Abstract
A low level of inflammation is an integral part of the balance between the immune system and the microbiota in the high antigen environment of the gastrointestinal tract and maintains homeostasis. A failure of this balance can lead to chronic intestinal inflammation and increase the chances to develop colorectal cancer significantly. The underlying mechanisms that link inflammation and carcinogenesis are not clear but the molecular platforms of the inflammasomes have been implicated. Inflammasomes are molecule complexes that are assembled in response to microbial components or cellular danger signals and facilitate the production of bioactive pro-inflammatory cytokines. One inflammasome in particular, NLRP3, has been analysed extensively in its contribution to colitis and has been shown to be associated with the development of colitis-associated colorectal cancer. This review will summarise the role of NLRP3 in intestinal inflammation, discuss some of the triggers of inflammation in the gastrointestinal tract such as diet and introduce some opportunities to use this inflammasome as therapeutic target for the treatment of colitis and colitis-associated colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common malignancy worldwide (Ferlay et al. 2015) and presents with a high mortality rate (Siegel et al. 2016) due to rapid cancer progression with late diagnosis at an advanced tumour stage (Siegel et al. 2014). An inflammation-associated form of CRC, colitis-associated CAC has been recognised as a complication of inflammatory bowel disease (IBD) (Parian and Lazarev 2015; Triantafillidis et al. 2009). These chronic inflammatory, idiopathic disorders are characterised by significant inflammation of small intestine and colon and are becoming more prevalent and more severe due to global adoption of western diet, the increasing use of nonsteroidal anti-inflammatory medications and an ageing population (Taleban et al. 2015). Furthermore, the increasing incidence and prevalence of IBD in children makes it an important paediatric chronic disease (Nasiri et al. 2017) with an increased risk of developing CAC (Peneau et al. 2013).
The two primary types of IBD are Crohn’s disease (CD) and ulcerative colitis (UC). CD commonly affects the small intestine or colon, however it can affect all parts of the gastrointestinal tract from the oesophagus to the anus involving all layers of the intestinal wall. Complications characteristic for CD are strictures, fistulae and fissures. UC is characterised with inflammation that is limited to the mucosa of the colon and presents with bleeding ulcers and that can result in the perforation of the colon (Cosnes et al. 2011; Mulder et al. 2014).
Innate immunity, IBD and cancer
Crohn and Rosenberg first reported an ulcerative colitis case associated with colorectal cancer development in 1925 (Crohn and Rosenberg 1925). Subsequently, many studies have been published indicating gut inflammation as an important factor predisposing the development of colorectal cancer (Dupaul-Chicoine et al. 2010; Karin and Greten 2005). The cumulative risk of UC-associated cancer is estimated to be at 1.6% at 10 years, 8.3% at 20 years and 18.4% at 30 years (Eaden et al. 2001) correlating CAC directly to the extent and duration of colitis. Nevertheless the exact mechanism of how chronic inflammation is connected to the development of colitis-associated colorectal cancer (CAC) has yet to be established.
Chronic inflammation in IBD progresses to CAC with constant overproduction of pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α (Becker et al. 2004; Popivanova et al. 2008), chemokines and DNA damaging reactive oxygen and nitrogen species. These inflammatory effector molecules promote aberrant intestinal epithelial cell proliferation, survival and angiogenesis and lead to epithelial dysplasia and ultimately to a formation of invasive tumours (Grivennikov 2013). Therefore, understanding and modulating the mechanisms of chronic mucosal inflammation will be the key to preventing the progression to CAC (Foersch and Neurath 2014).
Although the exact aetiology of chronic intestinal inflammation is not yet known, recent studies support the hypothesis of a defective innate immune response as the primary mechanism in chronic mucosal inflammation (Asquith and Powrie 2010). The innate immune response controls the intestinal microbiota and provides initial resistance to invading pathogens whilst maintaining homeostasis (Ignacio et al. 2016). Beyond anatomical barriers such as the skin and mucosa, various cellular components of the innate immune system such as epithelial cells, macrophages, dendritic cells (DC) and neutrophils are located in the intestinal wall and intestinal lymphoid organs including Peyer’s Patches and mesenteric lymph nodes. These cell populations keep the microbial occupants of the intestines under surveillance with the help of extracellular and cytosolic pattern recognition receptors (PRRs). The PRRs comprise the pro-inflammatory membrane-bound Toll-like receptors (TLR) and the cytosolic sensory protein complexes consisting of NOD-like receptors (NLR), the RNA sensing retinoic acid-inducible gene-1 receptors (RLR) and the pyrin and HIN domain (PYHIN) receptor family which includes the AIM2-like receptor (ALR) and C-type lectins (Ranson et al. 2017). These receptors recognise pathogen-associated molecular patterns (PAMPs) or host derived danger-associated molecular patterns (DAMPs). Receptor engagement causes cellular activation of various effector mechanisms ranging from microbicidal molecules and phagocytosis to activation of large multiprotein complexes called inflammasomes.
Biology of inflammasomes
Inflammasomes have emerged as a central feature in innate immunity (Martinon et al. 2002) and are involved in pathogen clearance, maintain tissue homeostasis and stimulate an adaptive immune response that removes tumour cells. These large cytosolic protein complexes can be divided in the NLR, the PYHIN or the ALR family (Fig. 1) and comprise inflammatory caspases which undergo autocatalytic activation and initiate inflammatory signalling cascades that activate protease Caspase-1 and releases pro-inflammatory cytokines IL-1β and IL-18 (Latz et al. 2013; Ranson and Eri 2013). This sequence of activating events is culminating in the initiation of pyroptosis, a Gasdermin-D-mediated form of inflammatory programmed cell death (Man et al. 2017). The functionally most completely characterised family member of the NLR is the NOD-like receptor family, pyrin domain containing protein 3 (NLRP3) (Table 1).
PRRs that initiate innate immunity. PRRs can be membrane bound like TLRs, or present in the cytoplasm as sensory proteins. The families are termed nucleotide-binding domain leucine-rich repeat containing receptors (NLRs), RIG-I like receptors and pyrin and HIN domain (PYHIN) receptors. PHYIN receptors are further subdivided as absent in melanoma (AIM2) receptors and C-type lectins
The association of inflammasomes with intestinal inflammation and CAC has been demonstrated with expression analysis of human colon cancer samples which shows lower expression levels for NLRP and AIM2 family members (Liu et al. 2015; Ranson and Eri 2013). Genetic ablation of these inflammasomes in the DSS/AOM CAC murine model suggested that they act to suppress intestinal inflammation-associated tumourigenesis essentially through inhibiting cellular proliferation and driving cell death (Allen et al. 2010, 2012; Chen et al. 2011; Karki et al. 2017; Normand et al. 2011; Wilson et al. 2015; Zaki et al. 2011). Mice deficient in inflammasome components such as apoptosis-associated speck-like protein containing a Caspase activation and recruitment domain (ASC), Caspase-1 and Caspase-11, IL-18 or IL-18r, exhibit increased colitis and tumorigenesis compared to wild-type (WT) mice in the azoxymethane-dextran sulphate sodium (AOM-DSS) model (Dupaul-Chicoine et al. 2010; Salcedo et al. 2010; Williams et al. 2015; Zaki et al. 2010b). These studies highlight the importance of inflammasome pathways in the modulation of colitis and the suppression of CAC.
