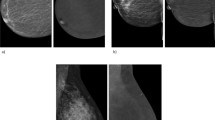
Abstract
Objectives
To summarize our institutional experience with contrast-enhanced mammography (CEM) exams reporting asymmetric background parenchymal enhancement (BPE).
Materials and methods
Consecutive CEMs performed between December 2012 and July 2023 were retrospectively reviewed to identify exams reporting asymmetric BPE. Associated factors, the level of reporting certainty, BI-RADS score, diagnostic workup, and clinical outcome were summarized. BPE grades and BI-RADS were compared between initial CEM vs. immediate MRI and 6-month follow-up CEM, when indicated, using the Sign test.
Results
Overall, 175/12,856 (1.4%) CEMs (140 female patients, mean age, 46 ± 8.0 years) reported asymmetric BPE. Reporting certainty was mostly high (n = 86), then moderate (n = 59) and low (n = 30). Associated factors included contralateral irradiation (n = 94), recent ipsilateral breast treatment (n = 14), and unilateral breastfeeding (n = 4). BI-RADS scores were 0 (n = 21), 1/2 (n = 75), 3 (n = 67), 4 (n = 3), and 6 (n = 1), or given for a finding other than asymmetric BPE (n = 8). Initial diagnostic-workup often included targeted-US (n = 107). Immediate MRI (n = 65) and/or 6-month CEM follow-up (n = 69) downgraded most cases, with a significant decrease in BPE grade compared to the initial CEM (p < 0.01 for both). On follow-up, two underlying cancers were diagnosed in the area of questionable asymmetric BPE.
Conclusion
Apparent asymmetric BPE is most often a benign finding with an identifiable etiology. However, rarely, it may mask an underlying malignancy presenting as non-mass enhancement, thus requiring additional scrutiny.
Clinical relevance statement
The variability in the diagnostic-workup of apparent asymmetric background parenchymal enhancement stresses the clinical challenge of this radiological finding. Further studies are required to verify these initial observations and to establish standardized management guidelines.
Key Points
-
Apparent asymmetric background parenchymal enhancement usually represents a benign clinical correlate, though rarely it may represent malignancy.
-
Evaluation of asymmetric background parenchymal enhancement varied considerably in the metrics that were examined.
-
Targeted US and MRI can be useful in evaluating unexplained asymmetric background parenchymal enhancement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Background parenchymal enhancement (BPE) refers to the normal fibroglandular tissue enhancement subsequent to contrast administration, as observed originally on the breast magnetic resonance imaging (MRI) scans of healthy volunteers, with fluctuating enhancing foci during the menstrual cycle [1, 2]. On breast MRI, BPE is typically portrayed as having a “cortical” or “picture framing” configuration, owing to the peripheral vascular supply of the breast [3], and is graded qualitatively into one of four ordinal categories, of minimal, mild, moderate and marked, using the BI‐RADS four‐point scale [4].
In recent years, contrast-enhanced mammography (CEM) has emerged as a viable alternative to breast MRI, facilitating perfusion breast imaging [5] at reduced costs and at potentially greater availability [6]. BPE on CEM has shown moderate to substantial inter-reader agreement [7,8,9] and significant associations with mammographic density, menopausal status, prior breast radiation, and hormonal therapy [8,9,10]; in addition, it was suggested to be an independent risk factor for developing breast cancer [11, 12].
The fifth addition of the Breast Imaging-Reporting and Data System (BI-RADS) lexicon advocated reporting whether BPE on MRI is symmetric or asymmetric [13], and more recently, in 2022, the CEM supplement to the BI-RADS lexicon has followed this instruction [14]. Indeed, BPE is usually symmetric and less frequently asymmetric [3]. Symmetric BPE manifests as a mirror-image enhancement of both breasts which provides reassurance regarding the physiological nature of this enhancement [15]. On the contrary, asymmetric BPE manifestation occurs in cases in which a more prominent and broader distribution of enhancement appears in one breast when compared with the other breast. To date, only scant scientific evidence exists about asymmetric BPE, including its prevalence, clinical significance, and management. Thus, the purpose of this study was to summarize our institutional experience with CEM exams reporting asymmetric BPE across a ~ 10-year period. In particular, we sought to evaluate the extent of this radiological finding, associated factors, diagnostic workup, and clinical outcome.
