Abstract
Male F1 hybrids between inbred strains and Mus macedonicus have very small testes and are sterile. Cytological analysis of testes shows very few meioses. To determine the genetic basis for this sterility, (C57BL/6J × Mus macedonics) F1 females were mated to males from C57BL/10J. In about half the male progeny no meiosis I was observed. About half of the animals that progressed through meiosis I showed other indications of low fertility and the balance appeared fertile. QTL analysis of the progeny suggested that loci on proximal Chrs 17 and X were involved in the sterility and a locus on Chr X in variation of body weight. There is also evidence that X//Y dissociation of the pseudo-autosomal region occurs. The QTLs on Chrs X and 17 together account for about 37% of the variance for testis weight. Congenic lines B.MAC-X(1-38), and B.MAC-17(1-23) have been constructed using a modified speed congenic approach. Testis tubules from B.MAC-X(1-38) are narrow and vacuolated. They contain only Sertoli cells and mitotically dividing spermatogonia. Very occasionally a meiotic metaphase can be observed, but no sperm are produced. Homozygous males from B.MAC-17(1-23) are sterile, producing sperm heads but no complete sperm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several loci affecting hybrid fertility have been reported. Five of these (Hst1, Hst4, Hst5, Hst6, Hst7) are on the proximal portion of mouse Chromosome (Chr) 17, and two (Hst3 and Ihtw1) are on Chr X. Hybrid sterility 1 (Hst1) affects fertility in male F1s between inbred strain C57BL/10, carrying Hst1s and M. musculus. Similar F1 hybrids between C3H, carrying Hst1f, and M. musculus are fertile (Forejt and Ivanyi 1974; Trachtulec et al. 1997). Hst4 (Pilder et al. 1991) and Hst5 and Hst6 (Pilder et al. 1993) were found by analyzing crosses involving laboratory strains, M. spretus, and mice carrying t-mutations. Hst6 has been identified as due to species differences in axonemal dynein (Samant et al. 1999; Fosella et al. 2000). Hst7 is on proximal Chr 17 (Pilder et al. 1997). Hst3 is located at the distal end of Chr X (Guenet et al. 1990). It has been studied in crosses between M. spretus and C57BL/6 and probably is located in the pseudo-autosomal region (PAR) (Matsuda et al. 1991). We have reported a locus Ihtw1 (interspecific hybrid testis weight 1) on proximal Chr X, with a phenotype that affects both testis weight and hybrid fertility (Elliott et al. 2001).
Hybrids between C57BL/6J and M. spretus are sterile. This is probably due to lack of X//Y pairing in the PAR. Seminiferous tubules from these hybrids contain spermatocytes, some showing meiotic metaphase I, although mature sperm are not found. As with interspecies crosses involving M. spretus, male F1 hybrids between inbred strains andM. macedonicus are sterile. However, seminiferous tubules from adult hybrids show almost no meiotic metaphase I.
The studies reported here were designed to determine the genetic basis for this sterility. They utilized a backcross in which (C57BL/6J × M. macedonicus) F1 females were mated to C57BL/10J males. In about half the male progeny no meiotic metaphase I was observed. About half of the animals that progressed through meiosis I showed other indications of low fertility and the balance appeared fertile. A genomewide scan was performed and quantitative trait loci (QTLs) for testis weight and body weight have been identified on proximal to central Chr X. X//Y association shows a QTL at distal Chr X, suggesting that, as in the F1 hybrids with M. spretus, the PAR on distal Chrs X and Y is also involved. On Chr 17 there is a QTL for testis weight. To confirm and extend these findings, congenic lines for the proximal ends of Chrs 17 and X have been constructed.