Mechanism of action: NLRP3 in the innate immune response
Polymorphisms of the NLRP3 gene are associated with poor survival in CAC patients and the specific role of NLRP3 in the processes leading to tumorigenesis is not well understood (Ungerbäck et al. 2012). Therefore, we will discuss the current literature on the specific contribution of NLRP3 to the modulation of the intestinal microbiota and intestinal pathologies of colitis and CAC.
The NLRP3 inflammasome is a multiprotein platform comprising the NLRP3 protein, the adaptor protein ASC and pro-Caspase-1. The NLRP3 protein itself contains a nucleotide-binding and oligomerization domain (NBD or NACHT), carboxy-terminal leucine-rich repeat (LRR) at the C-terminus and a PYD (Leemans et al. 2011). The inflammasome assembly is inhibited by the LRR domain. This activity is disabled by the activating signal either from PAMPS or DAMPS, whereas the NBD is required for homo- or hetero-oligomerization that leads to the activation of Caspase-1 from the inactive zymogen pro-Caspase-1 and the subsequent auto-cleavage and secretion of the pro-inflammatory cytokines IL-1β and IL-18 (Martinon et al. 2002, Schroder and Tschopp 2010).
Triggers for activation of NLRP3 inflammasome include a diversified array of unrelated molecular structures (PAMPs) such as microbial cell wall components including lipopolysaccharide (LPS) and muramyl dipeptide (MDP), nucleic acids, pore-forming toxins, DAMPs, ATP and crystalline substances such as uric acid, oxidised mitochondrial DNA. As the NLRP3 inflammasome assembles in response to these molecules, it has been proposed that it responds to a common cellular distress signal, instead of a direct interaction with cognate ligands (Halle et al. 2008; Mariathasan et al. 2006; Martinon et al. 2006; Shimada et al. 2012) (Fig. 2). Three key mechanistic pathways have been suggested for triggering the activation of NLRP3 inflammasome. Firstly, pore formation and potassium efflux (Petrilli et al. 2007), secondly, lysosomal destabilisation (Okada et al. 2014) and thirdly, mitochondrial reactive oxygen species (mROS) generation (Gurung et al. 2015). Importantly, the presence of ROS has been implicated in the activation of the NLRP3 inflammasome and has been linked to cancer promotion (Fang et al. 2009).
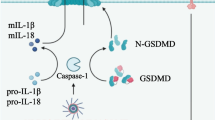
Canonical and non-canonical activation of NLRP3. The canonical pathway needs two signals to initiate NLRP3 inflammasome activation. Signal 1 is also termed priming and is triggered by pathogen-associated molecular patterns (PAMPs) from extra cellular environment. Signal 1 upregulates the availability of NLRP3 protein, pro-IL-18 and -IL-1β via NF-κβ mediated transcription. Signal 2 is activated by intracellular danger-associated molecular patterns (DAMPs) of the host. This leads to oligomerization of NLRP3 proteins and to a recruitment of ASC and Caspase-1 thus completing the NLRP3 inflammasomes assembly and activation. Activated NLRP3 inflammasomes will cleave pro-IL-18 and -IL-1β precursor cytokines into bioactive IL-18 and IL-1β and initiate the inflammatory response. The non-canonical pathway does not requires signal 1 or priming but is activated by intracellular DAMPs such as ROS. Upon activation its activity follows the canonical activation pathway
Canonical activation of NLRP3 occurs in two steps via both transcriptional and post-transcriptional processes. The first signal (Signal 1) is provided predominantly in a PAMPs-dependent manner. A frequently used model is the lipopolysaccharide (LPS)-induced activation of the TLR4/NF-κB pathway. This step is termed “priming” and causes a transcriptional up-regulation of the transcription of nlrp3 mRNA and for pro-il-1β and -il-18 (Latz et al. 2013) (Fig. 2). In a second step, intracellular sensing of specific ligands (DAMPs) leads to the recruitment and oligomerization of the key adaptor protein, ASC, which, through its a Caspase activation and recruitment domain (CARD) facilitates the subsequent recruitment and activation of Caspase-1. In a final activating step this protease catalyses the proteolytic cleavage of inactive pro-IL-1β or pro-IL-18 proteins into secreted bioactive cytokines which initiate a plethora of potent inflammatory responses. Furthermore, activation of Caspase-1 induces Gasdermin-D-mediated pyroptosis, a form of cell death frequently observed during invasion by gram-negative and gram-positive pathogens (Fig. 2).
In addition to canonical inflammasomes comprising NLRP3, ASC and Caspase-1, recent studies have identified an alternative non-canonical NLRP3 inflammasome which consists of Caspase-11 (Caspase-4 and Caspase-5 in human) and not Caspase-1. The non-canonical pathway is seen in infections with gram-negative bacteria, where Caspase-11 binds directly to cytosolic LPS. This promotes inflammasome-independent pyroptosis and the assembly of the NLRP3 inflammasome and activation of Caspase-1 to cleave pro-IL-1β and pro-IL-18 into secreted bioactive cytokines (Kayagaki et al. 2011; Rathinam et al. 2012) (Fig. 2).
IL-1β and IL-18 are important pro-inflammatory mediators of the mucosal inflammatory response. The presence of IL-1β can induce various cellular activities, including the proliferation, differentiation and apoptosis of both immune and non-immune cells (Huber et al. 2012; Vela et al. 2002). Additionally, IL-1β can co-stimulate IL-6 production which acts as a growth factor for B cell proliferation and initiate the release of other pro-inflammatory cytokines such as TNF-α, IL-23 (and IL-6) that can polarise the adaptive immune response to a Th2 or a Th17 response depending on the antigenic environment (Dinarello 2009).
The importance of IL-1β in the pathogenesis of colitis has been well established (Ning et al. 2015). In addition, numerous studies have revealed that secretion of IL-1β is elevated in the sera of patients with IBD (Sartor 1994) and mice subjected to DSS-induced colitis (Bauer et al. 2010). Importantly inhibition of IL-1β has shown to alleviate DSS-induced colitis (Siegmund 2002).
