Abstract
In the present study, dissection of genetic bases of testis weight in mice was performed. Autosomes and the X chromosome were searched using traditional quantitative trait locus (QTL) scans, and the Y chromosome was searched by association studies of Y-consomic strains. QTL analysis was performed in ♀DDD × ♂CBA F2 mice; the inbred mouse DDD has the heaviest testes, whereas the inbred mouse CBA has the lightest testes. Two significant testis weight QTLs were identified on chromosomes 1 and X. A DDD allele was associated with increased and decreased testis weight at the locus on chromosomes 1 and X, respectively. In the reciprocal cross ♀CBA × ♂DDD F2 mice, QTL on chromosome 1, and not on chromosome X, had a significant effect on testis weight. The DDD allele at the X-linked locus could not sustain testis weight in combination with the Y chromosome of the CBA strain. The Y chromosome per se had a significant effect on testis weight, i.e., DH-Chr YDDD had significantly heavier testes than DH-Chr YCBA. On the basis of the results of Y-chromosome-wide association studies using 17 Y-consomic strains, variations in Uty, Usp9y, and Sry were significantly associated with testis weight. Thus, testis weight is a complex quantitative phenotype controlled by multiple genes on autosomes and sex chromosomes and their interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testis weight is a physiologically important reproductive phenotype because of its direct connection with male fertility, i.e., spermatogenic ability. Indeed, the sperm production rate depends on testis weight, e.g., testis weight in polygamous males is higher than that in monogamous males in primates (Harcourt et al. 1981). A seasonal change in testis weight is reported in wild animals (seasonal breeders); they tend to have a higher testis weight during their breeding season (Leader-Williams 1979).
Testis weight is a representative quantitative phenotype that varies widely among inbred mouse strains. For example, at age 80 days, the average paired testis weight in the DDD/Sgn strain is 296.5 mg, whereas that in the CBA/N strain is only 105.2 mg (Suto 2008; unpublished data). This difference is not due to a difference in body weight because body weight does not vary significantly between these two strains, suggesting the existence of genes that primarily determine testis weight. Several investigators have examined the genetic basis of testis weight by quantitative trait locus (QTL) analysis, and many testis weight QTLs have been identified on mouse chromosomes (Zídek et al. 1998; Le Roy et al. 2001; Elliott et al. 2004; Oka et al. 2004; Storchová et al. 2004; Bolor et al. 2006; L’Hôte et al. 2007; Good et al. 2008; Vyskočilová et al. 2009; Otsuka et al. 2010). Analysis of a series of mouse mutants has also provided direct evidence for the presence of genes associated with testis weight (Mouse Genome Informatics [MGI], http://www.informatics.jax.org). Most of these genes or loci are autosomal or X-linked. Genes on the Y chromosome have also been implicated in the control of testis weight, although this result is controversial to some extent (Hayward and Shire 1974; Herrick and Wolfe 1977; Hunt and Mittwoch 1987; Chubb 1992; Le Roy et al. 2001). Hayward and Shire (1974), Hunt and Mittwoch (1987), and Le Roy et al. (2001) reported results supporting a Y chromosome effect. In contrast, Herrick and Wolfe (1977) and Chubb (1992) claimed that the Y chromosome is unlikely to have a substantial effect. To resolve this controversy, comprehensive analyses of testis weight were performed in Y-consomic strains in which the Y chromosome from various inbred mouse strains was inserted into an inbred DH/Sgn strain background (Suto 2008). My findings support the role of Y-linked genes in the control of testis weight because statistically significant differences were observed among the Y-consomic strains.
The first objective of this study was to identify additional autosomal and/or X-linked QTLs for testis weight. Novel testis weight QTLs could be identified by analyzing F2 intercrosses produced using the above-mentioned DDD and CBA strains. The second objective was to identify the Y-linked gene or genes involved in the control of testis weight. Genes that control testis weight in Y-consomic mouse strains might be identified by searching for associations between gene polymorphisms and testis weight.
Materials and methods
Mice
Inbred mouse strains DH/Sgn (hereafter referred to as DH), DDD/Sgn (DDD), CF1/Sgn (CF1), RR/Sgn (RR), and SS/Sgn (SS) were maintained at the National Institute of Agrobiological Sciences (NIAS, Tsukuba, Japan). Inbred strains A/J (A), CAST/EiJ (CAST), AKR/J (AKR), RF/J (RF), SJL/J (SJL), and SWR/J (SWR) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Inbred strains BALB/cA (BALB), C3H/HeJ (C3H), C57BL/6 J (B6), DBA/2 J (DBA), and KK/Ta (KK) were purchased from Clea Japan (Tokyo, Japan), and the inbred strain CBA/N (CBA) was purchased from Japan SLC (Hamamatsu, Japan).
The Y chromosome of these strains was successively backcrossed to the DH strain for at least 15 generations. The following Y-consomic strains were assessed: DH-Chr YA, DH-Chr YAKR, DH-Chr YB6, DH-Chr YBALB, DH-Chr YC3H, DH-Chr YCAST, DH-Chr YCBA, DH-Chr YCF1, DH-Chr YDBA, DH-Chr YDDD, DH-Chr YDH (this is identical to DH), DH-Chr YKK, DH-Chr YRF, DH-Chr YRR, DH-Chr YSJL, DH-Chr YSS, and DH-Chr YSWR.
