Abstract
In this article, a new configuration of MOX gas sensor was designed, fabricated, and characterized, with a particular design including a micro-heater and a sensing electrode on the same plane using uncomplicated and low-cost steps. A spiral form of the micro-heater and sensing electrode were fabricated in 5 mm × 5 mm surface on a Si/SiO2/Al-0.5Cu. Owing to its high sensitivity in gas sensing, the SnO2 layer was deposited on the top of the sensor. The performances of the fabricated sensor were performed by electrical characterization and sensing behavior under ethanol, LPG, and natural gas. The thermal characterization gave a TCR of 3.2 × 10−3 °C−1 for SnO2/Al-0.5Cu micro-heater. The evolution under gases of the typical device at different temperatures ranging from 218 to 280 °C and relative humidity from 40% to more than 80% showed high response, stability, and reproducibility. The selectivity of the fabricated sensor towards ethanol makes it a perfect candidate for ethanol sensing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the interest in gas sensing is still growing and opens a boundless field in the domain of research. There is a variety of these sensors used in several areas in daily life such as industry or domestic uses [1] fabricated with different technologies [2]. Sensor technology has become increasingly popular due to the demand for detection systems with high sensitivity and stability, at low cost and weight. Gas sensors are micro-technological products frequently used in the physical, chemical and biological fields to detect gases, or generally to monitor changes in the ambient gas atmosphere [3, 4].
Particularly, the MOX gas sensors are widely used due to their sensitivity, low cost, easy utilization and portability [5, 6], and they are manufactured with semiconductor and MEMS technologies. They provide many advantages such as the integration in intelligent systems [7], and the development of several matrix detection platforms on the same chip, to move towards the integrated electronic nose [2, 8]. These devices are sensitive to some toxic and combustible gases, such as CO, H2, CH4, C3H8, NO, NO2, H2S, and SO2 [3]. Moreover, they have been used successfully in climate, security, and medical equipment [9, 10]. The MOX gas sensors are developed with various processes on different substrates, such as semiconductor sensors on silicon, ceramic, and recently on flexible substrates [11,12,13].
It is well known that the working principle of the MOX sensors is based on the variation of their conductivity in the presence of gases. Their response depends on the nature and the concentration of the gas, and the type of the metal oxide used as sensitive layer. The structure of the metal–oxide semiconductor MOX sensors is essentially based on two important elements, which are the micro-hotplate and the sensitive layer material. The former is composed of electrodes for measuring the conductivity of the metal oxide and a heating platform for activating the sensitive layer towards detection [14]. The principal element of the platform is the heater that is electrically isolated from the electrodes using an insulating layer. The heater is required to be stable and uniform at high temperature for the stability of the sensor [15]. Several metallic materials have been used as the heating elements, such as platinum [16, 17], aluminum, copper [18], polysilicon [19], and nichrome [3, 19]. Whilst the sensitive layer materials interact with the target gas, and set the operating temperatures of sensors up to 800 °C [20].
Since the 70s [10], ZnO [4, 21, 22], SnO2 [4, 23,24,25,26], In2O3, TiO2, and WO3 [2, 4, 7] have been listed as the most sensing films for conductometric or chemiresistive gas sensors [27]. These materials were chosen for their ability to change their properties depending on the ambient gases. During the interaction of the sensitive layer with a reducing gas, like di-hydrogen, ethanol, and acetone, the first effect is the variation of its electrical resistance using n- or p-type materials. In contrast, when the sensitive layer is exposed to an oxidizing gas, like carbon dioxide, its electrical resistance increases for n-types and decreases for p-types [10]. SnO2 thin film is n-type semiconductor with a direct band gap (~ 3.6 eV). It is one of the most important materials used in several applications [28]. Recently, Tin oxide was widely used as a sensitive material in MOX gas sensor for detecting various gases due to its suitable physicochemical properties and lower cost [27,28,29,30,31,32,33]. In the present work, SnO2 was selected as the sensitive layer for the detection of vapors and combustible gases [23,24,25,26].
At present, the typical design of MOX gas sensor including heater and measurement electrodes is designed in two separated planes with a thin-film insulator such as SiO2, Si3N4 assembled on suspended membrane and integrated in a single chip [14, 15]. Unfortunately, they faced serious problems because of their complicated and expensive fabrication processes, such as chemical and reactive ion etching (RIE) process [28, 29], lift-off process [18], and physical deposition technique, such as evaporation, sputtering [32], and PECVD/LPCVD of different materials [33]. Another important problem is the material used for the fabrication of the micro heaters and sensors. The cost of manufacturing using these processes is in general very high.