Other studies have indicated that in contrast to IL-1β, IL-18 plays a major role in suppressing colitis and CAC. Polymorphisms of the il-18 gene have confirmed a strong association of this cytokine with an increased susceptibility to CD (Tamura et al. 2002). This suggests that IL-18 signalling provides protection against a development of intestinal inflammation. Mice deficient for IL-18 and IL-18 receptor were hyper-susceptible to DSS-induced colitis, which was associated with higher mortality rates and more severe histopathological changes (Takagi et al. 2003). In a similar study, IL-18−/− and IL18r−/− mice also developed severe DSS-induced colitis with high lethality and more histopathological abnormalities and were more susceptible to AOM/DSS-induced colon tumorigenesis as compared to WT mice, suggesting an essential and protective role of IL-18 signalling in colorectal cancer (Salcedo et al. 2010). MyD88 KO mice, which are defective in both IL-1β and IL-18 production and the downstream signalling of their respective receptors, showed increased colonic epithelial proliferation and colorectal tumorigenesis (Salcedo et al. 2010). The explanation proposed was that the increase in tumorigenesis was caused by a decrease in DNA damage response genes causing insufficient response to DNA damage. Additionally, IL-18 is a key mediator in epithelial regeneration by the up-regulation of adhesion molecules (Stuyt et al. 2003) during the early stages of colitis (Allen et al. 2010; Hirota et al. 2011; Nowarski et al. 2015; Zaki et al. 2010a). Besides its indirect tumour-suppressive role in CAC, IL-18 has been associated with a T helper 1-skewed immune-stimulatory, anti-tumorigenic response through its ability to induce IFN-γ (Okamura et al. 1995) and its effects on enhancing the cytosolic activity of cytotoxic T cells and NK cell response (Chaix et al. 2008; Novick et al. 2013; Takeda et al. 1998). Furthermore, high levels of IL-18 have been detected in lamina propria mononuclear cells (LPMCs) and colon epithelial cells of patients with Crohn’s disease (Monteleone et al. 1999; Pizarro et al. 1999).
In an experimental T cell-mediated colitis model administration of a recombinant IL-18 antisense-expressing adenoviruses was able to reduce IL-18 and suppress IFN-γ thus ameliorating colitis in vivo (Wirtz et al. 2002). The connection between IL-18 and IFN-γ was supported by an analysis of LPMCs from IL-18−/− mice which featured an exacerbated form of DSS colitis and produced a significantly higher amount of IFN-γ (Takagi et al. 2003). Furthermore, the in murine colitis models neutralisation of IL-18 has shown to ameliorate intestinal inflammation (Siegmund et al. 2001a; Sivakumar et al. 2002) and may therefore an interesting candidate for a targeted immunotherapy of human intestinal inflammatory diseases. These experiments have highlighted the dual role of IL-18 in intestinal homeostasis and colitis. Early in the mucosal immune response, its expression by IECs and LPMCs indicates a protective, local role through epithelial regeneration and proliferation in response to injury. In chronic inflammation its excessive production can enhance inflammation, which potentially promotes tumorigenesis and tumour growth (Reuter and Pizarro 2004).
The role of NLRP3 in colitis-associated and colitis-associated cancer: lessons from mouse models
NLRP3 inflammasome and its activation in intestinal pathologies have been investigated predominantly using mouse models of chemically induced intestinal inflammation and CAC specifically the 2,4,6-trinitrobenzene sulphonic acid (TNBS) and the dextran sulphate sodium (DSS) models of inflammation, and the azoxymethane (AOM or AOM/DSS) model of CAC induction respectively (Table 2).
In murine of TNBS models the chemical compound is administered intrarectally and mediates a T cell-mediated immune response, similar to chronic colonic inflammation as described in CD (Wirtz and Neurath 2007). In the DSS colitis model, DSS is dissolved in the drinking water and is ingested. The chemical causes significant damage to the epithelial barrier by causing acute colonic crypt destruction and mucosal ulceration. The compromised epithelial barrier is invaded by gut microflora which enters to the lamina propria resulting in massive infiltration of inflammatory cells and up-regulation of pro-inflammatory cytokines comparable to human ulcerative colitis (Chassaing et al. 2014; Ni et al. 1996). Administering DSS in weekly cycles alternating it with water results in a condition that is similar to clinically observed conditions of active and remission phases of UC. Finally, AOM a potent genotoxic carcinogen that causes DNA damage in epithelial cells is used in combination with repeated DSS administration. The resulting chronic inflammation promotes the development of colorectal cancer in cells carrying mutations generated by AOM (Tanaka et al. 2003).
The role of NLRP3 in colitis and CAC is still controversial with some studies showing a protective role while other studies demonstrate a detrimental effect of NLRP3 activation (Fig. 2; Table 2). Studies of individual inflammasome components in colitis models before the inflammasome was identified as complex molecular platform questioned the role of Caspase-1 (Dupaul-Chicoine et al. 2010; Siegmund et al. 2001b). The phenotype of Caspase-1−/− DSS colitis mice showed reduced acute and chronic colitis (Siegmund et al. 2001b). Both Caspase-1−/− and ASC−/− mice showed an increased susceptibility to DSS colitis and disease severity was linked to reduced IL-18 production. Conversely, the administration of exogenous IL-18 completely reversed severity of colitis (Dupaul-Chicoine et al. 2010). Intestinal epithelial cells rather than lamina propria cells were shown to be the source of IL-18 that was needed for early induction of tissue repair and epithelial cell regeneration.
The discrepancy in the above studies was explained by the novel discovery of two confounding aspects of the Caspase-1−/− mouse model. First, Caspase-1−/− mice additionally lacked Caspase-11 (Kayagaki et al. 2011). Therefore all results based on previous studies of this mouse model during DSS colitis is complicated by the contribution of Caspase-11 to disease pathogenesis.
The second controversy revolves around the fact that the Caspase-1−/− Caspase-11129mt/129mt mice harbour colitogenic microbiota that have shown to enhance the severity of DSS colitis (Elinav et al. 2011). A recent study characterised the intrinsic functions of Caspase-1 in vivo. The study generated Caspase-1−/− and Caspase-11−/− mice on a pure C57BL/6N genetic background with a non-dysbiotic intestinal microbiota. Using these gut microbiota it could be shown that that canonical Caspase-1 activation, not Caspase-11, is responsible for exacerbating DSS-induced colitis (Blazejewski et al. 2017).
The protective role of NLRP3 in colitis was suggested by experiments that showed enhanced colitis in NLRP3−/− mice mediated by a loss of epithelial barrier integrity and reduction of IL-18 (Zaki et al. 2010a), defective antimicrobial mechanism leading to bacterial dysbiosis and increased susceptibility to DSS- and TNBS-induced colitis (Hirota et al. 2011).