Methods
Animals and DNA
Mice were bred in the colony maintained at Roswell Park Cancer Institute (RPCI). M. macedonicus had been trapped in Bulgaria in 1984 and sent by Dr. Francois Bonhomme to Dr. Verne Chapman. They were maintained at RPCI until 2003. C57BL/6J and C57BL/10J have been bred at RPCI from animals purchased from The Jackson Laboratory. Progeny from the backcross (C57BL/6J × Mus macedonicus) F1 × C57BL/10 were dissected at 8–20 weeks. Body weight and weight of pairs of testes were obtained and spleen and kidneys were frozen for DNA isolation. A genomewide screen using 92 simple single length polymorphisms (SSLPs) was performed, and further SSLPs were typed on Chrs 17 and X. The data were analyzed using Map Manager QT ( Manly and Olsen 1999). A permutation test of 1000 permutations was used to establish the suggestive, significant, and highly significant critical values.
Testis cytology was performed using the air-drying method of Imai et al. (1981) as modified by Matsuda et al. (1991). Briefly, seminiferous tubules were dissociated into fixative and observed using either a fluorescence microscope or a light microscope after Giemsa staining. Necropsies were performed at the Research Animal Diagnostic Laboratory (RADIL), University of Missouri. Histology and PCR were performed as in Elliott et al. (2001).
Results
Hybrid sterility
Testes of (C57BL/6J × M. macedonicus) F1 males were very small, weighing about 0.06 g, and histological sections showed that tubules contained only Sertoli cells and spermatogonia. Average testis weights for C57BL/6J, M. macedonicus, and (C57BL/6J × M. macedonicus) F1 are 0.230 ± 0.013 g (n = 11), 0.22 ± 0.035 g (n = 3), and 0.060 ± 0.012 g (n = 22). This is a more extreme phenotype than that seen in (C57BL/6J × M. spretus) F1 hybrid males. To determine whether hybrid sterility in (C57BL/6J × M. macedonicus) F1 males is due to mispairing in the PAR, as with hybrids involving M. spretus, or whether other genes were involved, the following backcross was generated, (C57BL/6J × M. macedonicus) F1 × C57BL/10J. C57BL/10 was used because it differs from C57BL/6 for several markers in the PAR. Testes from 140 backcross progeny were weighed and analyzed by cytology.
Figure 1 shows a histogram of testis weights among the backcross progeny. There is an eightfold weight range, from 0.039 to 0.315 g between the smallest and largest testis weights. Mean weight ± SD is 0.108 ± 0.06 g, and there is a mode at 0.06 g, close to the mean weight of the F1 testis. This skewed distribution suggests that more than one locus is affecting testis weight in the backcross progeny. Cytological analysis of the very small testes showed no meiotic metaphase I. Of those testes that progressed to meiosis I, about half were small and produced either no sperm, very few sperm, or malformed sperm. The rest of the testes were of normal weight and contained normal sperm. These results are also consistent with the involvement of more than one locus. To determine the genetic positions of these loci a genomewide scan was performed followed by QTL analysis on testis weight, body weight, and X//Y dissociation in the PAR.
Histogram of testis weights. Testis weights were obtained from male progeny of backcross (C57BL/6 × M. macedonicus) F1 × C57BL/10 when they were at least 10 weeks old. Weights among 140 backcross males range from 0.039 to 0.315 g, with the major peak at about 0.06 g. Testis weights from C57BL/6 and M. macedonicus parents are both about 0.2 g.
QTL analysis
A genomewide scan was performed using 92 MIT markers, chosen so that each chromosome was typed for at least 3 loci, with loci close to each chromosomal end and one or more centrally placed loci. Only Chrs X and 17 showed significant associations with testis weight and more markers were then typed on these chromosomes. Figure 2 shows the QTL analysis of testis weight on Chr 17. The proximal and distal markers, at consensus positions 1 and 57.6 cM, flank the full length of Chr 17. A comparison of the marker order with a consensus map of Chr 17 indicates that the markers are in the same order as markers in the consensus map. The positions of the markers on the map in Figure 2 represent recombinational distances determined in the cross, not consensus map positions. The curve represents values of the likelihood ratio statistic (LRS). A fairly broad peak covers the proximal half of Chr 17 and its maximum LRS of 23.2 is above the highly significant value of 21.8 (LOD = 5.0). The peak is found at consensus position 16 cM, near D17Mit198. This is proximal to the H-2 region and close to the previously mapped Hst4 and Hst6 loci. This QTL accounts for 15% of testis weight variation.