Therefore, to reduce this problem, a particular design was proposed by including the micro-heater and the sensing electrodes on the same plane. This design can simplify the process by reducing its steps [22]. In this case, the process was simplified using one-step lithography.
In the present work, we propose a new and simple design of MOX gas sensor that combines the low-cost fabrication and the reduction of the process steps. Prior to utilization, the new fabricated device with the heating structure and sensing electrodes in-plane was presented. The structures were realized on oxidized Silicon and metal aluminum 0.5Cu issued from the Complementary metal–oxide–semiconductor (CMOS) metallization process. The sensitive layer SnO2 was deposited using spray pyrolysis method. This chemical process was advantageous in simplicity, low cost, and process yield [34]. The properties of our films were characterized by atomic force microscopy (AFM) and X-ray diffraction (XRD). The thermoelectric characterization of the micro-heater was also reported. Then, the experimental characterization of the device was investigated in the presence of ethanol and some domestic combustible gases, such as butane, methane, and propane. Besides, the influence of relative humidity (RH) on the gas response was examined.
2 Experimental details
2.1 Design and fabrication
In this work, we present a new device of MOX gas sensor and provide solutions to those problems encountered in its conception, to reduce the process steps and the cost of the manufacturing. The non-suspended co-planar micro-heater for gas sensor was designed and manufactured as shown in Fig. 1a, on Si/SiO2/Al-0.5Cu wafer by choosing the spiral shape with an area of 5 mm × 5 mm and a track width of 300 µm with a spacing of 150 µm, due to the uniformity and stability of the temperature at the sensor surface., [28]. Both the sensing electrode and micro-heater were in the same plane. The change in resistance of the gas-sensitive layer was assessed by the sensing electrode which allowed the conductivity variation induced by the change of the gas atmosphere. They are composed of two discontinuous antisymmetric pads; the sensitive layer has been deposited on top of the co-planar pad, to obtain electrical continuity. Firstly, the fabrication of the co-planar micro-heater and sensing electrode platform was based on Si wafer substrate with an electrically insulating layer of SiO2. The Al-0.5Cu metal was then deposited by MRC643 sputtering of the CMOS process with a thickness of 1 µm. The manufacturing process consisted of a single contact photolithography with a mask printed on a transparent mylar paper. It was thereafter transferred to the surface, using coated HIPR 6512 positive photoresist as a protective layer and patterned by UV lithography. The Al-0.5Cu micro-heater and sensing electrode pattern appeared after etching in PWS 80-16-4(65) Puranal (Aluminum Etching Solution), as shown in Fig. 1b.
2.2 Processing of sprayed sensing materials
The deposition of SnO2 was performed in ambient atmosphere using the spray pyrolysis process, with a concentration of 0.1 M at temperature 350 °C, using a starting transparent solution of thin chloride diluted in methanol (99.99% pure) as solvent. The distance between the substrate and the spray gun nozzle was fixed at 33 cm. Figure 1c illustrates the scheme of the spray pyrolysis apparatus for the synthesis of the SnO2 thin film on the co-planar platform the micro-heater and the sensing electrode pads were hidden with Kapton polyimide film, to protect them from the deposition of the SnO2 thin film. The scheme of the final device is displayed in Fig. 1d.
2.3 Microstructural characterization
The surface profile of co-planar platform was carried out using the Mechanical Dektak-IIa Profilometer. The measurement display ranges from 0.020 to 65.5 µm, and the scan length 0.050–30 mm were utilized, with the aim of determining the geometric parameters. The morphology of the synthesized SnO2 thin film was characterized and analyzed by AFM (JEOL JSPM 5200). The structural properties of the SnO2 was performed by XRD (D8 Advance Bruker, using the Cu Kα radiation; λ = 0.15406 Å).
2.4 Electrical characterization of micro-heater
The characterization of the micro-heater consisted in measuring the temperature produced on the surface as a function of the power supplied. This characterization allowed the determination of the maximum temperature, the current density, and the thermal stability of the elaborated device as a function of time. The electrical measurements were made in ambient air at temperature 19 °C. Simple measurement was performed by implementing two-wire method using the micro-heater pads for both voltage sense and induced current [23]. A DC power supply (MCP_M10 OPP3203) was connected to the heater pads. The digital multi-meter (GDM-8255A) was connected to the temperature sensor (type-k) to measure the temperature changes. After introducing the DC fixed current to the micro-heater pads, the micro-heater voltage was measured.
