Abstract
Tetragonal-phase BaTiO3 powders of particle size 370 nm were synthesized by microwave sintering at 850 °C. The raw materials were BaCO3, TiO2, and alanine. SiC microspheres were used as microwave conductors. The effects of the holding time, sintering aids, and SiC addition on the preparation of BaTiO3 were investigated. The results indicate that the addition of SiC as a microwave acceptor leads to formation of microwave micro-regions. This enables uniform heating of the raw materials and decreases the calcination temperature needed to obtain BaTiO3. Alanine coordinates with Ba, and this loosens the metal–CO3 bond and promotes separation of CO2, decreases the BaCO3 decomposition temperature, and provides a higher nucleation site density. It gives an idea about the microwave solid-state synthesis of BaTiO3 powder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Perovskite oxide has many properties, e.g., piezoelectric, dielectric, and ferroelectric activities [1,2]. BaTiO3 is an important perovskite structure material. It is used in multilayer ceramic capacitors (MLCCs), semiconductors, and electroluminescent panels [3,4]. The trend toward miniaturization of components in the electronics industry has increased interest in perovskite oxide nanopowders. For example, the fabrication of high-capacitance, small MLCCs requires the solid-state production of tetragonal-phase BaTiO3 nanopowders with small highly dispersed particles [5]. The development of methods for decreasing the particle size and improving the uniformity of the BaTiO3, while decreasing the synthesis temperature, is therefore a key issue.
BaTiO3 can be synthesized by sol–gel [6,7], solid-state [8,9], hydrothermal [10,11], coprecipitation [12], and microwave methods [13]. BaTiO3 prepared by liquid-phase methods has hydroxyl lattice defects, and this leads to MLCC porosity during sintering [14]. BaTiO3 has been synthesized by a solid-state method below 1000 °C, with BaCO3 and TiO2 as the raw materials [15]. Although solid-state methods are cheap and simple, the products have a large average particle size, high agglomeration, and poor chemical homogeneity, and are not suitable for use in miniaturized electronic devices [16]. However, BaTiO3 powder synthesized by a solid-state method is crystalline and has fewer surface defects than BaTiO3 prepared by liquid-phase methods. It has good dielectric properties, and its use ensures reliability of MLCCs. Companies such as Taiyo Yuden in Japan are therefore still developing improved solid-state methods for preparing tetragonal BaTiO3 with small particles. Ando et al. [17] tried to solve the problems associated with solid-state reactions by adding bovine serum albumin to decrease the decomposition temperature of BaCO3 in the presence of TiO2; this decreases the calcination temperature in BaTiO3 synthesis. Rui et al. [18] synthesized BaTiO3 via energy ball milling, which decreased the particle size, increased the uniformity of the raw materials, and decreased the reaction temperature. Microwave solid-state synthesis is a new method that has emerged in recent years. Gromov et al. [19] placed a high-purity graphite pellet under a crucible as a secondary acceptor to absorb microwave radiation, which decreased the calcination temperature, to obtain BaTiO3 powders. Rataro et al. [20] used ultrasonication and microwave irradiation, respectively, instead of classical ball mixing and synthesis steps, to obtain BaTiO3. The synthesis of highly dispersed BaTiO3 nanopowders is therefore a challenge.
In this work, we used a combination of a solid-state method and microwave irradiation to prepare tetragonal BaTiO3 from BaCO3, TiO2, and alanine as the raw materials. Alanine has C=O and > NH groups, which coordinate preferentially with the ligand field of Ba. This lowers the BaCO3 decomposition temperature and decreases the calcination temperature. Because BaCO3 and TiO2 are weak microwave acceptors, a secondary acceptor, namely SiC, was added. This additive must efficiently absorb microwave radiation, be chemically inert, decrease the synthesis temperature, and be readily separable after completion of the process. Unlike traditional solid-state methods, this method enables calcination below 900 °C and provides tetragonal BaTiO3. The product has promising applications in miniaturization of electronic devices.