All mice were maintained in a specific-pathogen-free facility with a regular light cycle and controlled temperature and humidity. Food (CRF-1, Oriental Yeast Co. Ltd., Tokyo) and water were freely available throughout the experimental period. All animal experiments were performed in accordance with guidelines approved by the Institutional Animal Care and Use Committee of NIAS.
Phenotyping
At age 80 days, the mice were weighed on an electric balance to the nearest 0.01 g. They were then killed, and their testes were removed and placed in physiological saline. After being rinsed and wiped using wet chromatography paper, the paired testes were weighed on the electric balance to the nearest 1 mg. Both “absolute (paired) testis weight” and “relative testis weight (testis weight relative to body weight)” were assessed. The relative testis weight was calculated by dividing testis weight (mg) by body weight (g). Spleen weight was similarly determined for QTL analysis because it served as a reference for a parenchymatous organ. Trait names were abbreviated as follows: Tw for absolute testis weight, rTw for relative testis weight, Sw for absolute spleen weight (mg), and rSw for relative spleen weight [Sw (mg)/body weight (g)]. Tw and rTw and Sw and rSw were simultaneously referred as “testis weight” and “spleen weight,” respectively.
Genotyping of microsatellite markers, single nucleotide polymorphisms (SNPs), and Sry
Microsatellite sequence length polymorphisms were identified after PCR amplification of genomic DNA by 10% polyacrylamide gel electrophoresis and visualized by ethidium bromide staining. SNP genotyping was performed by direct sequencing of the PCR product of the genomic region containing the SNP site. The nucleotide sequence of Sry was also determined by direct sequencing of the PCR product. Sry has nine known polymorphisms that differ between YMus and YDom (Eicher 1994). These include polymorphisms in the number of CAG repeats in Sry.
QTL analysis
As mentioned above, DDD is a mouse strain having the heaviest testes, whereas CBA is a strain having the lightest testes. In this study, QTL analysis was performed in F2 mice produced by crossing DDD and CBA strains.
A total of 96 selected ♀DDD × ♂CBA F2 mice, including 35 mice with the highest Tw, 33 mice with the lowest Tw, 33 mice with the highest rTw, and 39 mice with the lowest rTw, were genotyped for the following 72 microsatellite marker loci: D1Mit316, D1Mit214, D1Mit334, D1Mit390, D1Mit33, D1Mit362, D2Mit312, D2Mit297, D2Mit75, D2Mit274, D2Mit346, D3Mit46, D3Mit217, D3Mit45, D4Mit149, D4Mit214, D4Mit26, D4Mit190, D5Mit200, D5Mit239, D5Mit30, D5Mit223, D6Mit116, D6Mit188, D6Mit111, D7Mit76, D7Mit253, D7Mit15, D8Mit64, D8Mit248, D8Mit215, D9Mit90, D9Mit229, D9Mit212, D9Mit1003, D10Mit188, D10Mit183, D10Mit261, D11Mit231, D11Mit242, D11Mit214, D12Mit109, D12Mit158, D12Mit141, D13Mit207, D13Mit64, D13Mit233, D13Mit130, D13Mit171, D14Mit11, D14Mit120, D14Mit64, D14Mit194, D14Mit165, D15Mit175, D15Mit63, D15Mit159, D16Mit32, D16Mit4, D16Mit139, D17Mit176, D17Mit180, D17Mit93, D18Mit123, D18Mit142, D18Mit25, D19Mit46, D19Mit91, D19Mit71, DXMit141, DXMit172, and DXMit239. Genome-wide scans were performed on ♀DDD × ♂CBA F2 mice, and some of the results were confirmed in ♀CBA × ♂DDD F2 mice. All ♀DDD × ♂CBA F2 mice (n = 253) were genotyped for the underlined microsatellite markers. All ♀CBA × ♂DDD F2 mice (n = 113) were genotyped for microsatellite markers D1Mit33, DXMit141, DXMit172, and DXMit239.
Normality of trait data distribution in F2 mice was tested using the Shapiro–Wilk W test (JMP 8.0.2, SAS Institute Japan, Tokyo). If the trait values did not follow a normal distribution, they were appropriately transformed.
A genome-wide scan was performed in ♀DDD × ♂CBA F2 mice for single QTLs using the R/qtl (Broman et al. 2003; Broman and Sen 2009). Threshold LOD scores for suggestive (P < 0.63) and significant (P < 0.05) linkages were determined by performing 1,000 permutations for each trait. In particular, since selective genotyping was used in this study, stratified permutation tests were applied to the data set as recommended. Furthermore, X-chromosome-specific threshold LOD scores were independently determined. After single QTL scans, pairwise evaluations of the potential interaction between marker loci were performed. At this stage, threshold LOD scores were based strictly on the recommended ones according to “A brief tour of R/qtl” by Broman (http://www.rqtl.org).
Next, data on ♀DDD × ♂CBA F2 mice and ♀CBA × ♂DDD F2 mice were combined and analyzed. To assess evidence for the interaction between X-linked QTLs and the Y chromosome, QTL × covariate interaction was tested with the cross-direction (pgm) included as an interactive covariate.