The role of the NLRP3 inflammasome in the pathogenesis of UC was analysed in an oxazolone-induced murine colitis model. Intrarectal delivery of oxazolone (OXA) created a relevant UC pathogenesis model mediated by Th2 cytokines. In this model a reduction of mature IL-1β and IL-18 production induced a higher severity of colitis in OXA-treated NLRP3−/− mice when compared to WT mice. Conversely this increase in severity could be prevented by exogenous administration of IL-1β or IL-18. This study shows that NLRP3 inflammasome-derived IL-1β and IL-18 may play a protective role against OXA-induced colitis (Itani et al. 2016).
Controversially, competing studies showed that DSS-induced colitis was attenuated in NLRP3−/− mice potentially mediated by a local reduction of pro-inflammatory cytokines IL-1β, TNF-α and IFN-γ (Bauer et al. 2010). Exposure to DSS and concurrent treatment with the Caspase-1 inhibitor Pralnacasan ameliorated colitis (Bauer et al. 2007). An attenuation of colitis in NLRP3-deficient mice has been confirmed by others supporting the function of NLRP3 as a negative regulator of the inflammatory process (Elinav et al. 2011).
Genetic models of NLRP3−/−, Caspase-1−/− and ASC−/− mice exposed to AOM/DSS have an increased tumour burden (Allen et al. 2010). Using bone marrow chimaeras, it was demonstrated that tumorigenesis suppressive signalling by NLRP3 was located in the hematopoietic and not in the epithelial compartment. This correlated colitis-associated tumours with a defective production of IL-18 during the initiation of inflammation in the AOM/DSS model. Consequently, the role of NLRP3 in CAC has mainly been reported as a negative regulator in colitis-associated tumorigenesis (Allen et al. 2010; Zaki et al. 2010b).
Consistent with a protective role of NLRP3 in tumorigenesis NLRP3−/− mice were highly susceptible to AOM/DSS-induced inflammation and the treatment caused increased tumours in the colon The mechanism proposed was that NLRP3-dependent IL-18 secretion is required for STAT1 activation and IFN-γ induction leading to decreased immune tumour surveillance in the absence of IL-18. This phenotype was reduced with administration of recombinant IL-18 (Zaki et al. 2010b).
The ability of NLRP3 to release bioactive IL18 is a potential mechanism explaining the protective role of this cytokine against CAC development. However, another study found no difference in CAC between NLRP3-deficient and WT mice in a AOM/DSS model (Hu et al. 2011). The reason for these discrepancies observed in the Nlrp3−/− colitis or CAC model phenotype is not clear, but could be due to differences in length and concentration of DSS treatment or to baseline differences in the composition of the intestinal microbiota in experimental mouse colitis.
Human and murine studies suggest that bacterial dysbiosis promotes inflammation in human and mouse colitis and CAC models (Kitajima et al. 2001; Richard et al. 2018). Studies in mice deficient in inflammasome genes have provided evidence that specific inflammasomes like NLRP6 and NLRP3 are major regulators of commensal microbiota. A study in Nlrp3−/− mice suggested that the NLRP6/ASC inflammasome acts as a crucial regulator of the gut microbiota (Elinav et al. 2011). Another study that observed a significant increase in bacterial counts in stool, colon, mesenteric lymph node and liver in DSS colitis of Nlrp3−/− mice compared to DSS colitis in wild-type mice demonstrated that NLRP3 plays a role in controlling commensal overgrowth and bacteraemia (Zaki et al. 2010a).
How NLRP3 regulates the microbial composition in the intestines is not clear. This question was addressed by comparing faecal microbiota of Nlrp3−/− deficient mice to their WT littermates (Hirota et al. 2011). Only NLRP3−/− mice were shown to carry a specific bacterial composition with potentially pathogenic members of the family Enterobacteriaceae including the species Citrobacter, Proteus and Shigella (Hirota et al. 2011). Furthermore, the unique genera Mycobacterium, Collinsella, Clostridium and Ralstonia were identified which were not detectable in WT littermates. Additionally, it could be shown that colon crypt secretions obtained from NLRP3−/− mice ex vivo had decreased bactericidal activity against E. coli. The significant difference in composition of the intestinal microbiota in the NLRP3−/− mice could explain the increased susceptibility to DSS- and TNBS-induced colitis (Hirota et al. 2011).
Further studies have demonstrated that the distinct commensal bacterial species Proteus mirabilis induces a robust Il-1β secretion via NLRP3 inflammasome activation in newly recruited intestinal Ly6Chigh monocytes during DSS colitis. This could be linked to an increased severity of disease (Seo et al. 2015). However, the molecular pathways by which intestinal bacterial populations in NLRP3 inflammasome-deficient mice promote intestinal inflammation are still elusive. It has been speculated that this could be due to their ability to upregulate the generation of pro-inflammatory chemokines and cytokines such as CCL5 (Elinav et al. 2011), IL-6 and TNF-α (Hu et al. 2013).
NLRP3−/− mice show a more severe colitis when compared to WT mice after infection with the intestinal pathogen Citrobacter rodentium (Song-Zhao et al. 2013). This could be due to a lack of downstream cytokines IL-1β and IL-18 in the gene-deficient mice and allows the conclusion that activation of the NLRP3 inflammasome is necessary for an attenuation of Citrobacter rodentium driven intestinal inflammation.
In contrast to gene-deficient models, the NLRPp3R258W mouse model displays an enhanced NLRP3 inflammasome signalling. These mice develop an auto-inflammatory response in the skin (Meng et al. 2009). In the intestine they maintain homeostasis and remain strongly resistant to experimental colitis and subsequently, colorectal cancer. This due to a distinct microbiota in NLRP3R258W mice with an increased presence of bacterial species such as Clostridium XIVa and Lactobacillus murinus and also a significant reduction of colitogenic bacteria such as Akkermensia muciniphila which promote the local differentiation of Tregs, that contribute to homeostasis in the gut (Yao et al. 2017). Further work needs to be done to elucidate the signalling pathways by which resident bacteria stimulate the NLRP3 inflammasome to induce colitis in IBD.