Materials and methods
This retrospective Health Insurance Portability and Accountability Act-compliant review was approved by our institutional review board. The necessity for informed consent was waived.
Study sample
A computational search of a prospectively maintained database within our institutional radiology information system (RIS) was conducted, targeting consecutive CEM exams performed between December 2012 and July 2023. Our search parameters included specific filter terms such as “asymmetric” and “greater” (e.g., “marked BPE, right greater than left”) to identify cases reporting asymmetric BPE [14].
Subsequently, to validate our final study cohort, a case-by-case review was undertaken to exclude any potentially misclassified cases, particularly those erroneously collected as representing an “asymmetry” (on low energy views), rather the “asymmetric (BPE)”.
CEM technique
All CEM examinations were acquired using a dual-energy mammography system (Senographe Essential; GE Medical Systems). Per our institutional protocol, intravenous iohexol (Omnipaque 350; GE Healthcare) at a dose of 1.5 mL/kg up to a maximum dose of 150 mL was automatically injected at a rate of 3 mL/s, followed by a saline flush. Two minutes after contrast injection, breasts were imaged using two standard views, mediolateral oblique (MLO) and craniocaudal (CC), with almost simultaneous low- (26–30 kVp) and high- (45–49 kVp) energy exposures. The low-energy exposure serves to provide the equivalent of digital mammography, whereas, the combined information of the low and high-energy exposures serves to construct the so-called recombined or iodine image, showing areas of contrast uptake [16].
CEM interpretation and data assessment
The official CEM reports, each of which was originally dictated by one of the breast radiologists at our institution, were used for data collection including indication for imaging, mammographic density BI-RADS category, BPE classification, including the dominant side of BPE and the grade of BPE at that dominant side (i.e., grade 0, minimal; grade 1, mild; grade 2, moderate and grade 3, marked), suspicious findings and the modality (LE, recombined images, or both) on which they were detected, any additional imaging performed (i.e., additional mammographic views and/or targeted US, etc.), and final BI-RADS score, assigned for both modalities (LE and recombined) together. Since 2013, the reporting of BPE has been an integral component of our institutional contrast-enhanced breast imaging reporting template (including both CEM and breast MRI), adhering closely to the Breast Imaging-Reporting and Data System (BI-RADS) lexicon standards.
In addition, the level of certainty with which asymmetric BPE was reported was recorded as follows: high, for cases in which asymmetric BPE was defined (i.e., “consistent with”); moderate, for cases in which asymmetric BPE was suggested to be “probable”; and low, for cases in which asymmetric BPE was suggested to be “possible” [17].
The diagnostic workup and follow-up results of cases reporting of asymmetric BPE were reviewed and summarized. In cases where further investigation by MRI and/or 6-month follow-up CEM was performed, the outcome, BI-RADS score, and BPE characteristics of those exams, as originally appeared on the of official radiology report, were recorded and compared to those of the initial CEMs. Medical records were reviewed to obtain information on age, risk factors, biopsy results, tumor pathology, and clinical information possibly related to asymmetric BPE.
Statistical analysis
Descriptive statistics were summarized by using frequencies and percentages. For the evaluation of BPE, ordinal numbering was assigned to BPE grades, with 0, 1, 2, and 3 representing minimal, mild, moderate, and marked grades, respectively. BPE grades on the initial CEM vs. immediate MRI and 6-month follow-up CEM were compared and correlated using the two-tailed Sign test and Spearman’s rank correlation coefficient test, respectively (R: A language and environment for statistical computing, 2020). Statistical significance was defined as p < 0.05.
Results
CEM exam and patient characteristics
Overall, asymmetric BPE appeared in 175 (of 140 patients) out of 12,856 CEM exams in the time span between December 2012 and July 2023, accounting for 1.4% of exams. Of the exams with asymmetric BPE, slightly more than a third (69/175, 39.4%) were performed in 2022–2023, reflecting both the increase in CEM utility in our institution in recent years, as well as the adherence to the 2022 CEM BI-RADS lexicon requirement to report the symmetry or asymmetry of BPE [14]. The indication for CEM was mostly screening (n = 159/175, 90.9%), followed by 6-month follow-up (n = 13/175, 7.4%), evaluation of a palpable concern (n = 2/175, 1.1%), and extent of disease evaluation (n = 1/175, 0.6%). The mean age of the patients was 46 ± 8.0 years. The majority of patients (132/140, 94.3%) were at elevated risk for developing breast cancer (Table 1). In terms of mammographic density, breasts were most commonly heterogeneously dense (n = 104/175, 59.4%), followed by extremely dense (n = 62/175, 35.4%) and a scattered fibroglandular pattern (n = 9/175, 5.1%).