Y-chromosome-wide association studies
Y-linked genetic variations controlling testis weight were identified using the following three-step approach. First, the effects of genes on autosomes and X chromosomes were eliminated using Y-consomic strains, and the net phenotypic effects of Y-linked genes were assessed. Second, whether a trait was indeed Y-linked was determined using Dunnett’s multiple-comparison tests with the background DH strain as a reference. Third, data from all strains were assembled on the basis of SNP genotypes, and the statistical significance of differences was assessed. Two groups partitioned by genotype were compared using Student’s or Welch’s t-test, and three groups were compared by one-way ANOVA. On the basis of the number of SNP loci (n) genotyped, the significant threshold P value was determined as 0.05/n by Bonferroni correction.
Other statistics
Statistical differences between two and three groups were analyzed using Student’s or Welch’s t-test and one-way ANOVA. Multiple comparisons were performed using Tukey–Kramer HSD tests. P < 0.05 was considered statistically significant.
Broad-sense heritability was determined according to the method of Massett et al. (2009). Briefly, heritability (H 2) was calculated with the formula: H 2 = [V F2 - (1/2V F1 + 1/4V P1 + 1/4V P2)]/V F2, where V P1 and V P2 are the variances of the parental strains, V F1 is the variance of the F1 generation, and V F2 is the variance of the F2 generation.
Results
Testis weight in parental and Y-consomic strains and in F1 and F2 mice
Figure 1a shows scatterplots of Tw in parental DDD (n = 25), CBA (n = 10), DDD × CBA F1 (n = 9), CBA × DDD F1 (n = 14), DDD × CBA F2 (n = 253), CBA × DDD F2 (n = 113), DH-Chr YDDD (n = 41), and DH-Chr YCBA (n = 21) mice.
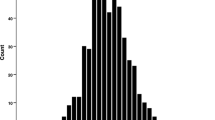
a Scatterplots of paired testis weight in parental DDD and CBA strains, Y-consomic strains, and reciprocal F1 and F2 mice. Each point represents the testis weight of an individual mouse. Horizontal bar indicates the average for the strain. b A histogram showing distribution of paired testis weight in DDD × CBA F2 mice. The mean ± standard error is 176.9 ± 2.1 mg
In parental mice, DDD mice had significantly higher Tw than CBA mice (average 296.5 mg vs. 105.2 mg, P < 6.4 × 10−24). In F1 mice, CBA × DDD F1 mice had significantly higher Tw than DDD × CBA F1 mice (232.5 mg vs. 192.2 mg, P < 0.00003). In F2 mice, Tw in CBA × DDD F2 mice had significantly higher Tw than DDD × CBA F2 mice (198.9 mg vs. 176.9 mg, P < 2.3 × 10−10). In Y-consomic mice, DH-Chr YDDD mice had significantly higher Tw than DH-Chr YCBA mice (204.5 mg vs. 191.6 mg, P < 0.00004).
A broad-sense heritability of Tw was 0.76 in DDD × CBA F2 mice and 0.77 in CBA × DDD F2 mice.
QTL analysis in DDD × CBA F2 mice
Figure 1b shows a histogram of the distribution of Tw in DDD × CBA F2 mice. Trait values of Tw, and not of rTw, Sw, and rSw, were normally distributed (data not shown); therefore, Box–Cox transformation was applied to these data to achieve a normal distribution.
Table 1 shows the results of single QTL scans. Two significant and six suggestive QTLs were identified for Tw. A significant QTL on chromosome 1 was designated Twdq2 because Twdq1 was previously assigned to QTL on chromosome 9 (Fig. 2a) (Suto 2008). At Twdq2, the DDD allele was associated with increased Tw (Table 2). A significant QTL on the X chromosome was designated Twdq3 (Fig. 2a). At Twdq3, the CBA allele was associated with increased Tw. The distal end of 95% CI could not be defined because its position extended distally and no available microsatellite markers were found distal to DXMit239. A suggestive QTL on chromosome 9 was coincidental with Twdq1; therefore, the same gene symbol was assigned to this locus. Except for a locus on chromosome 2, the DDD allele was associated with increased Tw at all suggestive loci. Two significant and three suggestive QTLs were identified for rTw. A significant QTL on chromosome 1 was designated Rtwdq3 because Rtwdq1 and Rtwdq2 were previously assigned to chromosomes 14 and 17, respectively (Fig. 2a) (Suto 2008). At Rtwdq3, the DDD allele was associated with increased rTw (Table 2). A significant QTL on the X chromosome was designated Rtwdq4 (Fig. 2a). At Rtwdq4, the CBA allele was associated with increased rTw. The distal end of 95% CI could not be defined again. Except for a locus on chromosome 6, the DDD allele was associated with increased rTw at all suggestive loci.
LOD score plots for testis weight QTLs on chromosomes 1 and X (a) and for spleen weight QTLs on chromosomes 1 and X (b). X axis represents the microsatellite marker position and Y axis represents the LOD score. LOD score plots for Tw and Sw are indicated by a solid line, while those for rTw and rSw are shown by a dashed line
Coincidental QTLs for testis weight and spleen weight were unexpectedly identified on distal X chromosome (Table 1). Highly significant QTLs for Sw and rSw were designated Swdq2 and Rswdq1, respectively (Fig. 2b) [Swdq1 was previously assigned to QTL on chromosome 11 (Suto 2008)]. At Swdq2 and Rswdq1, the DDD allele was associated with increased spleen weight (Table 2).