2.5 Gas testing
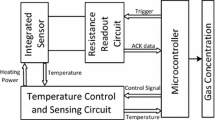
Gas detection tests were performed using a home-made bench top test chamber through a static approach, as illustrated in Fig. 2a. The gas sensor was placed in an upright position and in clean air.in a test chamber with a volume of 8.5 L. The gas inlet valve was fitted on the top of lid. The gas concentration inside the static system was achieved by injecting a known volume of the test gas inside the gas injecting syringe. The performances of the gas sensor when exposured to LPG, natural gas and ethanol vapor gas were investigated. To obtain a desired concentration of ethanol gas, a specific amount of liquid ethanol was evaporated in a 2.5 L container. Subsequently, this amount was injected into the test chamber. The LPG and natural gas were obtained from gas bottles. To ensure the preheating of the fabricated sensor, a DC current was introduced into the micro-heater pads by means of a programmable power supply. The sensing output (Vout) is then measured using a GDM-8255A digital multi-meter connected to the gas sensor. All tests were carried out in ambient atmosphere with an average relative humidity of 50% measured by the DHT21 humidity sensor. Vout was measured by a voltage-dividing circuit. The circuit used for powering the gas sensor as shown in Fig. 2b was set in series: the co-planar gas sensor resistance (RGas sensor) in series with the dividing resistance (RLoad = 10 kΩ). The test voltage (Vcc) was set to 5 V. The load resistance adjusted the stabilized voltage value Vout as suggested by Kim et al. [26]
According to the literature [1, 2, 35], the characteristics and performances of MOX gas sensor are heavily influenced by the temperature of the metal oxide thin film. The gas sensor operating temperature must be between 25 and 500 °C, to activate the adsorption phenomena. Our sensor was powered to reach a temperature lying in the range of 200–300 °C and kept constant in clean air for 40 min for stabilization before starting the detection. Once stable, the desired amount of gas will be injected. When the sensing output (Vout) became stable, the gas chamber was opened for recovering in air. This procedure was repeated for different gas concentrations, humidities, and temperatures. The gas sensor response (S) was estimated according to Eq. 2 [36]
where RAir and RGas sensor are the resistances measured from the sensor in clean and contaminated atmospheres, respectively.
3 Results and discussion
3.1 Mechanical profilometer Dektak-IIa
The surface profile characterization of our device was obtained using mechanical profilometer Dektak-IIa. The spiral micro-heater structure had tracks with 319 µm in width and 132 µm in spacing. As illustrated in Fig. 3a, the distances measured so far showed a widening of the tracks of few microns due to the impression of the mask on the mylar.
3.2 XRD analysis
The investigation of structural properties of the SnO2 was performed by XRD. The XRD pattern of SnO2 as sensitive layer deposited by spray pyrolysis technique is illustrated in Fig. 3b. This investigation can clearly reveal the formation of SnO2 nanoparticles with polycrystalline phase making in evidence the rutile structure compared to JCPDS Card No: 41-1445, a = b = 4.743 Å, c = 3.1859 Å. The crystal planes (110) are the most intense peak. Whilst, other peaks are also observed and assigned to (101), (200), (211), and (301) orientations indicating SnO2 thin-film formation. The highest density of the atoms can explain the highest intensity of the peak (110) in all planes of the rutile crystal structure; therefore, the surface energy of the (110) plane is the lowest. The crystallite size was determined using the Scherer’s formula
where K = 0.9, D is the crystallite size, λ = 0.15406 nm, the mean wavelength of Cu Kα radiation, and β is the full-width at half maximum (FWHM) of Bragg peak observed at Bragg angle θ (rad). The formula yields a calculated value of D equal to 13 nm.
3.3 AFM analysis
The surface morphologies of the SnO2 films were analyzed using AFM. The 3D-surface images showed the surface roughness variations of SnO2 with 0.1 M deposited on the top of the sensor shown in Fig. 3c, d. The surface roughness values Ra and Rq are 7.41 nm and 9.53 nm, respectively. It can be seen clearly from the XRD and AFM characterizations that the average particle size of SnO2 lies in the range of 8–13 nm.
