)N\(_{y}\) with interstitial and mixed doping are shown in Fig. 13. The DOS results of the undoped ZrN and substitute doping ZrN\(_{0.75}\)C\(_{0.25}\) are also shown as contrast. The results show that effect of interstitial and mixed doping on the DOS is similar to substitute doping. Compared to the DOS of ZrN\(_{0.75}\)C\(_{0.25}\), the valence bands of ZrNC\(_{0.25}\) and ZrN\(_{0.75}\)C\(_{0.5}\) shift more towards Fermi surface, and the characteristic energy are lower. There are possibly more interstitial C-related defects in the films prepared with high \(E_{a}\) and \(J_{a}\).
5 Conclusions
ZrC\(_{x}\)N\(_{y}\) films with different carbon and nitrogen content were prepared with DIBD, in which the chemical content were modulated by flow ratio of the CH\(_{4}\) and N\(_{2}\). The structure and plasma properties of the films, and the effect of assisting ions were studied. The electronic state were studied with first principle calculation.
The XRD results show that a solid solution of fcc-ZrN and fcc-ZrC was obtained. All the films are (111)-oriented. With the increase of the C content, the lattice constant increases, and the crystal grain size decreases. As the incident wavelength increases, all the ZrC\(_{x}\)N\(_{y}\) films turn from dielectric to metallic. With the increase of carbon content, the screened plasma frequency \(\hbar\) \(\omega\) \(_\textrm{c}\) and the carrier density n of the film decreases, and the maximum of the energy loss of the film shows a red shift. The energy \(E_\textrm{a}\) and current density \(J_\textrm{a}\) of assisting ion beam can give significant influence on the composition and plasmonic properties of the films. As \(E_\textrm{a}\) and \(J_\textrm{a}\) increases, \(\hbar\) \(\omega\) \(_\textrm{c}\) and the n of the film initially increase, and then decrease. The behavior of plasmonic properties with \(E_\textrm{a}\) and \(J_\textrm{a}\) varying is related to the atomic composition. After all, as \(E_\textrm{a}\) and \(J_\textrm{a}\) increases, the carbon content of the film initially decreases, and then increases. The mechanism of C-doping affecting the permittivity is confirmed by the electronic states. The calculation of electronic DOS shows that carbon doping elevates the N-2p band and lowers the interband transition energy, and so lowers the screened plasma energy. Additionally, the C-doping defect type is another factor affecting the properties of the films.
The study shows that metal carbonitride is a kind of more tunable plasmonic material in visible and infrared region, and DIBD is an effective method to prepare and modulate the metal carbonitride films.
References
T. von Woedtke, S. Reuter, K. Masur, K.D. Weltmann, Plasmas for medicine. Phys. Rep. 530, 291–320 (2013). https://doi.org/10.1016/j.physrep.2013.05.005
S. Bekeschus, P. Favia, E. Robert, T. von Woedtke, White paper on plasma for medicine and hygiene: Future in plasma health sciences. Plasma Process. Polym. 16, 1800033 (2018). https://doi.org/10.1002/ppap.201800033
H.A. Atwater, A. Polman, Plasmonics for improved photovoltaic devices. Nat. Mater. 9, 205–213 (2010). https://doi.org/10.1038/nmat2629
B. Anasori, M.R. Lukatskaya, Y. Gogotsi, 2D metal carbides and nitrides(MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017). https://doi.org/10.1038/natrevmats.2016.98
B. Li, F. Zhang, W. Liu, X. Chen, Y. Gao, F. Wang, X. Zhang, X. Yan, T. Cheng, An ultraviolet sensor based on surface plasmon resonance in no-core optical fiber deposited by Ag and ZnO film. Surf. Interfaces 31, 102074 (2022). https://doi.org/10.1016/j.surfin.2022.102074