QTL analysis of testis weight on Chr 17. QTL analysis of testis weights of 137 progeny was performed using Map Manager QT. A genomewide scan of 92 markers was carried out for all 137 progeny and 15 additional markers were typed on Chr 17. Typed markers range from 2.7 to 56.7 cM on the consensus map. The positions of five previously identified Hst genes are shown for comparison. The curve represents the values of the likelihood ratio statistic (LRS). The LRS for suggestive (6.6), significant (12.5), and highly significant (21.8) critical values are indicated by vertical lines. The LRS value associated with the peak is 23.2.
Figure 3A shows the QTL analysis of testis weight on Chr X. The proximal and distal markers are at positions 1 and 72, including the whole chromosome except the PAR. A comparison of maps for Chr X indicates that the marker order is the same as the consensus map. However, recombination is decreased at the proximal end of the map shown in Figure 3, making the whole map somewhat short. Analysis of testis weight gives a broad peak that extends over the proximal half of Chr X, with a maximum near Hprt, greater than the highly suggestive LRS of 21.8. The breadth of this peak suggests that more than one gene may be involved. This QTL accounts for 22% of testis weight variation.
QTL analysis of testis weight and X//Y dissociation on Chr X. (A) QTL analysis of testis weight of 137 progeny was performed using Map Manager QT. A genomewide scan of 92 markers was carried out for all 137 progeny and 21 additional markers were typed on Chr X. Typed markers range from 1.4 to 72 cM on the consensus map. The curve represents the values of the LRS. Suggestive (6.6), significant (12.5), and highly significant (21.8) critical values are indicated by vertical lines. The LRS value associated with the highest peak was 36.5 (LOD = 7.9). (B) Cytological analysis of 39 animals was used to determine whether X//Y dissociation had occurred. At least ten meioses were examined for each animal and the numbers of meioses with X//Y dissociation was determined. QTL analysis was performed using the genomewide typing above. The curve represents the values of the LRS. Suggestive (6.9) and significant (13.8) critical LRS values are indicated by vertical lines. The maximum LRS value was 21, less than the highly significant critical value of 30.4.
To evaluate the involvement of recombination in the pseudo-autosomal region, testis cytology was used to examine meiosis in male progeny. Most progeny did not show any meiotic figures. However, X//Y dissociation of the PAR could be examined in 39 progeny. QTL analysis was performed on these animals and Figure 3B shows a peak with LOD = 4.6 at DXMit31 (72 cM), the most distal marker typed. The peak is above the significant critical value (LRS = 13.8) but below the highly significant critical value (LRS = 30.4). The QTL accounts for 42% of the variation of X//Y dissociation. This distal peak suggests that for the subset of backcross progeny that did enter meiosis, hybrid sterility involved a lack of pairing in the PAR, as has been found for hybrids involving M. spretus.
At the time of dissection, body weight was determined. This ranged from 21.1 to 60.1 g with a mean of 30.7 ± 7 g. QTL analysis of body weight was performed, and only Chr X showed a significant association. Figure 4 shows the QTL analysis of body weight, which has a peak near the center of Chr X. Maximum peak height is 19.3 (LOD = 4.2), which lies above the critical value for highly significant (LRS = 17.4). This QTL accounts for 12% of body weight variation.
QTL analysis of body weight. QTL analysis of body weight was performed using Map Manager QT. Body weight was obtained from animals 8–20 weeks old. Correlation coefficient between age and body weight was 0.14. QTL analysis was performed using genomewide scan described in Figure 3. The curve represents LRS values. Suggestive (6.3), significant (12.2), and highly significant (17.4) LRS critical values are shown by vertical lines. The maximum LRS value was 19.3.