3.4 Electrical characterization of the micro-heater
To study the stability of the micro-heater and the temperature produced, different currents were applied. The results are illustrated in Fig. 4a. The extracted resistance–temperature plots illustrated in Fig. 4b are well described by a first-order function, which corresponds to the typical temperature-dependent relationship for the resistance of metals [23]
where R and R0 are the heater resistances at temperature T and the room temperature T0 measured at the beginning of the experiment, respectively; TCR is the temperature coefficient of resistance at T. It is important to know the TCR value to estimate the micro-heater temperature based on the voltage applied. The experimental value of TCR for Al-0.5Cu was 3.2 × 10–3 °C−1. After determining the TCR coefficient of the material and using the voltage variations, the temperature at the surface of the micro-heater was calculated using Eq. 4. We find that both calculated and measured temperature values are in good agreement, as illustrated in Fig. 4c. The results show that the temperature of the micro-heater becomes approximately constant after 10 min.
3.5 Sensor response under gas testing
The performances of the fabricated sensor were studied at room temperature T = 19 °C and RH = 50%. Figure 5a shows the starting measurement voltage of our device. An increasing voltage value before stabilization can be observed, explaining the behavior of the material under oxygen atmosphere. The sensitivity of gas sensor depends on the reaction of the gases and operating temperature. In gas sensing, oxygen adsorption plays an important role in the electrical properties of the adsorption phenomena. Oxygen ion adsorption removes the conduction electrons and enhances the resistance of sensitive layer [1]. Figure 5b shows the gas sensing tests at different operating temperatures, from 218 to 280 °C. For C2H5OH, C3H8, C4H10 and CH4, the response value increases when the temperature increases from 218 to 256 °C. However, the response values become to decrease when the temperature increases further. The result confirmed that 256 °C was the optimal operating temperature of the fabricated sensor.
a The starting voltage measured across the load resistance at 218 °C, b calculated gas response of the fabricated sensor at 7000 ppm of C2H5OH C3H8, C4H10, and CH4 within the operating range of 218–280 °C c the sensing output (Vout) for different concentrations of C2H5OH C3H8, C4H10, and CH4 at 218 °C. d Repeatability of the fabricated sensor at different temperatures and ppm levels
Considering the energy consumption and materials used in the manufacture of the micro-heater. Also taking into account that the operating temperature for detecting reducing gases is higher, and increasing the power supplied to reach it can damage the device. We estimated that 218 °C could be the test temperature, to conserve our device.
Figure 5c shows transient response/recovery curves the fabricated sensor exposed to different concentrations of C2H5OH, C3H8, C4H10, and CH4 ranging from 700 to 300 ppm at an operating temperature of 218 °C. All these plots show that the sensor has fast response to all of the injected gases. Indeed, when gas was removed, the resistance of sensor returned back to the original state in function of time. The voltage increased and then the resistance decreased during the detection of C2H5OH and C3H8, C4H10, and CH4 gases because of the quasi-free electrons which increased the carrier concentration on the surface of sensitive materials SnO2. The response times were 30 s 39 s, 29 s and 35 s. Whilst, the recovery times were 76 s, 76 s, 62 s, and 82 s for C2H5OH, C3H8, C4H10, and CH4, respectively. These values indicate that the fabricated sensor show relatively short response and recovery times for all of the injected gases. The result confirms that our sensor is operational using the new configuration.
The dynamic response of the sensor exposed to two successive concentrations 7000 ppm and 1200 ppm of ethanol is shown in Fig. 5d. The mean value of the response was very stable and no visible deviation in amplitude was observed for three repeated cycles. The influence of relative humidity (RH) on the gas response was also examined from 40% to more than 80% RH at 218 °C under 7000 ppm of C2H5OH concentration, as illustrated in Fig. 6. The effect of the humidity variations on the baseline and the dynamic gas response showed a slight change.
3.6 Selectivity test
Sensitivity and selectivity are also important parameters. A selective sensor demonstrates its capability to detect the target gas in the presence of different gases. The selectivity of the fabricated sensor was investigated by exposing the sensor to different gases such as ethanol, C3H8, C4H10, and CH4. Figure 7 shows the response of the fabricated sensor at different operating temperatures towards 7000 ppm. It can be clearly seen that the sensor shows an optimal response to ethanol around 256 °C. The observed data suggest that the fabricated sensor exhibits temperature-dependent selectivity towards ethanol.
3.7 Stability test
Sensor response stability indicates the ability of the gas sensor to give the same results under the same conditions of gas detection concentrations. To test the long-term stability, the sensors response of the fabricated sensor was recorded in 8 days (20 days) with regular interval towards ethanol, C3H8, C4H10, and CH4 as shown in Fig. 8. The gas sensor exposed to different gas concentration shows a constant response, which confirms its stability.