D.D. Kumar, N. Kumar, S. Kalaiselvam, S. Dash, R. Jayavel, Wear resistant super-hard multilayer transition metal-nitride coatings. Surf. Interfaces 7, 74–82 (2017). https://doi.org/10.1016/j.surfin.2017.03.001
S. Veprek, M.J.G. Veprek-Heijman, Industrial applications of superhard nanocomposite coatings. Surf. Coat. Technol. 202, 5063–5073 (2008). https://doi.org/10.1016/j.surfcoat.2008.05.038
W.L. Barnes, A. Dereux, T.W. Ebbesen, Surface plasmon subwavelength optics. Nature 424(6950), 824–830 (2003). https://doi.org/10.1038/nature01937
Z.L. Yu Li, C. Chi, H. Shan, L. Zheng, Z. Fang, Plasmonics of 2D nanomaterials: properties and applications. Adv. Sci. 4, 1600430 (2017). https://doi.org/10.1002/advs.201600430
P.M.R. Yue-Jiao Zhang, X.-S. Zhou, H. Zhang, J.-L. Yao, J.-F. Li, Plasmonic core-shell nanomaterials and their applications in spectroscopies. Adv. Mater. 33, 2005900 (2021). https://doi.org/10.1002/adma.202005900
T. Chung, C.S.H. Hwang, M.-S. Ahn, K.-H. Jeong, Au/Ag bimetallic nanocomposites as a highly sensitive plasmonic material. Plasmonics 14, 407–413 (2018). https://doi.org/10.48550/arXiv.1806.10305
X. Fang, C.L. Mak, J. Dai, K. Li, H. Ye, C.W. Leung, ITO/Au/ITO sandwich structure for near-infrared plasmonics. ACS Appl. Mater. Interfaces 6, 15743–15752 (2014). https://doi.org/10.1021/am5026165
M. Bomers, A. Mezy, L. Cerutti, F. Barho, F. Gonzalez-Posada Flores, E. Tourni, T. Taliercio, Phosphonate monolayers on InAsSb and GaSb surfaces for mid-IR plasmonics. Appl. Surf. Sci. 451, 241–249 (2018). https://doi.org/10.1016/j.apsusc.2018.04.208
U. Guler, V.M. Shalaev, A. Boltasseva, Nanoparticle plasmonics: going practical with transition metal nitrides. Mater. Today 18, 227–237 (2015). https://doi.org/10.1016/j.mattod.2014.10.039
G.V. Naik, J. Liu, A.V. Kildishev, V.M. Shalaev, A. Boltasseva, Demonstration of Al:ZnO as a plasmonic component for near-infrared metamaterials. Proc. Natl. Acad. Sci. 109, 8834–8838 (2012). https://doi.org/10.1073/pnas.1121517109
G.V. Naik, J.L. Schroeder, X. Ni, A.V. Kildishev, T.D. Sands, A. Boltasseva, Titanium nitride as a plasmonic material for visible and near-infrared wavelengths. Opt. Mater. Express 2, 478–489 (2012). https://doi.org/10.1364/OME.2.000478
G.V. Naik, V.M. Shalaev, A. Boltasseva, Alternative plasmonic materials: beyond gold and silver. Adv. Mater. 25, 3264–3294 (2013). https://doi.org/10.1002/adma.201205076
P. Patsalas, N. Kalfagiannis, S. Kassavetis, G. Abadias, D.V. Bellas, C. Lekka, E. Lidorikis, Conductive nitrides: growth principles, optical and electronic properties, and their perspectives in photonics and plasmonics. Mater. Sci. Eng. R. Rep. 123, 1–55 (2018). https://doi.org/10.1016/j.mser.2017.11.001
L. Zhang, H. Liu, X. Suo, S. Tong, Y. Li, Z. Jiang, Z. Wang, Plasmonic properties of titanium nitride thin films prepared by ion beam assisted deposition. Mater. Lett. 185, 295–298 (2016). https://doi.org/10.1016/j.matlet.2016.08.147
L. Zhang, S. Tong, H. Liu, Y. Li, Z. Wang, Effects of sputtering and assisting ions on the orientation of titanium nitride films fabricated by ion beam assisted sputtering deposition from metal target. Mater. Lett. 171, 304–307 (2016). https://doi.org/10.1016/j.matlet.2016.02.100
G.V. Naik, B. Saha, J. Liu, S.M. Saber, E.A. Stach, J.M. Irudayaraj, T.D. Sands, V.M. Shalaev, A. Boltasseva, Epitaxial superlattices with titanium nitride as a plasmonic component for optical hyperbolic metamaterials. Proc. Natl. Acad. Sci. 111, 7546–7551 (2014). https://doi.org/10.1073/pnas.1319446111