Congenic strain construction
To confirm the findings of the QTL analysis and to begin to isolate the genes causing the hybrid sterility, congenic strains for proximal Chr X and proximal Chr 17 were constructed using a modified speed congenic approach. A new backcross, (C57BL/6 × M. macedonicus) F1 × C57BL/10, was generated. Ten female progeny carrying alleles from M. macedonicus at the proximal end of Chr X or Chr 17 were identified and backcrossed to C57BL/6J. Backcrossing was continued until the N6 generation was reached, always selecting for alleles from M. macedonicus on either proximal Chr X (using DXMit55, 23, and 63) or Chr 17 (using D17Mit19, 80, and 50). Distal markers DXMit31 and D17Mit123 were also checked until they lost the M. macedonicus genotype. Loci on other autosomes were typed, beginning at the N6 generation. Those females that were homozygous C57BL/6 for the greatest number of autosomal loci were selected for the N7 generation. Two lines for each chromosome were continued at this time. At N8 it was found that some females were homozygous for all typed markers on unselected chromosomes and these were used to establish congenic lines. The congenic interval on Chr X extended from 1.4 cM (DXMit55; 3,528,086 in the 2/03 assembly) to 38 cM (DXMit63; 78,970,802), so we have named the congenic strain B.MAC-X(1–38). The congenic interval on Chr 17 extended from 1 cM (D17Mit172; 3,164,162) to 23.2 cM (D17Mit50; 43,562,047), so it has been named B.MAC-17(1–23).
Analysis of congenic X males
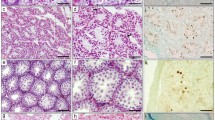
Males carrying the Chr X congenic interval were identified by typing DXMit55 (1.4 cM), DXMit23 (17 cM), and DXMit63 (38 cM). Average testis weight was 0.052 ± 0.012 g (n = 15). Cytological analysis of these 15 males showed either no meiotic metaphase I or only one metaphase in many fields. Mitotic metaphases were observed in spermatogonia of all but one of the samples. In most males no sperm heads were observed, although occasionally an abnormally shaped sperm head was seen. Histological sections of testes (Fig. 5A) showed that all tubules were narrow and contained large vacuoles. Tubules contained only Sertoli cells and spermatogonia. Controls included testes from five males obtained from progeny that had lost the congenic region and carried alleles from C57BL/6J for the proximal region of Chr X. These animals had normal testes both in histologic sections (Fig. 5B) and in cytological spreads. Many meioses were observed, and all males possessed many normal sperm. For these controls, average testis weight was 0.200 ± 0.005 g.
Histology of two congenic strains. Hematoxylin and eosin–stained sections of mouse testis (×200). (A) From B.MAC-X(1-38). Note vacuoles and tubule width, as well as general disorganization of tubule contents and large intertubular area. (B) From normal sib of A. All of Chr X is derived from C57BL/6J. Note tubule diameter and normal cellular organization. (C) From B.MAC−17(1-23). Note lack of lumen in several tubules.
To determine whether other organs are affected in these animals, a single adult male was sent to RADIL which performed a complete necropsy, comparing it with a C57BL/6 male from our colony. Fifty-seven organs were examined and, other than the testis, no significant lesions were detected. Clinical chemistries were also within normal ranges, as was the hematological analysis. Only platelet numbers were below normal. These results suggest that in the male the effect of the congenic region is limited to the changes we have observed in the testis.
Analysis of congenic 17 males
In the N2 generation, animals heterozygous for proximal Chr 17 had low weight testes. At the N3 generation, the hybrid congenic animals had testes of normal weight. Congenic construction was continued, however, with the intent of studying the interaction of the hybrid regions in Chrs X and 17. At the N8 generation all autosomes other than Chr 17 were homozygous for typed alleles from C57BL/6. Intercrossing was performed with the intention of generating a congenic strain homozygous for alleles from M. macedonicus over the whole Chr 17 interval. The homozygous hybrid congenic females were fertile, but homozygous congenic males were sterile. Four homozygous congenic males were mated with females that were either homozygous or heterozygous congenic or mated with C57BL/6 females. The females exhibited plugs, indicating normal mating behavior, but no progeny were produced. Average testis weight for the sterile males was 0.136 ± 0.025 (n = 4). Histology of the testes (Fig. 5C) shows that many testis tubules are completely filled, lacking lumen. Abnormally shaped sperm heads can be identified, but no sperm tails. This suggests that homozygous hybrid congenic males reach a block late in spermiogenesis, as some sperm precursors can be seen but no mature sperm.