2 Experimental
2.1 Sample fabrication
A BaTiO3 nanopowder was synthesized from commercially available BaCO3 (99.99%, Guangdong Fenghua Advanced Technology (HOLDING) Co., Ltd., Zhaoqin, China) and TiO2 (99.99%, Guangxi Jinmao Titanium Industry Co., Ltd., Wuzhou, China). A mixture of BaCO3 and TiO2 in water was ground by sand milling with 0.3 mm ZrO2 balls (BYZR-03, Shenzhen Chemical Boyi Industrial Co., Ltd., Shenzhen, China) at 2200 rpm for 6 h. The mixture was dried at 80 °C for 12 h. Then, 5 wt% alanine (99.99%, Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China) was added to BaCO3 and TiO2 in water under ball milling, and dried by spraying. SiC gravel (99.99%, Meiqilin New Materials Co., Ltd., Wuhan, China) was mixed with the spray-dried materials by mechanical stirring, and the mixture was transferred to a microwave furnace (HY-QS3016, Hunan Huaye Microwave Technology Co., Ltd., Hunan, China). The frequency was set at 2.45 GHz, and the mixture was heated to 850 °C at a rate of 30 °C/min and then held for 2 h. The temperature was measured with an infrared instrument. Figure 1 shows a schematic diagram of the interior of the microwave oven. The SiC was separated mechanically. Figure 2 shows a flow chart of the microwave synthesis of the BaTiO3 powder.
2.2 Characterization
The crystal phases of the BaTiO3 samples were investigated by X-ray diffraction (XRD; D8 Advance, Germany) with Cu Kα radiation and a step size of 0.02°/s in the 2θ range 20°–70°. Changes in the electronic states caused by alanine addition and milling were evaluated by X-ray photoelectron spectroscopy (XPS; Thermo ESCALAB250). Mass losses of the raw materials were investigated by thermogravimetry (TG). The sample microstructures were examined by field-emission scanning electron microscopy (FE-SEM; FEI Tecnai-450, USA). The BaTiO3 structure was examined by Raman spectroscopy (Rwlishes, France) and Fourier transform infrared (FTIR) spectroscopy (Nicolet 6800, The Netherlands). Impurity elements in BaTiO3 were identified by inductively coupled plasma atomic emission spectroscopy (ICP-AES; Varian, UK). The particle size distribution of the BaTiO3 powder was determined with a Mastersizer 2000 (UK).
3 Results and discussion
3.1 Effects on BaTiO3 microstructure of different holding times during microwave synthesis
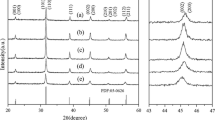
The effects on BaTiO3 of the weight percentage of SiC and holding time were investigated by synthesizing two groups of samples, group A and B, under different conditions; details are shown in Table 1. Figure 3 shows XRD and spectroscopic data for BaTiO3 nanopowders (group A) prepared by microwave solid-state sintering with different holding times. As the holding time increased from 0.5 to 4 h, the structure changed from cubic to tetragonal and the XRD peak intensities increased, as shown in Fig. 3a. For the samples prepared with holding times of 0.5 and 1 h, BaCO3 was detected and the patterns of the prepared BaTiO3 samples correspond to JCPDS card (31-0174). When the holding time was increased, the BaCO3 peak disappeared. The samples prepared with holding times of 2 and 4 h, i.e., S3 and S4, respectively, correspond to pure tetragonal BaTiO3 (PDF 75–0460). The BaTiO3 phases, i.e., cubic and tetragonal, are generally distinguished by the absence or presence in the XRD pattern of a double diffraction peak near 2θ \(\approx\) 45° [21]. Figure 3a shows that when the holding time was increased, the diffraction peak at 2θ \(\approx\) 45° eventually split into two peaks and became more apparent; these peaks correspond to the (200) and (002) planes of tetragonal-phase BaTiO3 [22]. This is because with increasing holding time, BaTiO3 undergoes a crystal-phase transformation. The lattice parameter a decreases and c increases, as shown in Table 1. Low-tetragonal cubic-phase BaTiO3 was obtained by composite-hydroxide-mediated synthesis, and tetragonal-phase BaTiO3 was obtained by solid-state synthesis. This suggests that the addition of SiC enabled uniform microwave sintering, which enables shortening of the holding time and promotes the phase transition to tetragonal. The tetragonality values of the group A samples (determined from the c/a ratio) are 1.0002, 1.0078, 1.0085, and 1.0093 for S1, S2, S3, and S4, respectively [23,24]. The content of the tetragonal phase was calculated by using the following formula [25]:
where θ1 and θ2 correspond to the (002) and (200) planes, respectively. Quantitative analysis of S1, S2, S3, and S4 by using the MDI Jade 5.0 software showed that the tetragonal-phase content increased from 18.2 (S1) to 97.3% (S4). The presence of BaCO3 in the samples was detected by FTIR spectroscopy; the spectra of BaTiO3 samples S1–S4 are shown in Fig. 3b. The spectra of S1 and S2 have two weak absorption peaks at 1439.11 and 858.69 cm−1; these verify the presence of BaCO3 [26]. The BaCO3 peaks gradually disappeared with increasing holding time. The FTIR spectra confirm the results obtained by XRD. Tetragonal-phase BaTiO3 can be obtained by increasing the holding time.