Analysis in reciprocal CBA × DDD F2 mice
To confirm the results obtained in DDD × CBA F2 mice, all individuals were genotyped for microsatellite markers D1Mit33, DXMit141, DXMit172, and DXMit239. One-way ANOVA showed that D1Mit33 had significant effects on both Tw and rTw, and DDD alleles at these loci were associated with increased testis weight (Table 2). In contrast, DXMit239 had no significant effects on Tw and rTw, and the DDD allele tended to be associated with increased testis weight. DXMit239 had significant effects on both Sw and rSw, and the DDD allele was associated with increased spleen weight.
When the mean trait values from four F2 subgroups, DXMit239 DDDYDDD, DXMit239 DDDYCBA, DXMit239 CBAYDDD, and DXMit239 CBAYCBA, were simultaneously compared using a Tukey–Kramer HSD test, DXMit239 DDDYCBA showed significantly lower testis weight than the other three F2 subgroups (Table 3) (a significant difference between DXMit239 DDDYDDD and DXMit239 CBAYCBA was also observed for Tw).
To assess evidence for the interaction between X-linked QTL and Y chromosome, both F2 data sets were combined and QTL × covariate interaction was tested with the cross-direction (pgm) included as an interactive covariate. As summarized in Table 4, QTL × pgm interaction was significant for QTL on X chromosome, but was not for QTL on chromosome 1 with regard to both traits.
Y-chromosome-wide association studies
Of the Y-consomic strains, DH-Chr YA, DH-Chr YB6, DH-Chr YBALB, DH-Chr YC3H, DH-Chr YCBA, DH-Chr YCF1, DH-Chr YDBA, DH-Chr YDH, DH-Chr YKK, DH-Chr YRR, and DH-Chr YSS possess the Mus musculus musculus Y chromosome (YMus), whereas DH-Chr YAKR, DH-Chr YDDD, DH-Chr YRF, DH-Chr YSJL, and DH-Chr YSWR possess the Mus musculus domesticus Y chromosome (YDom). The strains were classified on the basis of the following criteria: (1) a C-to-T transitional substitution at nucleotide position (nt) 8491 in the high-mobility group (HMG) box of Sry (nucleotide numbering throughout this article is based on the GenBank entry X67204) (YMus had T and YDom had C) (Kunieda and Toyoda 1992); and (2) the presence of a C-to-T change that created a TAG termination codon at nt 9006 in the third major CAG repeat starting at nt 8985 in YDom (Coward et al. 1994). This was absent in YMus (mouse Sry has four major sites consisting of about 10 CAG repeats). Sry in DH-Chr YCAST had C at nt 8491, but it did not possess TAG at nt 9006. Therefore, YCAST was not classified.
Figure 3 shows a histogram of the distribution of Tw in 472 mice from among the 17 Y-consomic strains. Tw and rTw showed bell-shaped distribution curves (data not shown); however, they did not follow normal distribution in a strict statistical sense. Therefore, Box–Cox transformation was applied before subsequent analyses.
The raw phenotypic data on Tw was compared for each Y-consomic strain (Fig. 4). DH-Chr YC3H, DH-Chr YCBA, DH-Chr YDBA, DH-Chr YKK, DH-Chr YRR, and DH-Chr YSJL had significantly lower Tw than DH-Chr YDH. The results of the comparison of rTw are summarized in Table 5. DH-Chr YDBA, DH-Chr YC3H, and DH-Chr YSS had significantly lower rTw than DH-Chr YDH, whereas DH-Chr YKK had a significantly higher rTw than DH-Chr YDH.
Box (median value ± one quartile) and whisker (range between maximum and minimum values) plots for Tw for each Y-consomic strain are shown. Significant deviations, after Box–Cox transformation, are indicated by asterisks. Significance levels: *P < 0.05, **P < 0.01, ***P < 0.001. The strain names on the X axis apply only to the Y chromosome constitution in an otherwise DH background
Table 6 lists 30 SNPs and 9 Sry polymorphisms identified in the 16 Y-consomic strains (DH-Chr YDH was excluded). These SNP loci were selected on the basis of SNP data retrieved from the Mouse Phenome Database (MPD, http://phenome.jax.org). A high-density strain set, comprising 18 inbred strains, had 18 SNPs associated with nonsynonymous amino acid changes. One of them, rs51394161, which was located on exon 5 of Zfy2, could not be determined; therefore, 17 nonsynonymous SNPs were typed (nonsynonymous SNPs were preferentially selected based on the assumption that coding SNPs must be the causal variants). MPD search also yielded 25 synonymous SNPs, 13 of which were genotyped. Sry polymorphisms included nucleotide substitutions at seven sites and a number of major CAG repeats at two sites (Eicher 1994). Polymorphic patterns are color-coded as yellow, blue, and purple to facilitate discrimination. No polymorphisms were observed at 11 SNP loci; these cells are not color-coded (see online version for color codings).