To determine whether other organs are affected in this congenic strain, a single adult male, homozygous for the congenic region on Chr 17, was sent to RADIL for a complete necropsy. The same 57 organs were analyzed and the changes we had observed in the testis were found during the necropsy. Of the other organs, all were found to be normal but two. These were the bulbourethral gland, where fewer basophilic cells and more eosinophilic cells were reported, and the preputial gland, where atrophy of the parenchymal cells was reported. As above, the clinical chemistry and hematological results were normal. Thus, the testis and possibly two of the accessory sex glands appear to be the only organs affected in this hybrid congenic male.
Discussion
This study was initiated with the observation that meiotic metaphase I was missing from testes of (C57BL/6 × M. macedonicus) F1 hybrids and that testis weight was about 25% of normal. The first phase of the study involved a backcross of the F1 hybrid with C57BL/10J. QTL analysis of the progeny showed an association of genetic regions on proximal Chrs 17 and X with testis weight. In the second phase, hybrid congenic strains were constructed for these chromosomal regions. Preliminary analysis of the congenic strains has shown that loci on proximal Chr X appear to be primarily responsible for the extremely low testis weight and the lack of meiotic metaphase I. The testis defect associated with Chr 17 acts much later in sperm development and requires homozygosity of the hybrid region.
Hybrids between inbred strains and M. spretus have been analyzed previously in a number of laboratories. The major cause of male infertility in these hybrids appears to be the lack of pairing in the PAR (Guenet et al. 1990; Matsuda et al. 1991). Metaphase I is completed in these animals, but spermatocyes die before metaphase II. A secondary cause of decreased male fertility lies more proximal on Chr X (Elliott et al. 2001).
QTL analysis of the cross with M. macedonicus reported here shows the potential for involvement of X//Y dissociation between chromosomes from C57BL/6 and M. macedonicus. This is the cause for infertility in M. spretus hybrids. However, most hybrids involving M. macedonicus do not show meiotic metaphase I. Thus, the likely immediate cause of their infertility appears to be due to loci that are more proximal on Chr X and act before X//Y pairing occurs.
The QTL involving body weight was obtained from animals ranging from 8 to 20 weeks of age. Mice reach much of their adult weight by 8 weeks and increase weight only slightly during the next 12 weeks. Furthermore, we showed a very low correlation between body weight and age. The weight range we observed could not be explained by the 12-week variation in age. There are other reports of a QTL for body weight in this genetic region (Dragani et al. 1995; Brockman et al. 1998; Liu et al. 2001), and it is possible that all represent the same one or two QTLs.
Markers for Chr 17 and Chr X in Figures 2–4 show the same order as these markers in their respective consensus maps, obtained from the most recent Chromosome Committee maps in Mouse Genome Informatics (MGI). Furthermore, they have the same order as these markers in sequence-based maps for these chromosomes (Elliott 2003) (found at ftp://ftp.informatics.jax.org/%2Fpub/datasets/index.html). The similarity between the map in Figure 2 and the sequence-based map for Chr 17 suggests that the organization of this chromosome is like C57BL/6J rather than M. spretus. However, the decrease in recombination frequency at the proximal end of Chr X suggests that there may be an inversion between M. macedonicus and C57BL/6J in this region.
During the process of generating the congenic strains, the females were genotyped and used for breeding. Male progeny of breeders were checked for the phenotype of low testis weight associated with alleles on Chr X from M. macedonicus. This phenotype was retained throughout the breeding process and and was a valuable and rapidly ascertained phenotype that was used in conjunction with the genotyping. In the last three generations of breeding, the loci examined included markers that were at the extreme ends of chromosomes as well as indicated internal markers. Care was taken to avoid double recombinations resulting from adjacent recombination in different generations. However, as the line is maintained by backcrossing and progeny testing, any undetected double recombinants would be lost over time as breeding proceeds.