Diet, microbiota and the activation of NLRP3: bowel cancer as an end stage of inflammation
Environmental factors, together with the individual genetic make -up and the innate immune response play role in development of IBD (Marion-Letellier et al. 2016). Two central environmental factors are diet and the composition of symbiotic microorganisms that live in our gastrointestinal tract (GI), the so-called microbiome. Microbiota composition has been shown to modulate metabolism-associated conditions like obesity and inflammatory diseases of the bowel like IBD and associated cancers such as CAC and CRC (Requena et al. 2018). A healthy diet helps to maintain a balanced and healthy microbiota and consequently, immune homeostasis in the gut. Unbalanced consumption of nutrients can lead to a dysbiosis of the microbiota and inflammation (Geuking et al. 2014; Marion-Letellier et al. 2016). Chronic insults from dietary metabolites activate NLRP3 and IL-1β production and thus progress disease pathogenesis (Camell et al. 2015). Consequently, diet has been identified as an important driver of the development a large percentage (50–90%) of tumours of the bowel (Kasdagly et al. 2014). For example, diet rich in fat increases the amount of bile acid which consists to 58% of deoxycholic acid (DCA). DCA has been found to disrupt epithelial integrity due to Cathepsin B activation of NLRP3 and leads to barrier disruption observed in DSS-induced colitis. In mice deficient for NLRP3 or Caspase 1 DSS-induced colitis could not be established which underlines the role of NLPR3 in the development of colitis in a high fat environment (Zhao et al. 2016). Another chronic insult by the ingested diet is the presence of dietary cholesterol in the intestines. In an AOM-treated mouse model this has been associated with a hyperactivity of NLRP3 resulting in an increase in IL-1β (Du et al. 2016). In general, saturated lipids, ceramide (Vandanmagsar et al. 2011) and uric acid can act as DAMPs that induce unwanted NLRP3 activation by initiating the production of ROS (Camell et al. 2015). On the other hand, some metabolites like omega-fatty acids prevent activation of NLRP3 and thus have an anti-inflammatory effect (Yan et al. 2013). These examples allow an insight how inflammation, colitis and CAC can be supported by various dietary parameters.
Under normal physiological conditions the symbiotic microbiota support the host by breaking down complex polysaccharides in dietary fibre into short chain fatty acids (SCFA) with less than six carbon atoms (Nieuwdorp et al. 2014). The predominant forms are acetate (C2), propionate (C3) and butyrate (C4). SCFA are the major products of bacterial fermentation and important as energy source for colonic epithelial cells (Huycke and Gaskins 2004). SCFA induce a favourable intestinal environment in a variety of ways and high fibre diet boosts bacterial species which produce SCFA (Desai et al. 2016; Kelly et al. 2015). The presence of dietary fibre and SCFA also protects in DSS directly through activation of NLRP3 possibly in colonic epithelial cells (Macia et al. 2015). While the molecular pathways of this interaction are still elusive it demonstrates the important role of diet in intestinal homeostasis.
Furthermore, butyrate as one of the main SCFA induces functional Tregs via intrinsic up-regulation of the Foxp3 gene (Furusawa et al. 2013). The extrathymic differentiation of Tregs in the periphery is enhanced via the conserved non-coding sequence 1 (CNS-1)-dependent pathway in the presence of butyrate and propionate (Arpaia et al. 2013). Butyrate activates the intestinal butyrate GPR109A receptor which drives the differentiation of Tregs and modulates immune responses by up-regulation of the production of IL-10, suppressing colonic inflammation and carcinogenesis in a mouse model of intestinal inflammation associated with CAC (Singh et al. 2014). Increased Treg initiate an anti-inflammatory response and act as antagonist to NLRP3 thus maintaining the homeostasis in experimental colitis and CAC (Yao et al. 2017).
Future directions of CAC therapy by intervention with NLRP3 activation
An improved understanding of the mechanistic interactions between diet and microbiota, and inflammasomes such as NLRP3 will reveal new potential therapies that target these pathways (Fig. 3). Here we outline some opportunities for interventions (Table 3).
Different therapeutic interventions targeting NLRP3 assembly or activation. Small molecule inhibitors modify or prevent NLRP3 assembly. Associated target therapy targets NLRP3 activation through changes in diet and microbiota, or modulation of macrophages. Therapy against novel stimulation of NLRP3 such as activation by mitochondria
Diet/metabolic immunomodulators
Metabolite targeted therapy can be used to modulate the hyper activation/blockage of NLRP3. Metabolic product-induced inflammatory mediators like plasminogen activator inhibitor (PA)-1, sphingosine-1-phosphate and ceramide-1-phosphate constitute a link that allows modulation of NLRP3 activity. Targeting these mediators results in a therapy of colitis. Lipoxygenase is needed to recruit monosodium urate which activates NLRP3 in gout. This mechanism needs to be investigated in colitis and CAC models (Amaral et al. 2012). A further interesting possibility is the inhibition of ceramide synthesis by increasing fatty acid oxidation to reduce fatty acid inflammation (Schilling et al. 2013).
Inhibition of de novo activation of NLRP3
The cytoskeleton protein α-microtubulin is an activator of NLRP3. This protein supports NLRP3 assembly by recruiting ASC from mitochondria. This supporting role of NLRP3 activation requires an assistance from dynein (Akira et al. 2013). The microtubules have to undergo post-translational modification by acetylation by the Mec17 gene product in order to activate NLRP3 (Akira et al. 2013). An activation of the enzyme SIRT2 which is α-tubulin deacetylase is required by DAMPs in order to activate NLRP3. A targeted therapy against this mechanism of NLRP3 assembly could offer an interesting treatment opportunity.
Small molecule targeted therapy
Novel small molecule specific inhibitors such as MCC950, CY-09, BHB has been used to pharmacologically target NLRP3 activation and successfully treat a variety of inflammatory disorders (Coll et al. 2015; Jiang et al. 2017; Youm et al. 2015). These inhibitory molecules target various protein components such as ASC or Caspase-1 or inhibit production of NLRP3-mediated effector cytokines such as IL-1β (Mangan et al. 2018). At present there are no specific NLRP3 inhibitors used in clinical IBD therapy. However a few compounds that show inhibition of NLRP3 activity have emerged as potential therapeutics for IBD (Perera et al. 2017). Most of these are nonspecific with unknown mechanisms, limiting their progression to clinical usage for long term application in chronic IBD but specific NLRP3 inhibitors have shown promise in IBD patient tissues and colitis mouse models. A recent study using specific inhibitor MCC950 in the spontaneous chronic IBD mouse model Winnie, illustrated the potent therapeutic effect of NLRP3 blockade. Established colitis was ameliorated by a 3 week treatment of orally administered MCC950 (Perera et al. 2018). Another study used Glyburide alleviating colitis and preventing disease onset in IL10−/− mice. This drug also inhibited pro-inflammatory cytokines in mucosal explants from Crohn’s patients (Liu et al. 2016). Furthermore, the benzimidazole-containing synthetic small molecule inhibitor Fc11a-2 alleviated colitis in a DSS-induced colitis mouse model (Liu et al. 2013). In this study the mechanism of action was found to be inhibition of cleavage of pro-Caspase-1 following a reduction in production of IL-1β and IL-18 suppressing the activation of NLRP3 inflammasome. Finally, a non-cytotoxic, novel acrylate derivative inhibitor INF39 which does not block Caspase 1. Oral administration of INF39 in a TNBS-induced rat colitis model attenuated intestinal inflammation (Cocco et al. 2017). The use of small molecule inflammasome inhibitors also shows promise when it comes to prevention of CAC. A study on the small molecule andrographolide (Andro) shows that it is protective against AOM/DSS induced colon carcinogenesis in mice by inducing mitophagy in macrophages which leads to a reversed mitochondrial membrane potential collapse that inactivates the NLRP3 inflammasome and prevents the development of CAC (Guo et al. 2014). A further example is the small-molecule AMPK activator GL-V9 which resolves colitis and is protective against tumorigenesis in colitis-associated colorectal cancer. GL-V9 acts by triggering autophagy in cells leading to activation of the NLRP3 inflammasome (Zhao et al. 2018). In summary the regulation of chronic inflammation in the intestines through pharmacological intervention of small molecule NLRP3 inhibitors in IBD patients could be a potential therapeutic option for preventing CAC.