Asymmetric BPE evaluation and associated factors
The grade of the dominant asymmetric BPE was mainly moderate (n = 90/175, 51.4%), followed by mild (n = 50/175, 28.6%), marked (n = 33/175, 18.9%), and minimal (n = 1/175, 0.6%). In one case, the grade was not reported, due to the inability to determine whether the questionable enhancement was asymmetric BPE or not. The side of the dominant asymmetric BPE was evenly distributed for the right (n = 89/175, 50.9%) and left (n = 86/175, 49.1%) breast. Asymmetric BPE certainty was most commonly high (n = 86/175, 49.1%), followed by moderate (n = 59/175, 33.7%) and low (n = 30/175, 17.1%). In most CEM exams, patients had a prior vascular breast imaging exam (n = 99/175, 56.6%), of either CEM alone (n = 33), MRI alone (n = 32), or both (n = 66). In most cases, asymmetric BPE was attributed to an identifiable factor, including history of breast irradiation contralateral to the dominant BPE (n = 94/175); recent breast treatment, including surgery and irradiation, ipsilateral to the dominant BPE (n = 14/175); and unilateral breastfeeding, ipsilateral to the dominant BPE (n = 4/175) (Table 1). Representative images of asymmetric BPE attributed to these respective associated factors are given in Figs. 1–3.
Asymmetric background parenchymal enhancement (BPE) on contrast-enhanced mammography (CEM) in a patient with a history of unilateral breast irradiation. Recombined mediolateral oblique (a, b) and craniocaudal (c, d) CEM images of a representative 47-year-old patient with a remote history of right breast cancer treated with lumpectomy and chemoradiation. Longstanding post-treatment changes with asymmetric BPE, which is marked on the left (b, d) and minimal on the right (a, c), are demonstrated and represent changes attributed to right breast irradiation
Asymmetric background parenchymal enhancement (BPE) on early post-treatment contrast-enhanced mammography (CEM) in a patient with recent breast treatment. Recombined mediolateral oblique images of early (a, b) and 1-year follow-up (c, d) CEM images of a representative 39-year-old patient with a recent history of right breast cancer treated with lumpectomy and chemoradiation. Early post-treatment changes (6 months after surgery) with moderate BPE are visible on the right (a) whereas minimal BPE is exhibited on the left (b). At 1-year follow-up, a dramatic decrease in the right breast BPE is observed, with bilateral minimal BPE (c, d)
Asymmetric background parenchymal enhancement (BPE) on contrast-enhanced mammography (CEM) in a unilateral breastfeeding patient. Recombined mediolateral oblique (a, b) CEM images of a representative 38-year-old patient lactating from the left breast only, with a remote history of right breast cancer. Post-treatment changes with minimal BPE are visible on the right, previously irradiated breast (a), whereas diffuse marked BPE is exhibited on the left lactating breast, one-month postpartum (b). Asymmetric BPE is also demonstrated on the post-contrast subtracted maximum intensity projection MRI image (c) performed for further evaluation. Upon breastfeeding cessation and follow-up, a dramatic decrease in the left breast BPE is observed (d)
CEM BI-RADS and diagnostic workup recommendations
Most cases were scored as BI-RADS 1/2 (n = 75/175, 42.9%), followed by BI-RADS 3 (67/175, 38.3%), BI-RADS 0 (21/175, 12%), BI-RADS 4 (3/175, 1.7%), BI-RADS 6 (1/175, 0.6%), or a BI-RADS score was given for a finding other than asymmetric BPE (n = 8/175, 4.6%).