A similar approach was used for the Chr 17 congenic strain. However, loss of testis weight phenotype for male progeny at generation N3 meant that animal choices were made purely on genotyping. As there was no male sterility in the heterozygotes, both males and females were used for breeding. At the N8 generation an intercross was performed and the phenotype of male infertility was observed in males homozygous for M. macedonicus alleles on proximal Chr 17. Again, as the line is maintained by backcrossing heterozygotes to C56BL/6J, any residual double crossovers would disappear with time. For both lines, markers close to the centromere were typed. We could not determine whether Chr 17 or Chr X centromeres from M. macedonicus are included in the congenic interval, but it is likely that they are included.
Both congenic strains described here show male sterility, but the reasons for sterility appear to be quite different. In B.MAC-X(1–38) testis weight is 25% of normal adult testis weight and does not exceed that for 3-week-old males. Tubules are narrow and contain only Sertoli cells and mitotically dividing spermatogonia. Cells undergoing meiosis very occasionally can be detected, but no normal spermatozoa have been found. Thus, entry into meiosis appears to lead to death of the germ cells. In B.MAC-17(1–23) testis weight is less than normal but in the range of 0.17–0.13 g. Meiosis proceeds normally but spermiogenesis is affected and there is no evidence for the formation of sperm tails. This is similar to a phenotype described for Hst6 (Fossella et al. 2000) involving Dnahc8, which makes this locus a good candidate for the phenotype described here.
Many mutants that affect testis development cause the formation of tubules with vacuoles, for example, Spc3 (Yuan et al. 2000), and include the synthetic mutant we have described in a congenic strain containing a short region of Chr X from M. spretus (Elliott et al. 2001). The congenic strain formation and analysis described above has allowed the identification of new synthetic mutants affecting sterility. These synthetic mutants map on proximal Chrs X and 17.
References
GA Brockmann CS Haley U Renne SA Knott M Schwerin (1998) ArticleTitleQuantitative trait loci affecting body weight and fatness from a mouse line selected for extreme high growth Genetics 150 369–381 Occurrence Handle1:CAS:528:DyaK1cXmtFKgsLc%3D Occurrence Handle9725853
TA Dragani ZB Zeng F Canzian M Gariboldi MT Ghilarducci et al. (1995) ArticleTitleMapping of body weight loci on mouse chromosome X Mamm Genome 6 778–781 Occurrence Handle1:CAS:528:DyaK28XktVWlug%3D%3D Occurrence Handle8597632
Elliott RW (2003) Mouse genome sequence-based maps for Chrs 17 and X. ftp://ftp.informatics.jax.org/%2Fpub/datasets/index.html
RW Elliott DR Miller RS Pearsall C Hohman Y Zhang et al. (2001) ArticleTitleGenetic analysis of testis weight and fertility in an interspecies hybrid congenic strain for Chr X Mamm Genome 12 45–51 Occurrence Handle10.1007/s003350010234 Occurrence Handle1:CAS:528:DC%2BD3MXptlGqug%3D%3D Occurrence Handle11178743
J Forejt P Ivanyi (1974) ArticleTitleGenetic studies on male sterility of hybrids between laboratory and wild mice (Mus musculus L.) Genet Res 24 189–206 Occurrence Handle1:STN:280:CSqC3MzkslY%3D Occurrence Handle4452481
J Fosella SA Samant LM Silver SM King KT Vaughan et al. (2000) ArticleTitleAn axonemal dynein at the hybrid sterility 6 locus: implications for t-haplotype-specific male sterility and the evolution of species barriers Mamm Genome 11 8–15 Occurrence Handle10.1007/s003350010003 Occurrence Handle10602986