Combination therapy
Combination of small molecule therapy along with diet modulators could be tried to increase effectiveness. For example various combinations of diet modulators can be used such as 5-aminosalicylic acid together with fish oil. This combination lowered the inflammatory score compared to treatment with 5-aminosalicylic acid alone in rats with TNBS-induced colitis (Mbodji et al. 2013). In human tissues the combination of glutamine and arginine have been shown to reduce TNF-α production in colonic biopsies of colitis patients (Lecleire et al. 2008).
Conclusion
There is clear evidence supporting the NLRP3 inflammasome as key player in the inflammatory response in colitis (Schoultz et al. 2009; Villani et al. 2009) with ultimate implications for CAC development (Allen et al. 2010). However, it is also clear from the conflicting evidence outlined in this review that involvement of the NLRP3 inflammasome in inflammatory processes leading to tumour development is complex and may be context dependent. Therefore carefully designed follow up experiments are warranted, where conditions for the induction of colitis including controlled intestinal microbiota, are carefully controlled. Unravelling the role of the NLRP3 inflammasome in intestinal inflammation will provide insights for the role of NLRP3 in intestinal epithelial cells and the pathways employed by the mucosal immune system to modulate the microbiota and integrate with the adaptive immune response to defend the integrity of the gut mucosa. Understanding the mechanisms that underpin these interactions also more broadly builds on the current strong interest in understanding the immune pathways underpinning chronic inflammation in tumourigenesis.
References
Akira S, Misawa T, Satoh T, Saitoh T (2013) Macrophages control innate inflammation. Diabetes Obes Metab 15(Suppl 3):10–18
Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP (2010) The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 207:1045–1056
Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, Jobin C, Rogers AB, Ting JP (2012) NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity 36:742–754
Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, Soriani FM, Silveira TN, Cunha LD, Zamboni DS, Quesniaux V, Peres RS, Cunha TM, Cunha FQ, Ryffel B, Souza DG, Teixeira MM (2012) NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum 64:474–484
Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD (2012) NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488:389–393
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455
Asquith M, Powrie F (2010) An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med 207:1573–1577
Bauer C, Loher F, Dauer M, Mayer C, Lehr HA, Schönharting M, Hallwachs R, Endres S, Eigler A (2007) The ICE inhibitor pralnacasan prevents DSS-induced colitis in C57BL/6 mice and suppresses IP-10 mRNA but not TNF-α mRNA expression. Dig Dis Sci 52:1642–1652
Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M (2010) Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59:1192–1199
Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF (2004) TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21:491–501
Blazejewski AJ, Thiemann S, Schenk A, Pils MC, Galvez EJC, Roy U, Heise U, de Zoete MR, Flavell RA, Strowig T (2017) Microbiota normalization reveals that canonical caspase-1 activation exacerbates chemically induced intestinal inflammation. Cell Rep 19:2319–2330
Camell C, Goldberg E, Dixit VD (2015) Regulation of Nlrp3 inflammasome by dietary metabolites. Semin Immunol 27:334–342
Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T (2008) Cutting edge: priming of NK cells by IL-18. J Immunol 181:1627–1631
Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M (2014) Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104:15–25
Chavarria-Smith J, Vance RE (2015) The NLRP1 inflammasomes. Immunol Rev 265:22–34
Chen GY, Liu M, Wang F, Bertin J, Núñez G (2011) A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol 186:7187
Cocco M, Pellegrini C, Martinez-Banaclocha H, Giorgis M, Marini E, Costale A, Miglio G, Fornai M, Antonioli L, Lopez-Castejon G, Tapia-Abellan A, Angosto D, Hafner-Bratkovic I, Regazzoni L, Blandizzi C, Pelegrin P, Bertinaria M (2017) Development of an acrylate derivative targeting the NLRP3 inflammasome for the treatment of inflammatory bowel disease. J Med Chem 60:3656–3671
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248–255
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A (2011) Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140:1785–1794
Crohn BB, Rosenberg H (1925) The sigmoidoscopic picture of chronic ulcerative colitis (non-specific). Am J Med Sci 170:220–228
Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Nunez G, Martens EC (2016) A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167:1339–1353.e1321
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550
Du Q, Wang Q, Fan H, Wang J, Liu X, Wang H, Wang Y, Hu R (2016) Dietary cholesterol promotes AOM-induced colorectal cancer through activating the NLRP3 inflammasome. Biochem Pharmacol 105:42–54
Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, Vallance BA, Saleh M (2010) Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32:367–378
Eaden JA, Abrams KR, Mayberry JF (2001) The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48:526–535
Elinav E, Strowig T, Kau Andrew L, Henao-Mejia J, Thaiss Christoph A, Booth Carmen J, Peaper David R, Bertin J, Eisenbarth Stephanie C, Gordon Jeffrey I, Flavell Richard A (2011) NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145:745–757
Fang J, Seki T, Maeda H (2009) Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev 61:290–302
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Foersch S, Neurath MF (2014) Colitis-associated neoplasia: molecular basis and clinical translation. Cell Mol Life Sci 71:3523–3535
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450
Geuking MB, Köller Y, Rupp S, McCoy KD (2014) The interplay between the gut microbiota and the immune system. Gut Microbes 5:411–418
Grivennikov S (2013) Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 35:229–244
Guo W, Sun Y, Liu W, Wu X, Guo L, Cai P, Wu X, Wu X, Shen Y, Shu Y, Gu Y, Xu Q (2014) Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy 10:972–985
Gurung P, Lukens JR, Kanneganti TD (2015) Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med 21:193–201
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9:857–865
Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, McCafferty DM, Rioux KP, Ghosh S, Xavier RJ, Colgan SP, Tschopp J, Muruve D, MacDonald JA, Beck PL (2011) NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 17:1359–1372
Hu B, Elinav E, Flavell RA (2011) Inflammasome-mediated suppression of inflammation-induced colorectal cancer progression is mediated by direct regulation of epithelial cell proliferation. Cell Cycle 10:1936–1939
Hu B, Elinav E, Huber S, Strowig T, Hao LM, Hafemann A, Jin CC, Eisenbarth SC, Flavell RA (2013) Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci USA 110:9862–9867
Huber S, Gagliani N, Flavell RA (2012) Life, death, and miracles: Th17 cells in the intestine. Eur J Immunol 42:2238–2245
Huycke MM, Gaskins HR (2004) Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med (Maywood) 229:586–597
Ignacio A, Morales CI, Camara NOS, Almeida RR (2016) Innate sensing of the gut microbiota: modulation of inflammatory and autoimmune diseases. Front Immunol 7:54