J. Wang, W. Zhang, M. Zhu, K. Yi, J. Shao, Broadband perfect absorber with titanium nitride nano-disk array. Plasmonics 10, 1473–1478 (2015). https://doi.org/10.1007/s11468-015-9962-x
W.M. Kima, S.H. Kimb, K.-S. Leea, T.S. Leea, I.H. Kim, Titanium nitride thin film as an adhesion layer for surface plasmon resonance sensor chips. Appl. Surf. Sci. 261, 749–752 (2012). https://doi.org/10.1016/j.apsusc.2012.08.093
M.M. Larijani, M. Kiani, E. Jafari-Khamse, V. Fathollahi, Temperature dependence of the optical properties of ion-beam sputtered ZrN films. Appl. Phys. A 117, 1179–1183 (2014). https://doi.org/10.1007/s00339-014-8495-z
H. Lu, Y. Ran, S. Zhao, L. Jia, C. Gao, Q. Guo, Z. Jiang, F. Yang, Z. Wang, Effects of assisting ions on the structural and plasmonic properties of ZrNx thin films. J. Phys. D Appl. Phys. 52, 245102 (2019). https://doi.org/10.1088/1361-6463/ab10fe
A. Rizzo, M.A. Signore, L. Mirenghi, D. Dimaio, Deposition and properties of ZrNx films produced by radio frequency reactive magnetron sputtering. Thin Solid Films 515, 1486–1493 (2006). https://doi.org/10.1016/j.tsf.2006.04.012
D. Valerini, M.A. Signore, A. Rizzo, L. Tapfer, Optical function evolution of ion-assisted ZrN films deposited by sputtering. J. Appl. Phys. 108, 083536 (2010). https://doi.org/10.1063/1.3493267
L. Chen, Y. Ran, Z. Jiang, Y. Li, Z. Wang, Structural, compositional, and plasmonic characteristics of Ti-Zr ternary nitride thin films tuned by the nitrogen flow ratio in magnetron sputtering. Nanomaterials (Basel) 10, 829 (2020). https://doi.org/10.3390/nano10050829
H. Lu, Y. Ran, S. Zhao, Q. Guo, C. Gao, Z. Jiang, F. Yang, Z. Wang, Modulation of the plasmonic characteristics of Ti-Zr ternary nitride thin films by assisting ions. Appl. Surf. Sci. 505, 144579 (2020). https://doi.org/10.1016/j.apsusc.2019.144579
W. Tianrun, R. Yujing, L. Tingting, G. Qian, G. Chang, J. Zhaotan, W. Zhi, Plasmonic and electronic characteristics of (Zr, Nb)Nx thin films with different metal content. Appl. Phys. A (2022). https://doi.org/10.1007/s00339-022-05777-6
Y. Ran, H. Lu, S. Zhao, L. Jia, Y. Li, Z. Jiang, Z. Wang, Effects of substrate bias and temperature on the structure and dielectric properties of TiZrN ternary nitride thin films. Surf. Coat. Technol. 359, 258–264 (2019). https://doi.org/10.1016/j.surfcoat.2018.12.023
Jos D. Castro, M.J. Lima, S. Carvalho, Corrosion resistance of Cu-Zr(O)N films in a simulated seawater environment. Surf. Coat. Technol. 451, 129050 (2022). https://doi.org/10.1016/j.surfcoat.2022.129050
L. Braic, N. Vasilantonakis, A. Mihai, I.J. Garcia, S. Fearn, B. Zou, N.M. Alford, B. Doiron, R.F. Oulton, S.A. Maier, A.V. Zayats, P.K. Petrov, Titanium Oxynitride Thin Films with Tunable Double Epsilon-Near-Zero Behavior for Nanophotonic Applications. ACS Applied Materials & Interfaces 9, 29857–29862 (2017). https://doi.org/10.1021/acsami.7b07660
Q. Guo, T. Wang, Y. Ren, Y. Ran, C. Gao, H. Lu, Z. Jiang, Z. Wang, Plasmonic properties of nonstoichiometric zirconium nitride, oxynitride thin films, and their bilayer structures. Phys. Rev. Mater. 5, 065201 (2021). https://doi.org/10.1103/PhysRevMaterials.5.065201
L. Jia, H. Lu, Y. Ran, S. Zhao, H. Liu, Y. Li, Z. Jiang, Z. Wang, Structural and dielectric properties of ion beam deposited titanium oxynitride thin films. J. Mater. Sci. 54, 1452–1461 (2018). https://doi.org/10.1007/s10853-018-2923-y
E. Grigore, C. Ruset, X. Li, H. Dong, Zirconium carbonitride films deposited by combined magnetron sputtering and ion implantation (CMSII). Surf. Coat. Technol. 204, 1889–1892 (2010). https://doi.org/10.1016/j.surfcoat.2009.11.012