4 Conclusion
This investigation describes the fabrication of a new configuration gas detection platform with simple design of MOX gas sensor combining low-cost manufacturing and reducing steps of process. This structure was produced on a silicon substrate with CMOS metal process. Typical design was used in which the heating structure and the sensing electrodes were fabricated on the same plane. The characterization of the micro-heater showed that depending on the supplied current, the temperature varied from ambient temperature up to 300 °C. The thermal characterizations gave a TCR of 3.2 × 10−3 °C−1 for SnO2/Al-0.5Cu micro-heater. The sensitive layer SnO2 was deposited on the top of sensing electrodes by chemical spray pyrolysis process. The device was tested under variation of ethanol, natural gas, and LPG. Besides, it was demonstrated that the evolution of the response of the typical device at different temperatures ranging from 218 to 280 °C and relative humidity (RH) from 40% to more than 80% showed higher response and reproducibility. The working temperature of our device started from 218 °C and the optimum temperature was 256 °C. The selectivity of the fabricated sensor towards ethanol makes it a perfect candidate for ethanol sensing. The manufactured sensor exposed to various gas concentrations presents a constant response, confirming its stability.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
A. Mirzaei et al., Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 126(24), 241102 (2019)
X. Liu, S. Cheng, H. Liu, S. Hu, D. Zhang, H. Ning, A survey on gas sensing technology. Sensors 12(7), 9635–9665 (2012)
S. Das, V. Jayaraman, SnO2: a comprehensive review on structures and gas sensors. Prog. Mater. Sci. 66, 112–255 (2014)
B. Firtat et al., Miniaturised MOX based sensors for pollutant and explosive gases detection. Sens. Actuators B Chem. 249, 647–655 (2017)
V.V. Krivetskiy, M.N. Rumyantseva, A.M. Gaskov, Chemical modification of nanocrystalline tin dioxide for selective gas sensors. Russ. Chem. Rev. 82(10), 917–941 (2013)
Y. Wang et al., Sputtered SnO2:NiO thin films on self-assembled Au nanoparticle arrays for MEMS compatible NO2 gas sensors. Sens. Actuators B Chem. 278, 28–38 (2019)
Z. Yuan, F. Yang, F. Meng, K. Zuo, J. Li, Research of low-power mems-based micro hotplates gas sensor: a review. IEEE Sens. J. 21(17), 18368–18380 (2021)
H. Liu, L. Zhang, K.H. Li, O.K. Tan, Microhotplates for metal oxide semiconductor gas sensor applications—towards the CMOS-MEMS monolithic approach. Micromachines 9(11), 1–24 (2018)
M. Righettoni, A. Amann, S.E. Pratsinis, Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 18(3), 163–171 (2015)
D. Zappa, V. Galstyan, N. Kaur, H.M.M. Munasinghe Arachchige, O. Sisman, E. Comini, Metal oxide -based heterostructures for gas sensors—a review. Anal. Chim. Acta 1039, 1–23 (2018)
J. Kroutil et al., Performance evaluation of low-cost flexible gas sensor array with nanocomposite polyaniline films. IEEE Sens. J. 18(9), 3759–3766 (2018)
M. Knoll, C. Offenzeller, B. Jakoby, P. Kulha, A. Laposa, W. Hilber, An inkjet printed ZNO based gas senor on a flexible high temperature substrate for no 2 sensing. In 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII) (2019), pp. 1250–1253
R. Alrammouz, J. Podlecki, P. Abboud, B. Sorli, R. Habchi, A review on flexible gas sensors: from materials to devices. Sens. Actuators A Phys. 284, 209–231 (2018)
S. Semancik et al., Microhotplate platforms for chemical sensor research. Sens. Actuators B Chem. 77(1), 579–591 (2001)
I. Das, R. Bhattacharyya, H. Saha, S. Ghosh, Enhanced response of co-planar MEMS microheater-based methane gas sensor. IEEE Sens. J. 20(23), 14132–14140 (2020)
Z.X. Cai, X.Y. Zeng, J. Duan, Fabrication of platinum microheater on alumina substrate by micro-pen and laser sintering. Thin Solid Films 519(11), 3893–3896 (2011)
D.S. Yoon et al., Precise temperature control and rapid thermal cycling in a micromachined DNA polymerase chain reaction chip. J. Micromech. Microeng. 12(6), 813–823 (2002)