Of the 75 BI-RADS 1/2 cases, the initial diagnostic workup included targeted US (n = 25), additional mammographic views (n = 6), or both (n = 2). Of the 67 BI-RADS 3 cases, the initial diagnostic workup included targeted US (n = 53), with (n = 4) or without additional mammographic views (n = 49). The BI-RADS 3 cases were followed by a recommendation of either an immediate MRI (n = 41) with (n = 34) or without (n = 7) 6-month CEM follow-up imaging, or simply 6-month CEM follow-up imaging alone (n = 25). In one BI-RADS 3 case, the asymmetric BPE was unchanged for one year and therefore one year CEM follow-up was recommended. Of the 21 BI-RADS 0 cases, the initial diagnostic workup included MRI (n = 20), with (n = 16) or without targeted US (n = 4). In one case targeted US was performed with a recommendation of 6-month CEM follow-up. Of the 3 BI-RADS 4 cases, all were followed by a recommendation for an immediate MRI, which resulted in one benign MRI-guided biopsy yielding pseudo-angiomatous stromal hyperplasia (PASH) and two cancelations of biopsy due to lesion non-visualization that remained negative on follow-up. One BI-RADS 6 case was managed with a targeted US to the area of asymmetric BPE and a recommendation of 6-month CEM follow-up.
For 8 cases, the BI-RADS score was given for a finding other than asymmetric BPE, including BI-RADS score of 3 (n = 3), 4 (n = 4) or 0 (n = 1). One of these cases resulted in a diagnosis of ductal carcinoma in situ for calcifications contralateral to the dominant asymmetric BPE, and four more cases resulted in a benign biopsy.
MRI BI-RADS and BPE
As part of the evaluation of asymmetric BPE, immediate MRI was performed in 65 cases (41 BI-RADS 3 cases, 21 BI-RADS 0 cases, and 3 BI-RADS 4 cases). The immediate MRI downgraded the suspicion regarding asymmetric BPE for the majority of cases (42/65, 64.6%), resulting in 37 BI-RADS 2 cases, 4 BI-RADS 3 cases (in which MRI was negative, but BI-RADS remained 3) and 1 BI-RADS 4 case, given for a new finding unrelated to asymmetric BPE area, consequently yielding a benign biopsy. Interestingly, in 26 BI-RADS 1/2 cases with a negative immediate MRI, 6-month CEM follow-up remained recommended to confirm stability. For those cases where immediate MRI revealed an area of questionable asymmetric and recommended a biopsy (n = 13, 20%), most MRI-guided biopsies revealed benign findings, including PASH (n = 7), benign breast parenchyma (n = 5), and one case yielded neoplasm (see below).
Upon intra-individual comparison, the BPE grade on the initial CEM examination reporting asymmetric BPE (mean ± SD BPE score of 1.98 ± 0.75) was significantly higher compared with the BPE grade on the consequent MRI (mean ± SD BPE score of 1.63 ± 0.86) (p = 0.008). In addition, the BPE grades on both exams exhibited a weak but significant correlation (r = 0.25, p = 0.04).
Follow-up and clinical outcome
Six-month follow-up imaging for asymmetric BPE was recommended in 69 cases and performed in 57 of them, using CEM (n = 48) or MRI (n = 9), mostly downgrading of the BI-RADS to 1/2 score (n = 38), followed by continued BI-RADS 3 (n = 19). For the remaining 12 cases, follow-up imaging is either pending (n = 5), cancelled (n = 5) or the patient was lost to follow-up (n = 2). Upon paired intra-individual comparison, the mean ± SD initial CEM BPE of 1.92 ± 0.72 was significantly higher in comparison with the 6-month follow-up CEM BPE of 1.56 ± 0.82 (p = 0.003), with a weak but significant correlation (r = 0.28, p = 0.03).
At least one year of follow-up was available for 141/175 exams (80.6%) (Table 2). In two of those cases, cancer was diagnosed within the area of questionable asymmetric BPE (1.4%). The first case was a 43-year-old patient with a remote history of right breast cancer. In her initial CEM, moderate BPE, left greater than right, was reported, and BI-RADS 2 was assigned for contralateral post-radiation changes. Same-day bilateral whole-breast screening US was also performed with no suspicious finding. Six months following her initial CEM, the patient returned with a palpable abnormality in the left breast. Targeted US and US-guided biopsy yielded invasive ductal carcinoma within the area of previously apparent “greater BPE”, as demonstrated on the post-biopsy mammogram (Fig. 4). The second case was a 57-year-old patient. Her initial CEM demonstrated left non-mass-enhancement, “possibly representing asymmetric BPE”, with a negative targeted breast US. BI-RADS 0 as given, with a recommendation of MRI and 6-month left breast CEM follow-up imaging. An MRI correlate was demonstrated, and MRI-guided biopsy was performed, yielding a benign result. On the next annual CEM, persistent clumped non-mass enhancement was found, lateral to the biopsy marker, for which BI-RADS 0 was reassigned, with the recommendation of an MRI prior to re-biopsy. This time, CEM-guided stereotactic biopsy was performed, yielding ductal carcinoma in situ (Fig. 5).