A. Kenzhegulov, A. Mamaeva, A. Panichkin, Z. Alibekov, B. Kshibekova, N. Bakhytuly, W. Wieleb, Comparative study of tribological and corrosion characteristics of TiCN, TiCrCN, and TiZrCN coatings. Coatings 12, 564 (2022). https://doi.org/10.3390/coatings12050564
M. Braic, V. Braic, M. Balaceanu, C.N. Zoita, A. Kiss, A. Vladescu, A. Popescu, R. Ripeanu, Structure and properties of Zr/ZrCN coatings deposited by cathodic arc method. Mater. Chem. Phys. 126, 818–825 (2011). https://doi.org/10.1016/j.matchemphys.2010.12.036
C.L. Chu, H.L. Ji, C. Guo, X.B. Sheng, Y.S. Dong, P.H. Lin, T. Hu, P.K. Chu, Surface nanomechanical behavior of ZrN and ZrCN films deposited on NiTi shape memory alloy by magnetron sputtering. J. Nanosci. Nanotechnol. 11, 11176–11180 (2011). https://doi.org/10.1166/jnn.2011.4051
R.W. Harrison, W.E. Lee, Processing and properties of ZrC, ZrN and ZrCN ceramics: a review. Adv. Appl. Ceram. 115, 294–307 (2016). https://doi.org/10.1179/1743676115Y.0000000061
Z. Qi, P. Sun, Z. Wang, Microstructure and mechanical properties of TiCN coatings prepared by MTCVD. Adv. Tribol. 404, 796–800 (2009). https://doi.org/10.1007/978-3-642-03653-8_263
J.H. Mokkath, Localized surface plasmon resonances and electric field confinement in titanium carbide (Ti3C2) MXene nanoclusters Phys. Chem. Chem. Phys. 23, 25807 (2021). https://doi.org/10.1039/D1CP03960A
M. Gonçalves, A. Melikyan, H. Minassian, T. Makaryan, P. Petrosyan, T. Sargsian, Interband, surface plasmon and fano resonances in titanium carbide (MXene) nanoparticles in the visible to infrared range. Photonics 8, 36 (2021). https://doi.org/10.3390/photonics8020036
Z. Jaksic, M. Obradov, D. Tanaskovic, O. Jaksic, D.V. Radovic, Electromagnetic simulation of MXene based plasmonic metamaterials with enhanced optical absorption. Opt. Quantum Electron. 52, 83 (2020). https://doi.org/10.1007/s11082-020-2227-8
W. Hume-rothery, Comments on papers resulting from Hume-Rothery’s Note-1965. Acta Metallur. 15, 567–569 (1967). https://doi.org/10.1016/0001-6160%2867%2990091-0
Y. Ran, H. Lu, S.A. Zhao, Q. Guo, C. Gao, Z. Jiang, Z. Wang, Stoichiometry-modulated dual epsilon-near-zero characteristics of niobium nitride films. Appl. Surf. Sci. 537, 147981 (2021). https://doi.org/10.1016/j.apsusc.2020.147981
G.V. Naik, J. Kim, A. Boltasseva, Oxides and nitrides as alternative plasmonic materials in the optical range. Opt. Mater. Express 6, 1090–1099 (2011). https://doi.org/10.1364/OME.1.001090
Y. Wang, A. Capretti, L. Dal Negro, Wide tuning of the optical and structural properties of alternative plasmonic materials. Opt. Mater. Express 5, 2415–2430 (2015). https://doi.org/10.1364/OME.5.002415
S. Adachi, M. Takahashi, Optical properties of TiN films deposited by direct current reactive sputtering. J. Appl. Phys. 87, 1264–1269 (2000). https://doi.org/10.1063/1.372006
A. Catellani, A. Calzolari, Plasmonic properties of refractory titanium nitride. Phys. Rev. B (2017). https://doi.org/10.1103/PhysRevB.95.115145
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.11774029) and the Fundamental Research Funds for the Central Universities(FRF-TP-22-144A1). We thank Ms Le Zhao of National Center for Nanoscience and Technology for the ellipsometry measurement.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, T., Ran, Y., Wang, T. et al. Structural, plasmonic and electronic properties of zirconium carbonitride thin films prepared by dual ion beam deposition. Appl. Phys. A 129, 453 (2023). https://doi.org/10.1007/s00339-023-06713-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06713-y