P. Deekla, R. Phatthanakun, S. Sujitjorn, N. Chomnawang, Al microheater and ni temperature sensor set based-on photolithography with closed-loop control. Int. J. Electr. Comput. Eng. 5(4), 849 (2015)
M.G. Ahmed, J.O. Dennis, M.H.M. Khir, A.A.S. Rabih, Characterization of micro-heater and temperature sensor in micro-electromechanical system device for gas detection. In 2018 International Conference on Computer, Control, Electrical, and Electronics Engineering (ICCCEEE) (2018), pp. 1–6
R. Prajesh, N. Jain, A. Agarwal, Low power highly sensitive platform for gas sensing application. Microsyst. Technol. 22(9), 2185–2192 (2016)
Y. Bakha, Y. Djeridane, W. Aouimeur, L. Menasri, A. Smatti, S. Hamzaoui, ZnO based gas sensor testing. In 2014 9th International Design and Test Symposium (IDT) (2014), pp. 1–3
P.K. Basu, S. Benedict, S. Kallat, N. Bhat, A suspended low power gas sensor with in-plane heater. J. Microelectromech. Syst. 26(1), 48–50 (2017)
M. Baroncini, P. Placidi, A. Scorzoni, G. C. Cardinali, L. Dori, S. Nicoletti, Characterization of an embedded micro-heater for gas sensors applications. In 2001 International Symposium on VLSI Technology, Systems, and Applications. Proceedings of Technical Papers (Cat. No.01TH8517) (2001), pp. 164–167
M. Parthibavarman, V. Hariharan, C. Sekar, High-sensitivity humidity sensor based on SnO2 nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng. C 31(5), 840–844 (2011)
Q. Kuang, C. Lao, Z.L. Wang, Z. Xie, L. Zheng, High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 129(19), 6070–6071 (2007)
I. Kim, K.W. Seo, Ultra-thin filmed SnO2 gas sensor with a low-power micromachined hotplate for selective dual gas detection of carbon monoxide and methane. In 2017 Eleventh International Conference on Sensing Technology (ICST) (2017), pp. 1–5
C. Wang, L. Yin, L. Zhang, D. Xiang, R. Gao, Metal oxide gas sensors: sensitivity and influencing factors. Sensors 10(3), 2088–2106 (2010)
A. Sharma, B. Sharma, Influence of microheater patterns: MoSi2–SnO2 as energy-saving chemiresistors for gas sensing applications. Sens. Actuators B Chem. 351(October), 2022 (2021)
Q.-H. Wu, J. Li, S.-G. Sun, Nano SnO2 gas sensors. Curr. Nanosci. 6(5), 525–538 (2010)
J.-H. Yoon, J.-S. Kim, Study on the MEMS-type gas sensor for detecting a nitrogen oxide gas. Solid State Ion. 192(1), 668–671 (2011)
I.-S. Hwang et al., Gas sensing properties of SnO2 nanowires on micro-heater. Sens. Actuators B Chem. 154(2), 295–300 (2011)
R.E. Cavicchi, S. Semancik, F. DiMeo, C.J. Taylor, Featured article: use of microhotplates in the controlled growth and characterization of metal oxides for chemical sensing. J. Electroceram. 9(3), 155–164 (2002)
F. Samaeifar, A. Afifi, H. Abdollahi, Simple fabrication and characterization of a platinum microhotplate based on suspended membrane structure. Exp. Technol. 40(2), 755–763 (2016)
Y. Bakha, H. Khales, A. Tab, A. Smatti, S. Hamzaoui, in Structural and Optical Parameters of SnO2 Nanoparticle Deposited by Spray Pyrolysis BT—ICREEC 2019 (2020), pp. 335–341.
N. Yamazoe, Y. Kurokawa, T. Seiyama, Hydrogen sensitive gas detector using silver added tin (IV) oxide. Chem. Lett. 11(12), 1899–1902 (1982)
A.V. Shaposhnik, P.V. Moskalev, K.L. Chegereva, A.A. Zviagin, A.A. Vasiliev, Selective gas detection of H2 and CO by a single MOX-sensor. Sens. Actuators B Chem. 334, 129376 (2021)
Acknowledgements
This work was supported by the General Directorate for Scientific Research and Technological Development (Algerian Ministry of Higher Education).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Materials preparation, data collection, and analysis were performed by YB, SMM, HK, MK, and AD. The first draft of the manuscript was written by YB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakha, Y., Merah, S.M., Khales, H. et al. Development of new co-planar platform configuration of MOX gas sensor. Appl. Phys. A 129, 363 (2023). https://doi.org/10.1007/s00339-023-06647-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06647-5