Cancer detected within an area initially diagnosed as asymmetric background parenchymal enhancement (BPE) on contrast-enhanced mammography (CEM). Images of a 43-year-old patient with a history of right breast lumpectomy and irradiation 8 years prior to her CEM. Recombined images (a–d) demonstrate post-treatment changes in the right breast, and asymmetric enhancement within the upper, slightly inner breast, posterior depth (arrow), which was initially interpreted as asymmetric moderate BPE, left greater than right. For post-treatment changes, BI-RADS 2 was given. Same-day bilateral whole-breast screening US was also performed with no suspicious finding. Six months following her CEM, the patient returned with a left breast palpable abnormality. Mammography was negative; however, targeted US revealed a 2.0-cm irregular hypoechoic mass (e). Subsequent US-guided biopsy (not presented) yielded invasive ductal carcinoma and post-biopsy mammography (f, g) demonstrated the biopsy clip within the area of previously apparent “greater BPE”. Of note, the left breast anterior clip denotes the area of the previously benign biopsy
Cancer detected within an area initially diagnosed as possibly asymmetric background parenchymal enhancement (BPE) on contrast-enhanced mammography (CEM). Images of a 57-year-old patient whose CEM (a–d) demonstrated “mild BPE with 2.5 cm enhancing asymmetry in the left central breast, possibly representing asymmetric BPE”. Note the zoomed craniocaudal image (e) demonstrating clumped slightly linear enhancement. For this, targeted US was performed with no suspicious finding. BI-RADS 0 was given, with the recommendation of further evaluation with MRI and a 6-month left breast CEM follow-up in case of negative MRI. On subsequent MRI (not presented), minimal BPE was reported with a 2.5-cm linear enhancement in the left breast, correlating with CEM, for which MRI-guided biopsy was recommended, yielding benign breast parenchyma with sclerosing adenosis. One year later, on screening CEM, persistent clumped non-mass enhancement with the biopsy marker slightly superior-lateral to it was noted (f, g). BI-RADS 0 was given again, with the recommendation of further evaluation with MRI prior to biopsy, which demonstrated a suspicious correlate (h). This time, CEM-guided stereotactic biopsy was performed, yielding low-to-intermediate grade ductal carcinoma in situ. Post-biopsy mammography (not presented) demonstrated the second biopsy clip within the exact area initially assessed as possible asymmetric BPE
Discussion
In this work, we report our institutional experience with cases reporting asymmetric BPE on CEM. The American College of Radiology BI-RADS lexicon has recommended the reporting of asymmetric BPE on both breast MRI and more recently CEM. However, to date, the phenomenon of asymmetric BPE has been the focus of only a small number of reviews and pictorials, in which all reported this phenomenon on breast MRI [3, 4, 18, 19]. To the best of our knowledge, this work is the first investigation of asymmetric BPE on CEM.
Based on our retrospective review spanning a period of about 10 years from December 2012 to July 2023, 175 cases reporting on asymmetric BPE on CEM were identified, representing 1.4% of all CEM exams during that period. Of note, since the patient population at our institution is enriched with patients with a personal history of treated breast cancer [20, 21], we had anticipated a higher rate of asymmetric BPE on CEM. Our study sample may have been underestimated for several reasons. Most may be due to different wording used in the CEM report to characterize asymmetric BPE which was not captured by our search filter words, underscoring the need for reporting standardization. In patients who had been treated for cancer, it is possible that the asymmetric uptake was considered a normal/expected finding and not mentioned. To a lesser extent, possible under-documentation in CEM reports is also possible, in cases without a clear clinical relevance [22], such as cases with mild or longstanding asymmetric BPE, as suggested by the high abundance of cases with moderate/marked BPE in our study sample.