Itani S, Watanabe T, Nadatani Y, Sugimura N, Shimada S, Takeda S, Otani K, Hosomi S, Nagami Y, Tanaka F, Kamata N, Yamagami H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Arakawa T (2016) NLRP3 inflammasome has a protective effect against oxazolone-induced colitis: a possible role in ulcerative colitis. Sci Rep 6:39075
Janowski AM, Kolb R, Zhang W, Sutterwala FS (2013) Beneficial and detrimental roles of NLRs in carcinogenesis. Front Immunol 4:370
Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W, Deng X, Zhou R (2017) Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med 214:3219–3238
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Karki R, Man SM, Malireddi RKS, Kesavardhana S, Zhu Q, Burton AR, Sharma BR, Qi X, Pelletier S, Vogel P, Rosenstiel P, Kanneganti T-D (2016) NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature 540:583
Karki R, Malireddi RKS, Zhu QF, Kanneganti TD (2017) NLRC3 regulates cellular proliferation and apoptosis to attenuate the development of colorectal cancer. Cell Cycle 16:1243–1251
Kasdagly M, Radhakrishnan S, Reddivari L, Veeramachaneni DN, Vanamala J (2014) Colon carcinogenesis: influence of Western diet-induced obesity and targeting stem cells using dietary bioactive compounds. Nutrition 30:1242–1256
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121
Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP (2015) Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17:662–671
Kitajima S, Morimoto M, Sagara E, Shimizu C, Ikeda Y (2001) Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim 50:387–395
Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA (2005) Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731–734
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411
Lecleire S, Hassan A, Marion-Letellier R, Antonietti M, Savoye G, Bole-Feysot C, Lerebours E, Ducrotte P, Dechelotte P, Coeffier M (2008) Combined glutamine and arginine decrease proinflammatory cytokine production by biopsies from Crohn’s patients in association with changes in nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways. J Nutr 138:2481–2486
Leemans JC, Cassel SL, Sutterwala FS (2011) Sensing damage by the NLRP3 inflammasome. Immunol Rev 243:152–162
Liu W, Guo W, Wu J, Luo Q, Tao F, Gu Y, Shen Y, Li J, Tan R, Xu Q, Sun Y (2013) A novel benzo[d]imidazole derivate prevents the development of dextran sulfate sodium-induced murine experimental colitis via inhibition of NLRP3 inflammasome. Biochem Pharmacol 85:1504–1512
Liu RR, Truax AD, Chen L, Hu PZ, Li ZS, Chen J, Song CJ, Chen LH, Ting JPY (2015) Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: correlation with cancer stages and inflammasome components. Oncotarget 6:33456–33469
Liu L, Dong Y, Ye M, Jin S, Yang J, Joosse ME, Sun Y, Zhang J, Lazarev M, Brant SR, Safar B, Marohn M, Mezey E, Li X (2016) The pathogenic role of NLRP3 inflammasome activation in inflammatory bowel diseases of both mice and humans. J Crohns Colitis 11(6):737–750
Loving CL, Osorio M, Kim YG, Nunez G, Hughes MA, Merkel TJ (2009) Nod1/Nod2-mediated recognition plays a critical role in induction of adaptive immunity to anthrax after aerosol exposure. Infect Immun 77:4529–4537
Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR (2015) Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6:6734
Man SM, Karki R, Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277:61–75
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17:588
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232
Marion-Letellier R, Savoye G, Ghosh S (2016) IBD: in food we trust. J Crohn’s Colitis 10:1351–1361
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
Mbodji K, Charpentier C, Guerin C, Querec C, Bole-Feysot C, Aziz M, Savoye G, Dechelotte P, Marion-Letellier R (2013) Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-kappaB in rats with TNBS-induced colitis. J Nutr Biochem 24:700–705
Meng G, Zhang F, Fuss I, Kitani A, Strober W (2009) A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity 30:860–874
Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, Luzza F, Fusco A, Pallone F (1999) Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol 163:143–147
Mulder DJ, Noble AJ, Justinich CJ, Duffin JM (2014) A tale of two diseases: the history of inflammatory bowel disease. J Crohns Colitis 8:341–348
Nasiri S, Kuenzig ME, Benchimol EI (2017) Long-term outcomes of pediatric inflammatory bowel disease. Semin Pediatr Surg 26:398–404
Ni J, Chen SF, Hollander D (1996) Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut 39:234–241
Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM (2014) Role of the microbiome in energy regulation and metabolism. Gastroenterology 146:1525–1533
Ning C, Li YY, Wang Y, Han GC, Wang RX, Xiao H, Li XY, Hou CM, Ma YF, Sheng DS, Shen BF, Feng JN, Guo RF, Li Y, Chen GJ (2015) Complement activation promotes colitis-associated carcinogenesis through activating intestinal IL-1beta/IL-17A axis. Mucosal Immunol 8:1275–1284
Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M (2011) Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci 108:9601
Novick D, Kim S, Kaplanski G, Dinarello CA (2013) Interleukin-18, more than a Th1 cytokine. Semin Immunol 25:439–448
Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, Low JS, Harman CCD, Graham M, Elinav E, Flavell RA (2015) Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163:1444–1456
Okada M, Matsuzawa A, Yoshimura A, Ichijo H (2014) The lysosome rupture-activated TAK1-JNK pathway regulates NLRP3 inflammasome activation. J Biol Chem. https://doi.org/10.1074/jbc.M114.579961
Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M (1995) Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378:88
Parian A, Lazarev M (2015) Who and how to screen for cancer in at-risk inflammatory bowel disease patients. Expert Rev Gastroenterol Hepatol 9:731–746
Peneau A, Savoye G, Turck D, Dauchet L, Fumery M, Salleron J, Lerebours E, Ligier K, Vasseur F, Dupas JL, Mouterde O, Spyckerelle C, Djeddi D, Peyrin-Biroulet L, Colombel JF, Gower-Rousseau C (2013) Mortality and cancer in pediatric-onset inflammatory bowel disease: a population-based study. Am J Gastroenterol 108:1647–1653
Perera AP, Kunde D, Eri R (2017) NLRP3 Inhibitors as potential therapeutic agents for treatment of Inflammatory Bowel Disease. Curr Pharm Des 23(16):2321–2327
Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AAB, Schroder K, Kunde D, Eri R (2018) MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep 8:8618
Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14:1583–1589
Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F (1999) IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol 162:6829–6835
Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N (2008) Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118:560–570
Ranson N, Eri R (2013) The role of inflammasomes in Intestinal Inflammation. Am J Med Biol Res 1:64–76
Ranson N, Kunde D, Eri R (2017) Regulation and sensing of inflammasomes and their impact on intestinal health. Int J Mol Sci 18:2379
Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA (2012) TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150:606–619
Requena T, Martinez-Cuesta MC, Pelaez C (2018) Diet and microbiota linked in health and disease. Food Funct 9:688–704
Reuter BK, Pizarro TT (2004) Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol 34:2347–2355