Table 7 summarizes the results of statistical analyses. Mice were divided into two or three groups according to SNP or polymorphism in Sry. Statistically significant differences in mean values between or among groups were then tested. Because 39 polymorphisms were examined, the significance threshold at α = 0.05 was 0.0013. Variation in the ubiquitously transcribed tetratricopeptide repeat gene, Y chromosome (Uty) gene (rs46947134) was significantly associated with Tw and rTw. DH-Chr YC3H, DH-Chr YCBA, DH-Chr YDBA, and DH-Chr YRR had a G allele, which was associated with decreased Tw and rTw. rs46947134 was a nonsynonymous SNP and was accompanied by an Asp-to-His amino acid change. Variation in the gene for ubiquitin-specific peptidase 9 Y chromosome (Usp9y) (rs51766109) was also significantly associated with rTw. DH-Chr YC3H, DH-Chr YCBA, DH-Chr YDBA, DH-Chr YRR, DH-Chr YCAST, and all DH-Chr YDom strains had the T allele, which was associated with decreased rTw. rs51766109 was a nonsynonymous SNP and was accompanied by a Gly-to-Glu amino acid change.
Twenty-five polymorphisms (color-coded green and red in Table 6) approximated the classification of YMus and YDom. These SNPs were significantly associated with Tw and rTw, i.e., YDom had a significantly higher Tw and rTw than YMus (Table 7). Strains with YDom had significantly higher Tw and rTw than strains with YMus.
The number of first major CAG repeats, starting at nt 8733 of Sry, was significantly associated with Tw and rTw; strains with 12 CAG repeats (DH-Chr YC3H, DH-Chr YCBA, DH-Chr YDBA, and DH-Chr YRR) had significantly lower testis weight than those with 9 or 11 repeats (Table 7). The number of second major CAG repeats, starting at nt 8811 of Sry, was also significantly associated with Tw and rTw; strains with 10 CAG repeats (DH-Chr YC3H, DH-Chr YCBA, DH-Chr YDBA, and DH-Chr YRR) had significantly lower testis weight than those with 12 or 13 repeats. These polymorphisms resulted in differences in the length of the polyglutamine stretch.
Discussion
The DDD allele was associated with increased Tw and rTw at Twdq2/Rtwdq3 on chromosome 1, whereas the CBA allele was associated with increased Tw and rTw at Twdq3/Rtwdq4 on the X chromosome. The low-testis-weight strain-derived allele was associated with increased testis weight. Zídek et al. (1998) identified one significant and two suggestive testis weight QTLs using recombinant inbred strains derived from C57BL/6J and DBA/2J. At two of these three QTLs, including a significant one, a low-testis-weight C57BL/6J strain-derived allele was associated with increased testis weight. The discrepancy was considered to be appropriately explained in this study because the CBA allele was associated with increased testis weight at the locus showing the highest LOD score. Several explanations account for the discrepancy. First, the DDD allele was associated with increased Tw at all suggestive loci except for a locus on chromosome 2, and the DDD allele was associated with increased rTw at all suggestive loci except for a locus on chromosome 6. Thus, cumulative contribution conferred by these suggestive loci should not be underestimated. In particular, suggestive loci on chromosome 9 for Tw and chromosome 14 for rTw were identified as significant QTLs in a previous study involving DDD × DH F2 mice (Suto 2008). Second, the DDD allele may not be able to sustain testis weight when combined with the Y chromosome from the CBA strain. Table 3 shows the results of comparisons of testis weight among mice with four possible genotypes with regard to a combination between X and Y chromosomes. It can be concluded that F2 mice with DXMit239 DDDYCBA have significantly lower values than mice with other genotypes. Furthermore, QTLs on X chromosome were shown to interact with cross-direction, i.e., Y chromosome (Table 4). This does not preclude the possibility that influences of mitochondrial or other genes cause the discrepancy.
Another question to be addressed is whether X-linked QTLs for testis weight and spleen weight are allelic because 95% CIs for both QTLs overlap. At a glance, the allele effect was in opposite directions between the two QTLs, i.e., the DDD allele at Twdq3/Rtwdq4 was associated with decreased testis weight, whereas the DDD allele at Swdq2/Rswdq1 was associated with increased spleen weight. This suggests that they are unlikely to be allelic. However, this is not conclusive because they involve different traits. Indeed, one gene can produce such opposite effects on the weight of different organs. For example, the A y allele at the agouti locus significantly increases the spleen weight but reduces the testis weight in mice (Suto 2009). The spleen weight QTL is probably due to the Bruton agammaglobulinemia tyrosine kinase (Btk, formerly designated as xid) mutation that specifically occurs in the CBA/N strain (Scher et al. 1975; Rawlings et al. 1993; Thomas et al. 1993). According to Marquis et al. (1985), the average spleen weight was significantly higher in the CBA/Ca than in the CBA/N strain. Thus, low spleen weight in CBA/N is probably due to the Btk mutation. The Btk locus is located at the 56.18-cM position on the X chromosome, and this is within 95% CI for Swdq2/Rswdq1. In contrast, low testis weight appears to be common among CBA substrains (Shire and Bartke 1972; Hayward and Shire 1974; Hunt and Mittwoch 1987; Le Roy et al. 2001). On the basis of these facts, the X-linked testis weight QTL (Twdq3/Rtwdq4) is unlikely to be allelic with X-linked spleen weight QTL (Swdq2/Rswdq1).
The distal end of the 95% CI for Twdq3/Rtwdq4 could not be defined. This 95% CI may extend to the pseudoautosomal region (PAR). PAR is a chromosomal region of sequence identity between the X and Y chromosomes. X–Y pairing has been suggested to play an important role in male fertility (Matsuda et al. 1991). Irrespective of the location of Twdq3/Rtwdq4, the reduction in testis weight can be hypothesized to be the consequence of incompatibility between genes on the X and Y chromosomes.