Figure 3c shows the Raman spectra of the BaTiO3 samples prepared with different holding times. The figure shows that the spectrum of the sample synthesized at 850 °C for 0.5 h contains no obvious peak from the BaTiO3 tetragonal phase, which indicates that it has no Raman activity [27]. When the cubic phase is transformed to the tetragonal phase, broad peaks appear near 260 and 520 cm−1; these are attributable to changes in the Ti4+ position in the cubic-phase BaTiO3 lattice and indicate Raman activity. The Raman peaks near 304 and 720 cm−1 are characteristic of tetragonal BaTiO3 [28]. As the holding time increases, the intensities of the Raman peaks at 304 and 720 cm−1 increase, which indicates increasing tetragonality of the BaTiO3 samples; this is confirmed by the XRD patterns.
Table 2 shows the particle sizes of BaTiO3 samples synthesized at 850 °C with different holding times. The synthetic process involves growth of BaTiO3 grains and a crystal transition from the cubic phase to the tetragonal phase. The solid-state reaction is not sufficient when the holding time is 0.5 h. When the holding time is too short, cubic-phase BaTiO3 is obtained. Laser particle size analysis of sample S1 shows that grain growth is not complete and BaCO3 impurities are still present in the product; this is consistent with the results shown in Fig. 3. With increasing holding time, the BaCO3 impurities disappear and grain growth is complete. The particle size of the BaTiO3 nanopowders increased linearly because of microwave micro-region sintering. The D50 values for S1, S2, S3, and S4 are 270, 300, 370, and 650 nm, respectively. The corresponding specific surface areas are 8.56, 5.93, 7.32, and 3.66 m2/g.
3.2 Effects of alanine sintering aid on BaTiO3
The solid-state reactions of TiO2 and BaCO3 can be represented by Eqs. (2) and (3) [29]:
Figure 4a shows that the TG curves do not reflect reactions (2) and (3). The sample weight loss when alanine was added was complete at 820 °C, and the sample weight loss without alanine addition was complete at 900 °C. This confirms that the experimental method involves reaction (2) almost entirely, because reaction (3) occurs above 1000 °C [30]. The thermal decomposition behaviors of alanine and mixtures of alanine with Ti and Ba inorganic compounds are compared in Fig. 4b. Complete decomposition of alanine occurs at 227 °C, but the differential TG curves of alanine mixed with the raw materials are multimodal; 38% of the organic matter was present up to 342 °C. The weight loss curve indicates that alanine decomposition occurs in stages; the portion that coordinates with the metal ions can survive to a higher temperature. In this study, the differential TG curves for alanine with BaCO3, TiO2, and a mixture of the two, show that weight loss begins at around 200 °C because the desorbed excess alanine does not directly coordinate with the metal species [31]; the details are shown in Table 3. The data in Table 3 show that the weight loss at around 200 °C for alanine–BaCO3–TiO2 equals the difference between those for alanine–BaCO3 and alanine–TiO2. This indicates that alanine can be adsorbed on the surfaces of BaCO3 and TiO2. The rest of the alanine is then combusted at around 280 °C for alanine–TiO2 and alanine–BaCO3–TiO2, and 332 °C for alanine–BaCO3. It can be assumed that a portion of the alanine is adsorbed or coordinated on the surfaces of BaCO3 and TiO2
Figure 5 shows the changes in the O1s and Ba3d binding energies after addition of alanine. Figure 5a shows that after alanine was added, the Ba3d peak shifted by 0.7 eV compared with the corresponding peak for BaCO3 without added alanine. This indicates that coordination of the added alanine with Ba2+ resulted in a decrease in the \({\text{CO}}_{3}^{2 - }\) bond energy. The O1s peak position and energy transfer after mixing TiO2 with alanine are not much different from those for TiO2. Figure 5b shows that the O1s binding energy of BaCO3 shifted by 0.5 eV after alanine addition [32]. These results indicate that alanine and Ba2+ play important roles in the process and confirm that alanine helps to lower the temperature of BaTiO3 synthesis.