Richard ML, Liguori G, Lamas B, Brandi G, da Costa G, Hoffmann TW, Pierluigi Di Simone M, Calabrese C, Poggioli G, Langella P, Campieri M, Sokol H (2018) Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes 9:131–142
Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’HUigin C, Marincola FM, Trinchieri G (2010) MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med 207:1625–1636
Sartor RB (1994) Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology 106:533–539
Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, Weber K, Ory DS, Schaffer JE (2013) Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem 288:2923–2932
Schoultz I, Verma D, Halfvarsson J, Torkvist L, Fredrikson M, Sjoqvist U, Lordal M, Tysk C, Lerm M, Soderkvist P, Soderholm JD (2009) Combined polymorphisms in genes encoding the inflammasome components NALP3 and CARD8 confer susceptibility to Crohn’s disease in Swedish men. Am J Gastroenterol 104:1180–1188
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832
Seo SU, Kamada N, Munoz-Planillo R, Kim YG, Kim D, Koizumi Y, Hasegawa M, Himpsl SD, Browne HP, Lawley TD, Mobley HL, Inohara N, Nunez G (2015) Distinct commensals induce interleukin-1beta via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity 42:744–755
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M (2012) Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36:401–414
Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64:104–117
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Siegmund B (2002) Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol 64:1–8
Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr HA, Hartmann G, Dinarello CA, Endres S, Eigler A (2001a) Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol 281:R1264–R1273
Siegmund B, Lehr H-A, Fantuzzi G, Dinarello CA (2001b) IL-1β-converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci 98:13249–13254
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139
Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL (2002) Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut 50:812–820
Song-Zhao GX, Srinivasan N, Pott J, Baban D, Frankel G, Maloy KJ (2013) Nlrp3 activation in the intestinal epithelium protects against a mucosal pathogen. Mucosal Immunol 7:763
Stuyt RJ, Netea MG, Geijtenbeek TB, Kullberg BJ, Dinarello CA, van der Meer JW (2003) Selective regulation of intercellular adhesion molecule-1 expression by interleukin-18 and interleukin-12 on human monocytes. Immunology 110:329–334
Takagi H, Kanai T, Okazawa A, Kishi Y, Sato T, Takaishi H, Inoue N, Ogata H, Iwao Y, Hoshino K, Takeda K, Akira S, Watanabe M, Ishii H, Hibi T (2003) Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol 38:837–844
Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S (1998) Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383–390
Taleban S, Colombel J-F, Mohler MJ, Fain MJ (2015) Inflammatory bowel disease and the elderly: a review. J Crohn’s Colitis 9:507–515
Tamura K, Fukuda Y, Sashio H, Takeda N, Bamba H, Kosaka T, Fukui S, Sawada K, Tamura K, Satomi M, Yamada T, Yamamura T, Yamamoto Y, Furuyama J, Okamura H, Shimoyama T (2002) IL18 polymorphism is associated with an increased risk of Crohn’s disease. J Gastroenterol 37(Suppl 14):111–116
Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H (2003) A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci 94:965–973
Triantafillidis JK, Nasioulas G, Kosmidis PA (2009) Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res 29:2727–2737
Ungerbäck J, Belenki D, Jawad ul-Hassan A, Fredrikson M, Fransén K, Elander N, Verma D, Söderkvist P (2012) Genetic variation and alterations of genes involved in NFκB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis 33:2126–2134
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17:179–188
Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C (2002) Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci 20:489–502
Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL (2004) Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5:1166–1174
Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton A, Gaudet D, Cohen A, Langelier D, Fortin PR, Wither JE, Sarfati M, Rutgeerts P, Rioux JD, Vermeire S, Hudson TJ, Franchimont D (2009) Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet 41:71–76
Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, Reed G, Mecsas JC, Iwakura Y, Bertin J, Goguen JD, Fitzgerald KA, Lien E (2012) The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37:96–107
Williams TM, Leeth RA, Rothschild DE, McDaniel DK, Coutermarsh-Ott SL, Simmons AE, Kable KH, Heid B, Allen IC (2015) Caspase-11 attenuates gastrointestinal inflammation and experimental colitis pathogenesis. Am J Physiol Gastrointest Liver Physiol 308:G139–G150
Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Muhlbauer M, Chou WC, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK, Ting JP (2015) Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med 21:906–913
Wirtz S, Neurath MF (2007) Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 59:1073–1083
Wirtz S, Becker C, Blumberg R, Galle PR, Neurath MF (2002) Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol 168:411–420
Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang J-P, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, Elinav E, Finlay BB, Flavell RA (2014) NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156:1045–1059
Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R (2013) Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38:1154–1163
Yao XM, Zhang CH, Xing Y, Xue G, Zhang QP, Pan FW, Wu GJ, Hu YX, Guo QH, Lu AL, Zhang XM, Zhou RB, Tian ZG, Zeng BH, Wei H, Strober W, Zhao LP, Meng GX (2017) Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat Commun 8:17
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD (2015) The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21:263–269
Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD (2010a) The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32:379–391
Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD (2010b) IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol 185:4912–4920
Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD (2011) The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 20:649–660
Zhang L, Mo J, Swanson Karen V, Wen H, Petrucelli A, Gregory Sean M, Zhang Z, Schneider M, Jiang Y, Fitzgerald Katherine A, Ouyang S, Liu Z-J, Damania B, Shu H-B, Duncan Joseph A, Ting Jenny PY (2014) NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity 40:329–341
Zhao Y, Shao F (2015) The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol Rev 265:85–102
Zhao S, Gong Z, Zhou J, Tian C, Gao Y, Xu C, Chen Y, Cai W, Wu J (2016) Deoxycholic acid triggers NLRP3 inflammasome activation and aggravates DSS-induced colitis in mice. Front Immunol 7:536
Zhao Y, Guo Q, Zhao K, Zhou Y, Li W, Pan C, Qiang L, Li Z, Lu N (2018) Small molecule GL-V9 protects against colitis-associated colorectal cancer by limiting NLRP3 inflammasome through autophagy. OncoImmunology 7:e1375640
Funding
The funding was provided by John Stewart Bowel Cancer Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Perera, A.P., Sajnani, K., Dickinson, J. et al. NLRP3 inflammasome in colitis and colitis-associated colorectal cancer. Mamm Genome 29, 817–830 (2018). https://doi.org/10.1007/s00335-018-9783-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-018-9783-2