For QTLs identified in this study, coincidental QTLs have been reported. Otsuka et al. (2010) identified a highly significant QTL that is associated with decreased testis weight in MRL mice for Twdq2/Rtwdq3 on chromosome 1. The 95% CI overlaps with that of Twdq2/Rtwdq3. For X-linked QTLs, 95% CIs for QTLs reported by Le Roy et al. (2001) and Bolor et al. (2006) overlap those for Twdq3/Rtwdq4. At these QTLs, the allele from the high-testis-weight strain was associated with increased testis weight. Elliott et al. (2004), Oka et al. (2004), Storchová et al. (2004), Good et al. (2008), and Vyskočilová et al. (2009) also reported X-linked testis weight QTLs as a part of their investigations on hybrid male sterility.
As mentioned above, the Y chromosome is a potential source of testis weight genes, and indeed, DH-Chr YDDD had significantly higher Tw and rTw than DH-Chr YCBA. Further in-depth studies were performed to identify Y-linked genetic variations that control testis weight. A three-step approach was developed for the following reasons: (1) to precisely assess the net phenotypic effects of Y-linked genes using Y-consomic strains to eliminate the effects of genes on autosomes and X chromosomes. Indeed, because major testis weight determinants are autosomal and X-linked (Suto 2008), comparison of the phenotypic effects of Y-linked genes is possible only when the Y chromosomes are isolated, i.e., Y-consomic strains; (2) to determine whether a trait was indeed Y-linked for which Dunnett’s multiple-comparison test was used, with the background DH (DH-Chr YDH) strain as a reference. Using this approach, Tw and rTw were found to be controlled by Y-linked genes. (3) Data from all strains were assembled on the basis of SNP genotypes and tested for the statistical significance of differences. Unlike with autosomes and X chromosomes, genetic mapping was not applicable to the Y chromosome; therefore, this appeared to be the only way to evaluate phenotypic effects. Genome-wide association studies (GWAS) to map genes linked to complex disease-related traits have been attempted in inbred mice (Grupe et al. 2001; Liu et al. 2006; Guo et al. 2007). However, unlike in humans, GWAS in inbred mice revealed spurious associations (Su et al. 2010). This is because of the intricate population genetic structure of laboratory mice (Su et al. 2010). Therefore, controversial arguments exist over the pertinence of performing GWAS in inbred mice (Darvasi 2001; Su et al. 2010). However, GWAS can be a powerful tool in mice when performed in combination with linkage analysis (Park et al. 2003; DiPetrillo et al. 2004; Cervino et al. 2005). In the present study, whether the trait was linked to the Y chromosome was initially determined. This was facilitated by the fact that the Y chromosome range was extremely narrow, and most Y-linked genes described in this study were located within a 2-Mbp region. These experimental steps corresponded to linkage mapping of autosomes. Using the above procedures, several significant associations between gene polymorphisms and testis weight were identified.
Uty and Usp9y encode distinct epitopes of the histocompatibility Y (HY) antigen (Greenfield et al. 1996; Vogt et al. 2000a, b; Graves 2010). In other words, the HY antigen is a mixture of epitopes encoded by different Y-linked genes, and Uty and Usp9y represent such HY-coding genes in mice and humans. Each HY-coding gene is ubiquitously expressed, but their physiological functions are largely unknown. The results of this study suggest possible roles for these genes. In humans, USP9Y has been implicated in infertility associated with oligospermia and azoospermia because of its localization (Sun et al. 1999; Krausz et al. 2006). Although, according to a recent study, USP9Y is not essential for normal spermatogenesis in humans (Luddi et al. 2009), it might play a role in testis weight determination.
Strains with YDom had significantly higher testis weight and relative testis weight than those with YMus. The classification between YDom and YMus is phylogenetically determined; therefore, polymorphisms that are well correlated with this classification (e.g., rs48685451 and rs48834187 in Kdm5d, Table 5) might be similar. However, Kdm5d, formerly called Smcy or jarid1d, was also known as an HY-coding gene (Scott et al. 1995; Wang et al. 1995).
Sry encodes a transcription factor that is a member of the HMG-box family of DNA-binding proteins. The HMG-box region is well conserved among species. In mice, Sry also contains a large CAG trinucleotide repeat region that encodes a glutamine-rich domain. Four major CAG repeat regions exist that contain about 10 CAG repeats. The C-to-T substitution at nt 9006 creates a premature TAG termination codon in the third major CAG repeat region of YDom (Table 6). Accordingly, polymorphisms in the first and second CAG repeat regions were investigated. A polymorphic stretch of CAG repeats in Sry is believed to be correlated with an increased incidence of B6.YDom sex reversal (Eicher et al. 1983; Coward et al. 1994). Intriguingly, Coward et al. (1994) postulated that variations in the polyglutamine amino acid sequence that result from trinucleotide repeats are associated with the degree of sex reversal/hermaphrodism, i.e., YDom that causes complete (severe) sex reversal has 11 repeats, YDom that causes partial (fetal) sex reversal has 13, and YDom that does not cause sex reversal has 12 repeats. This hypothesis has since been rejected by the same group (Carlisle et al. 1996): the severity of sex reversal is not completely correlated with the number of CAG repeats. In contrast, testis weight in this study was completely correlated with the number of CAG repeats. Testis weight decreased as the number of CAG repeats increased, starting at nt 8733, and increased as the number of CAG repeats increased, starting at nt 8811 (Table 6). Of note, the number of CAG repeats in Sry was not limited to the above-mentioned three classes (Miller et al. 1995).