Changes in the intensity of BPE are often related to hormonal factors [23], including physiologic (such as menstrual or menopausal status [24] and lactation [25]) and exogenous factors (such as intrauterine contraceptive devices [26], hormonal replacement therapy [27], and endocrine therapy [28]). The effect of the grade of BPE on the diagnostic performance of MRI remains questionable [29, 30]. Early breast MRI experimental works among premenopausal healthy volunteers suggested a relation between the BPE manifestation and the timing of the exam along the menstrual cycle [1, 2]. Accordingly, guidelines have recommended scheduling breast MRI examinations during the second week of the menstrual cycle, with the aim to screen during minimal BPE [31]. Yet, more recent data, dispute this longstanding recommendation, and suggest that neither BPE levels nor MRI performance metrics are associated with menstrual cycle phase or week. [32, 33]. As CEM is a newer technique there is less data regarding the effect of menstrual cycle on BPE. Nonetheless, observations suggest that the extent of BPE detected on CEM remains largely unaffected by the menstrual cycle’s timing [34]. In light of this, we also do not schedule CEM based on the menstruation phase. Regardless of the timing of breast imaging, the evidence continues to support the role of BPE as an imaging marker to predict the development of breast cancer and to predict treatment outcomes [11, 35].
In this study, the most common associated factor for asymmetric BPE was prior breast cancer and treatment with irradiation contralateral to the side of the dominant BPE, in agreement with previous studies that compared BPE on the irradiated breast before and after radiation therapy [36, 37]. Interestingly, we have also seen, to a lesser extent, an ipsilateral increase of BPE in the post-lumpectomy breast on CEMs performed within months after breast conservation surgery and irradiation. Indeed, post-treatment breast MRI often displays focal enhancement as well as edematous changes, seroma, and skin thickening [38], making it difficult for interpretation [39]. Moreover, we encountered asymmetric, mostly marked BPE in women with unilateral breastfeeding and with a prior history of contralateral breast cancer, as was previously described with breast MRI [40,41,42,43]. Hence, the changes of BPE imposed by these associated factors stress the importance of clinical correlation.
Indeed, in the absence of clinical correlation, the differentiation between asymmetric BPE and non-mass enhancement (NME) can be challenging [15]. NME encompasses a spectrum of enhancements that do not conform to a discrete mass lesion but rather manifest as regions of increased contrast uptake. Unlike BPE, NME can be associated with a broad range of pathologies with overlapping appearances, including benign conditions, high-risk lesions and breast neoplasms, especially ductal carcinoma in situ (DCIS) and invasive lobular carcinoma (ILC) [44]. Yet, few NME descriptors including the distribution (segmental, clumped, and linear patterns) and internal enhancement pattern (clustered ring enhancement) should raise suspicion of possible malignancy [45]. On MRI, unlike the static post-contrast images of CEM, the kinetic evaluation of the contrast enhancement may affect the differential diagnosis over and above the morphology. In particular, wash-in pattern afforded by ultrafast sequences could be of value in discriminating benign and malignant NME which usually appear gradual and fast, respectively [46].
The uncertainty regarding questionable asymmetric BPE is highlighted by the variability in reporting confidence that we encountered, the subsequent BI-RADS score, and the diagnostic workup. This was particularly evident in cases given BI-RADS scores of 0 or 3 which varied greatly in the initial and recommended imaging performed to evaluate area of questionable asymmetric BPE.
In terms of the diagnostic workup of questionable asymmetric BPE, it appears that a routine same-day bilateral whole-breast US has minimal value and could be misleading [47]. On the contrary, targeted US to the asymmetric enhancement area can be of value, and presumably, had it been performed in our study, might have detected what was eventually a 2.0 cm interval cancer, misinterpreted as BPE. Furthermore, additional evaluation with MRI could be of value, considering its extended multiparametric characterization capabilities [48]. Indeed, MRI which usually displays comparable BPE grades with CEM [8] was able to downgrade most cases with questionable asymmetric BPE. In cases with a negative immediate MRI, the practice of automatic 6-month CEM follow-up imaging to confirm stability was not useful in our study. As such, either MRI alone or 6-month CEM follow-up imaging could be of value, but the utility of 6-month follow-up following a negative immediate MRI is probably of limited value [49].