JL Guenet C Nagamine D Simon–Chazottes X Montagutelli F Bonhomme (1990) ArticleTitleHst−3: an X-linked hybrid sterility gene Genet Res 56 163–165 Occurrence Handle1:STN:280:By6C3cnnsFE%3D Occurrence Handle2272506
HT Imai Y Matsuda T Shiroishi K Moriwaki (1981) ArticleTitleHigh frequency for X–Y chromosome dissociation in primary spermatocytes of F1 hybrids between Japanese wild mice (Mus musculus molossinus) and inbred laboratory mice Cytogenet Cell Genet 29 166–175 Occurrence Handle1:STN:280:Bi6C1cjkslQ%3D Occurrence Handle7226897
X Liu F Oliver SD Brown P Denny PD Keightley (2001) ArticleTitleHigh-resolution quantitative trait locus mapping for body weight in mice by recombinant progeny testing Genet Res 77 191–197 Occurrence Handle10.1017/S0016672301004943 Occurrence Handle1:CAS:528:DC%2BD3MXktVGqsL4%3D Occurrence Handle11355574
KF Manly JM Olson (1999) ArticleTitleOverview of QTL mapping software and introduction to map manager QT Mamm Genome 10 327–334 Occurrence Handle10.1007/s003359900997 Occurrence Handle1:CAS:528:DyaK1MXhvFyqtLY%3D Occurrence Handle10087288
Y Matsuda T Hirobe VM Chapman (1991) ArticleTitleGenetic basis of X–Y chromosome dissociation and male sterility in interspecific hybrids Proc Natl Acad Sci USA 88 4850–4854 Occurrence Handle1:STN:280:By6B2s7ivFQ%3D Occurrence Handle2052565
SH Pilder MF Hammer LM Silver (1991) ArticleTitleA novel mouse chromosome 17 hybrid sterility locus: implications for the origin of t haplotypes Genetics 129 237–246 Occurrence Handle1:CAS:528:DyaK38XnslOkuw%3D%3D Occurrence Handle1936961
SH Pilder P Olds–Clarke DM Phillips LM Silver (1993) ArticleTitleHybrid sterility−6: a mouse t complex locus controlling sperm flagellar assembly and movement Dev Biol 159 631–642 Occurrence Handle10.1006/dbio.1993.1270 Occurrence Handle1:STN:280:ByuD3cbptlw%3D Occurrence Handle8405685
SH Pilder P Olds–Clarke JM Orth WF Jester L Dugan (1997) ArticleTitleHst7: A male sterility mutation perturbing sperm motility, flagellar assembly, and mitochondrial sheath differentiation J Androl 18 663–671 Occurrence Handle1:STN:280:DyaK1c%2Fpt1CgtA%3D%3D Occurrence Handle9432139
SA Samant J Fossella LE Silver S Pilder (1999) ArticleTitleMapping and cloning recombinant breakpoints demarcating the hybrid sterility 6-specific sperm tail assembly defect Mamm Genome 10 88–94 Occurrence Handle10.1007/s003359900950 Occurrence Handle1:CAS:528:DyaK1MXotVyntw%3D%3D Occurrence Handle9922385
Z Trachtulec M Mnukova–Fajdelova RMJ Hamvas S Gregorova WE Mayer et al. (1997) ArticleTitleIsolation of candidate hybrid sterility 1 genes by cDNA selection in a 1.1 megabase pair region on mouse chromosome 17 Mamm Genome 8 312–316 Occurrence Handle10.1007/s003359900430 Occurrence Handle1:CAS:528:DyaK2sXjtVOhsLc%3D Occurrence Handle9107673
L Yuan J-G Liu J Zhao E Brundell B Daneholt et al. (2000) ArticleTitleThe murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis and male fertility Mol Cell 5 73–83 Occurrence Handle10.1016/S1097-2765(00)80404-9 Occurrence Handle1:CAS:528:DC%2BD3cXpvFKntg%3D%3D Occurrence Handle10678170
Acknowledgments
This study was supported by NIH grant GM33160 to R.W. Elliott. The work was initiated by Verne Chapman. We thank Yoichi Matsuda for providing training in the cytological procedures. This project utilized core facilities partially supported by RPCI’s NCI-funded Cancer Center Support Grant P30 CA016056.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elliott, R.W., Poslinski, D., Tabaczynski, D. et al. Loci affecting male fertility in hybrids between Mus macedonicus and C57BL/6. Mamm Genome 15, 704–710 (2004). https://doi.org/10.1007/s00335-004-2388-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00335-004-2388-y