3.3 Effects of SiC content on microstructures of BaTiO3 powders
When a dielectric material is placed in a microwave field, it couples with the microwave field and electromagnetic energy is converted to thermal energy. BaCO3 and TiO2 are weak microwave acceptors at room temperature, which results in hysteresis in the microwave heat transfer efficiency. A secondary acceptor, i.e., SiC, was added to improve the microwave heat transfer efficiency. The dielectric loss of SiC therefore determines the efficiency of heating of the raw materials. In this study, a SiC plate was added to the underside of a corundum crucible, and SiC gravel was mixed with the powders by mechanical stirring to form a BaCO3–SiC–TiO2–alanine symbiotic system, and a uniform temperature distribution was achieved in the microwave field. This promoted formation of small, uniform particles of tetragonal-phase BaTiO3. We found that without addition of SiC to the raw materials, microwave heating for 30 min to reach 900 °C and holding for 2 h gave BaTiO3, but the tetragonality was poor. When SiC was added to the raw materials, thermal runaway occurred and the heating rate increased. This can be attributed to the microwave absorption capacity of SiC. The power (P) absorbed by the dielectric material is calculated by using Eq. (4).
where electrical conductivity is denoted by \(\sigma\), the amplitudes of the electric and magnetic fields are |E| and |H|, respectively, the applied microwave frequency is denoted by \(\omega\), the dielectric constant and permeability of free space are denoted by \(\varepsilon_{0}\) and \(\mu_{0}\), respectively, and \(\varepsilon_{{\text{r}}}^{{^{\prime\prime}}}\) and \(\mu_{{\text{r}}}^{{^{\prime\prime}}}\) are the relative dielectric constant and the imaginary part of the permeability, respectively. BaCO3 and TiO2 have poor absorption properties. Equation (4) expresses the total energy absorbed by SiC. Consequently, BaTiO3 was prepared in a short time, and the synthesis temperature was 850 °C, which is lower than the phase transition temperature (900 °C). However, the internal temperature of the sample can be higher than 850 °C because of the microwave heating mechanism. The core temperature of the raw materials cannot be directly measured because there is a distance between the sample and the infrared thermometer, and only the surface temperature can be measured. It can be assumed that the core temperature of the sample was higher than the phase transition temperature of BaTiO3.
In the experiments, BaTiO3 was prepared by microwave solid-state synthesis at 850 °C, but BaCO3, TiO2, and alanine are poor microwave acceptors, therefore, a secondary acceptor, namely SiC, was added. In this study, the effects on the sample properties of the amount of added SiC were investigated; the details are given in Table 1. Figure 6 shows the XRD patterns of the samples in group B, which were synthesized at 850 °C for 2 h with different SiC contents. The BaTiO3 sample prepared without added SiC is cubic phase and corresponds to JCPDS card (31-0174). When the SiC content was increased, the XRD patterns show that the obtained samples (S6–S8) correspond to BaTiO3 (JCPDS No. 75-0460). These results indicate that with increasing SiC content from 0 to 25 wt%, the tetragonality values of the synthesized BaTiO3 (group B samples) increased from 1.0001 to 1.0091. This is in agreement with the Raman spectroscopic results.