In conclusion, testis weight is a complex quantitative phenotype controlled by multiple genes on autosomes and sex chromosomes and their interactions.
References
Bolor H, Wakasugi N, Zhao WD, Ishikawa A (2006) Detection of quantitative trait loci causing abnormal spermatogenesis and reduced testis weight in the small testis (Smt) mutant mouse. Exp Anim 55:97–108
Broman KW, Sen Ś (2009) A guide to QTL mapping with R/qtl. Springer, New York
Broman KW, Wu H, Sen Ś, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890
Carlisle C, Winking H, Weichenhan D, Nagamine CM (1996) Absence of correlation between Sry polymorphisms and XY sex reversal caused by the M. m. domesticus Y chromosome. Genomics 33:32–45
Cervino AC, Li G, Edwards S, Zhu J, Laurie C, Tokiwa G, Lum PY, Wang S, Castellani LW, Lusis AJ, Carison S, Sachs AB, Schadt EE (2005) Integrating QTL and high-density SNP analyses in mice to identify Insig2 as a susceptibility gene for plasma cholesterol levels. Genomics 86:505–517
Chubb C (1992) Genes regulating testis size. Biol Reprod 47:29–36
Coward P, Nagai K, Chen D, Thomas HD, Nagamine CM, Lau YFC (1994) Polymorphism of a CAG trinucleotide repeat within Sry correlates with B6.YDom sex reversal. Nat Genet 6:245–250
Darvasi A (2001) In silico mapping of mouse quantitative trait loci. Science 294:2423
DiPetrillo K, Tsaih SW, Sheehan S, Johns C, Kelmenson P, Gavras H, Churchill GA, Paigen B (2004) Genetic analysis of blood pressure in C3H/HeJ and SWR/J mice. Physiol Genomics 17:215–220
Eicher EM (1994) Sex and trinucleotide repeats. Nat Genet 6:221–223
Eicher EM, Washburn LL, Whiteney JB, Morrow KE (1983) Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science 217:535–537
Elliott RW, Poslinski D, Tabaczynski D, Hohman C, Pazik J (2004) Loci affecting male fertility in hybrids between Mus macedonicus and C57BL/6. Mamm Genome 15:704–710
Good JM, Dean MD, Nachman MW (2008) A complex genetic basis to X-linked hybrid male sterility between two species of house mouse. Genetics 179:2213–2228
Graves JAM (2010) Sex chromosome evolution and the expression of sex-specific genes in the placenta. Placenta 24:S27–S32
Greenfield A, Scott D, Pennisi D, Ehrmann I, Ellis P, Cooper L, Simpson E, Koopman P (1996) An H-YDb epitope is encoded by a novel mouse Y chromosome gene. Nat Genet 14:474–478
Grupe A, Germer S, Usuka J, Aud D, Belknap JK, Klein RF, Ahluwalia MK, Higuchi R, Peltz G (2001) In silico mapping of complex disease-related traits in mice. Science 292:1915–1918
Guo Y, Lu P, Farrell E, Zhang X, Weller P, Monshouwer M, Wang J, Liao G, Zhang Z, Hu S, Allard J, Shafer S, Usuka J, Peltz G (2007) In silico and in vitro pharmacogenetic analysis in mice. Proc Natl Acad Sci USA 104:17735–17740
Harcourt AH, Harvey PH, Larson SG, Short RV (1981) Testis weight, body weight and breeding system in primates. Nature 293:55–57
Hayward P, Shire JGM (1974) Y chromosome effect on adult testis size. Nature 250:499–500
Herrick CS, Wolfe HG (1977) Effect of the Y-chromosome on testis size in the mouse (Mus musculus). Genetics 86:s27
Hunt SE, Mittwoch U (1987) Y-chromosomal and other factors in the development of testis size in mice. Genet Res 50:205–211
Krausz C, Degl’lnnocenti S, Nuti F, Morelli A, Felici F, Morelli A, Felici F, Sansone M, Varriale G, Forti G (2006) Natural transmission of USP9Y gene mutations: a new perspective on the role of AZFa genes in male fertility. Hum Mol Genet 15:2673–2681
Kunieda T, Toyoda Y (1992) Nucleotide sequence of mouse Sry gene is different between Y chromosome originating from Mus musculus musculus and Mus musculus domesticus. Genomics 13:236–237
L’Hôte D, Serres C, Laissue P, Oulmouden A, Rogel-Gaillard C, Montagutelli X, Vaiman D (2007) Centimorgan-range one-step mapping of fertility traits using interspecific recombinant congenic mice. Genetics 176:1907–1921
Le Roy I, Tordjman S, Migliore-Samour D, Degrelle H, Roubertoux PL (2001) Genetic architecture of testis and seminal vesicle weights in mice. Genetics 158:333–340
Leader-Williams N (1979) Age-related changes in the testicular and antler cycles of reindeer, Rangifer tarandus. J Reprod Fertil 57:117–126
Liu P, Wang Y, Vikis H, Maciag A, Wang D, Lu Y, Liu Y, You M (2006) Candidate lung tumor susceptibility genes identified through whole-genome association analyses in inbred mice. Nat Genet 38:888–895
Luddi A, Margollicci M, Gambera L, Serafini F, Cioni M, De Leo V, Balestri P, Piomboni P (2009) Spermatogenesis in a man with complete deletion of USP9Y. New Engl J Med 360:881–885
Marquis G, Montplaisir S, Pelletier M, Mousseau S, Auger P (1985) Genetic resistance to murine cryptococcosis: Increased susceptibility in the CBA/N XID mutant strain of mice. Infect Immun 47:282–287
Massett MP, Fan R, Berk BC (2009) Quantitative trait loci for exercise training responses in FVB/NJ and C57BL/6J mice. Physiol Genomics 40:15–22
Matsuda Y, Hirobe T, Chapman VM (1991) Genetic basis of X-Y chromosome dissociation and male sterility in interspecific hybrids. Proc Natl Acad Sci USA 88:4850–4854
Miller KE, Lundrigan BL, Tucker PK (1995) Length variation of CAG repeats in Sry across populations of Mus domesticus. Mamm Genome 6:206–208
Oka A, Mita A, Sakurai-Yamatani N, Yamamoto H, Takagi N, Takano-Shimizu T, Toshimori K, Moriwaki K, Shiroishi T (2004) Hybrid breakdown caused by substitution of the X chromosome between two mouse subspecies. Genetics 166:913–924
Otsuka S, Namiki Y, Ichii O, Hashimoto Y, Sasaki N, Endoh D, Kon Y (2010) Analysis of factors decreasing testis weight in MRL mice. Mamm Genome 21:153–161
Park YG, Clifford R, Buetow KH, Hunter KW (2003) Multiple cross and inbred strain haplotype mapping of complex-trait candidate genes. Genome Res 13:118–121
Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, Jenkins NA, Witte ON (1993) Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science 261:358–361
Scher I, Ahmed A, Strong DM, Steinberg AD, Paul WE (1975) X-linked B-lymphocyte immune defect in CBA/N mice. I. Studies of the function and composition of spleen cells. J Exp Med 141:788–803
Scott DM, Ehrmann IE, Ellis PS, Bishop CE, Agulnik AI, Simpson E, Mitchell MJ (1995) Identification of a mouse male-specific transplantation antigen, H-Y. Nature 376:695–698
Shire JGM, Bartke A (1972) Strain differences in testicular weight and spermatogenesis with special reference to C57BL/10J and DBA/2J mice. J Endocrinol 55:163–171
Storchová R, Gregorová S, Buckiová D, Kyselová V, Divina P, Forejt J (2004) Genetic analysis of X-linked hybrid sterility in the house mouse. Mamm Genome 15:515–524
Su WL, Sieberts SK, Kleinhanz RR, Lux K, Millstein J, Molony C, Schadt EE (2010) Assessing the prospects of genome-wide association studies performed in inbred mice. Mamm Genome 21:143–152
Sun C, Skaletsky H, Birren B, Devon K, Tang Z, Silber S, Oates R, Page DC (1999) An azoospermic man with a de novo point mutation in the Y-chromosomal gene USP9Y. Nat Genet 23:429–432
Suto J (2008) Genetic dissection of testis weight in a mouse strain having an extremely large testis: major testis weight determinants are autosomal rather than Y-linked on the basis of comprehensive analyses in Y-chromosome consomic strains. Proc Jpn Acad Ser B 84:393–406
Suto J (2009) The A y allele at the agouti locus reduces the size and alters the shape of the mandible in mice. Proc Jpn Acad Ser B 85:248–257
Thomas JD, Sideras P, Smith CI, Vorechovsky I, Chapman V, Paul WE (1993) Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science 261:355–358
Vogt MHJ, Goulmy E, Kloosterboer FM, Blokland E, de Paus RA, Willemze R, Falkenburg JH (2000a) UTY gene codes for an HLA-B60-restricted human male-specific minor histocompatibility antigen involved in stem cell graft rejection: characterization of the critical polymorphic amino acid residues for T-cell recognition. Blood 96:3126–3132
Vogt MHJ, de Paus RA, Voogt PJ, Willemze R, Falkenburg JH (2000b) DFFRY codes for a new human male-specific minor transplantation antigen involved in bone marrow graft rejection. Blood 95:1100–1105
Vyskočilová M, Pražanová Z, Piálek J (2009) Polymorphism in hybrid male sterility in wild-derived Mus musculus musculus strains on proximal chromosome 17. Mamm Genome 20:83–91
Wang W, Meadows LR, den Haan JM, Sherman NE, Chen Y, Blokland E, Shabanowitz J, Agulnik AI, Hendrickson RC, Bishop CE (1995) Human H-Y: a male-specific histocompatibility antigen derived from the SMCY protein. Science 269:1588–1590
Zídek V, Musilová A, Pintíř P, Šimáková M, Pravenec M (1998) Genetic dissection of testicular weight in the mouse with the BXD recombinant inbred strains. Mamm Genome 9:503–505
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Nos. 15500305 and 19500373).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suto, Ji. Genetic dissection of testis weight in mice: quantitative trait locus analysis using F2 intercrosses between strains with extreme testis weight, and association study using Y-consomic strains. Mamm Genome 22, 648–660 (2011). https://doi.org/10.1007/s00335-011-9353-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-011-9353-3