Figure 7 shows SEM images of BaTiO3 samples prepared at 850 °C for 2 h with different SiC contents. Figure 7a shows that the cubic-phase BaTiO3 sample, which was prepared without SiC, is severely agglomerated, with an average particle size of 240 nm. The particle size of the BaTiO3 powder prepared with 10 wt% SiC is not uniform. The particle size varies from 300 to 600 nm, and the average size is 510 nm. These results show that the SiC was not well mixed with the raw materials and thermal runaway during heating led to abnormal grain growth. Figure 7c shows that the powder particles prepared with 20 wt% SiC are rectangular with good dispersibility and an average size of 370 nm. Unlike traditional solid-state methods, microwave irradiation gives a rapid temperature rise and uniform heating. This leads to rapid crystal nucleation and shortens the crystallization time. When SiC as a secondary acceptor is mixed with the powder, a micro-region heat source center is formed under the action of the microwave field, and uniform, rapid heating of the powder is achieved. The impurity content in sample S7 was determined by ICP-AES; the results are shown in Table 4. The results show a Ba/Ti ratio of 1.005:1 and no SiC impurities. Comparisons with the compositions of the raw materials shows that the impurities CaO, SrO, and MgO are derived from BaCO3 and TiO2, and the impurity SiO2 is derived from SiC. The prepared samples meet the MLCC manufacturing requirements that are stipulated in the MLCC production manual. Figure 7d shows a SEM image of the sample prepared with 25 wt% SiC. The image shows that the average particle size of the sample is about 920 nm. The statistical distributions of the sample particle sizes are shown in Table 5; D10, D50, and D90 are the particle sizes corresponding to cumulative particle size distributions of 10%, 50%, and 90%, respectively. The specific surface areas were 8.13, 3.64, 7.32, and 1.87 m2/g for the samples prepared with SiC contents of 0, 10, 20, and 25 wt%, respectively. The average particle size of BaTiO3 increased with increasing SiC content. This is ascribed to the effect of the SiC content on microwave absorption. When the SiC content is low, the raw materials are not uniformly heated, and particle agglomeration is severe. When the SiC content is increased, thermal runaway occurs, and the average particle size is around 1 μm. This shows that if the SiC content is too high, side effects occur.
4 Conclusions
A BaTiO3 powder of high tetragonality, with c/a = 1.0085, Ba/Ti = 1.005, and an average particle size of 370 nm was synthesized by microwave micro-region sintering at 850 °C for 2 h. Alanine facilitated the decomposition of BaCO3 and improved the nucleation density of BaTiO3. SiC effectively improved the uniformity of the synthesis temperature and enabled preparation of tetragonal-phase BaTiO3 at low temperatures. This improved microwave solid-state method produces tetragonal BaTiO3.
References
X. Luo, S. Yuan, X. Pan, C. Zhang, S. Du, Y. Liu, ACS Appl. Mater. Inter. (2017). https://doi.org/10.1021/acsami.7b02580
Y. Wang, K. Miao, W. Wang, Y. Qin, J. Eur. Ceram. Soc. (2017). https://doi.org/10.1016/j.jeurceramsoc.2017.01.035
N. Liu, W. Zhao, J. Rong, J. Am. Ceram. Soc. (2018). https://doi.org/10.1111/jace.15339
J. Li, K. Inukai, Y. Takahashi, A. Tsuruta, W. Shin, J. Asian Ceram. Soc. (2018). https://doi.org/10.1016/j.jascer.2017.05.001
P. Xue, Y. Hu, W. Xia, H. Wu, X. Zhu, J. Alloys Compd. (2017). https://doi.org/10.1016/j.jascer.2017.05.001
J. Li, K. Inukai, Y. Takahashi, A. Tsuruta, W. Shin, Materials (2018). https://doi.org/10.3390/ma11050712
Z. Zhu, W. Zhu, Curr. Appl. Phys. (2018). https://doi.org/10.1016/j.cap.2018.04.016886-892
R. Ashiri, RSC Adv. (2016). https://doi.org/10.1039/c5ra22942a
S.-S. Ryu, D.-H. Yoon, J. Mater. Sci. (2007). https://doi.org/10.1007/s10853-007-1537-6
Y.A. Huang, B. Lu, D.D. Li, Z.H. Tang, Y.B. Yao, T. Tao, B. Liang, S.G. Lu, Ceram Int. (2017). https://doi.org/10.1016/j.ceramint.2017.09.027
J. Li, K. He, Z.-H. Zhou, H. Huang, L. Zhang, C.-G. Lou, H.-Y. Yu, Ceram. Int. (2017). https://doi.org/10.1016/j.ceramint.2017.07.229
H. Jiao, K. Zhao, R. Shi, L. Ma, Y. Tang, Cryst. Res. Technol. (2018). https://doi.org/10.1002/crat.201700107
A.Z. Simões, F. Moura, T.B. Onofre, M.A. Ramirez, J.A. Varela, E. Longo, J. Alloys Compd. (2010). https://doi.org/10.1016/j.jallcom.2010.08.143
K. Hongo, S. Kurata, A. Jomphoak, M. Inada, K. Hayashi, R. Maezono, Inorg. Chem. (2018). https://doi.org/10.1021/acs.inorgchem.8b00381
S. Ahda, S. Misfadhila, P. Parikin, T.Y.S.P. Putra, IOP Conf. Ser. Mater. Sci. Eng. (2017). https://doi.org/10.1088/1757-899x/176/1/012048
S.-S. Ryu, J. Kore. Powd. Met. Inst. (2012). https://doi.org/10.4150/kpmi.2012.19.4.310
C. Ando, H. Kishi, H. Oguchi, M. Senna, J. Am. Ceram. Soc. (2006). https://doi.org/10.1111/j.1551-2916.2006.00917.x
R. Yanagawa, M. Senna, C. Ando, H. Chazono, H. Kishi, J. Am. Ceram. Soc. (2007). https://doi.org/10.1111/j.1551-2916.2007.01498.x
O.G. Gromov, A.P. Kuzmin, G.B. Kunshina, R.M. Usmanov, E.P. Lokshin, Russ. J. Appl. Chem. (2008). https://doi.org/10.1134/s1070427208110025
R. Rotaru, C. Peptu, P. Samoila, V. Harabagiu, J. Am. Ceram. Soc. (2017). https://doi.org/10.1111/jace.15003
H. Itasaka, K.I. Mimura, K. Kato, Nanomaterials (2018). https://doi.org/10.3390/nano8090739
M. Bi, Y. Hao, J. Zhang, M. Lei, K. Bi, Nanoscale (2017). https://doi.org/10.1039/c7nr05212j
T.-T. Lee, C.-Y. Huang, C.-Y. Chang, I.K. Cheng, C.-L. Hu, C.-Y. Su, C.-T. Lee, M. Fujimoto, Int. Appl. Ceram. Technol. (2013). https://doi.org/10.1111/ijac.12072
T.M. Khan, M. Zakria, R.I. Shakoor, S. Hussain, Appl. Phys. A (2016). https://doi.org/10.1007/s00339-016-9766-7
L. Zhang, J.X. Wen, Z.X. Zhang, J. Yang, H. Huang, Q.Y. Hu, H.R. Zhuang, H.Y. Yu, Phys. B (2019). https://doi.org/10.1016/j.physb.2019.02.002
Y. Zhang, L. Wang, D. Xue, Powder Technol. (2012). https://doi.org/10.1016/j.powtec.2011.11.043
H.-W. Lee, S. Moon, C.-H. Choi, D.K. Kim, S.J. Kang, J. Am. Ceram. Soc. (2012). https://doi.org/10.1111/j.1551-2916.2012.05085.x
K. Tsuzuku, M. Couzi, J. Mater. Sci. (2012). https://doi.org/10.1007/s10853-012-6310-9
J.C. Niepce, G. Thomas, Solid State lonics. (1990). https://doi.org/10.1016/0167-2738(90)90472-4
M.T. Buscaglia, M. Bassoli, V. Buscaglia, R. Alessio, J. Am. Ceram. Soc. (2005). https://doi.org/10.1111/j.1551-2916.2005.00451.x
C. Ando, H. Chazono, H. Kishi, Key Eng. Mater. (2004). https://doi.org/10.4028/www.scientific.net/KEM.269.161
C. Ando, K. Tsuzuku, T. Kobayashi, H. Kishi, S. Kuroda, M. Senna, J. Mater. Sci. Mater. Electron. (2008). https://doi.org/10.1007/s10854-008-9804-0
Acknowledgements
We thank the Science and Technology Major Project of Guangxi (AA18118001), Guangxi Key Laboratory of Information Materials Foundation (No. 171021-Z).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qian, H., Zhu, G., Xu, H. et al. Preparation of tetragonal barium titanate nanopowders by microwave solid-state synthesis. Appl. Phys. A 126, 294 (2020). https://doi.org/10.1007/s00339-020-03472-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-03472-y