The second case of cancer encountered in our study highlights the complexity that comes with evaluating a CEM-detected lesion with MRI and MRI-guided biopsy [50], which ended in an inaccurate biopsy. Fortunately, CEM-guided biopsy is now available which may prevent inaccurate targeting using MRI for a CEM finding [51].
Per definition, BPE is a benign phenomenon, with no malignant potential. However, distinguishing asymmetric BPE from multifocal or multicentric disease may be difficult, especially in cases with moderate or marked BPE [52]. Thus, if asymmetric BPE is seen without a known cause, or as seen in the first interval cancer in our cohort, with a reasonable explanation but with a focal presentation, it should be evaluated to rule out an underlying tumor [14]. Based on the positive predictive value of 1.4% found in our study sample for apparent asymmetric BPE (regardless of the official BI-RADS score), it appears that suspected asymmetric BPE (without an obvious explanation) falls exactly within the BI-RADS 3 score definition of a probably benign finding with less than 2% percent of malignant probability [13]. Further studies are needed to verify our observations. In addition, the discrimination between non-mass enhancement and BPE remains an important area for future research, including by utilizing advanced image analysis methods such as radiomics [53].
Several limitations of this study should be noted. This is a retrospective single-institution study and the identification of asymmetric BPE is most probably underestimated due to non-standardized terminology of asymmetric BPE. Second, our study sample included a relatively high portion of patients examined within the last year of the study period, for which 1 year of follow-up was unavailable at the latest time of submission. Finally, the retrospective nature of the study and the various approaches of diagnostic workup even for cases with the same BI-RADS score limit our ability to draw clear conclusions regarding the appropriate management of questionable asymmetric BPE, and stresses the complexity and lack of clear guidelines its evaluation.
To conclude, apparent asymmetric BPE is most often a benign finding with an associated clinical etiology. Nevertheless, in less than 2% of cases, it may mask an underlying malignancy. Thus, further evaluation of unexplained asymmetric BPE is warranted, probably with targeted US, MRI, or 6-month follow-up CEM. Further studies are required to verify these initial observations and to provide analytic tools for discriminating asymmetric BPE from breast cancer presenting as non-mass enhancement.
Abbreviations
- BPE:
-
Background parenchymal enhancement
- CEM:
-
Contrast-enhanced mammography
- NME:
-
Non-mass enhancement
References
Müller-Schimpfle M, Ohmenhäuser K, Stoll P et al (1997) Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 203:145–149
Kuhl CK, Bieling HB, Gieseke J et al (1997) Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 203:137–144
Giess CS, Yeh ED, Raza S, Birdwell RL (2014) Background parenchymal enhancement at breast MR imaging: normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 34:234–247
Liao GJ, Henze Bancroft LC, Strigel RM et al (2020) Background parenchymal enhancement on breast MRI: a comprehensive review. J Magn Reson Imaging 51:43–61
Cozzi A, Magni V, Zanardo M et al (2022) Contrast-enhanced mammography: a systematic review and meta-analysis of diagnostic performance. Radiology 302:568–581
Jochelson MS, Lobbes MBI (2021) Contrast-enhanced mammography: state of the art. Radiology 299:36–48
Wang S, Sun Y, You C et al (2023) Association of clinical factors and degree of early background parenchymal enhancement on contrast-enhanced mammography. AJR Am J Roentgenol 221:45–55
Sogani J, Morris EA, Kaplan JB et al (2017) Comparison of background parenchymal enhancement at contrast-enhanced spectral mammography and breast MR imaging. Radiology 282:63–73
Savaridas SL, Taylor DB, Gunawardana D, Phillips M (2017) Could parenchymal enhancement on contrast-enhanced spectral mammography (CESM) represent a new breast cancer risk factor? Correlation with known radiology risk factors. Clin Radiol 72:1085.e1–1085.e9
Karimi Z, Phillips J, Slanetz P et al (2021) Factors associated with background parenchymal enhancement on contrast-enhanced mammography. AJR Am J Roentgenol 216:340–348
Watt GP, Thakran S, Sung JS et al (2023) Association of breast cancer odds with background parenchymal enhancement quantified using a fully automated method at MRI: the IMAGINE study. Radiology 308:e230367
Sorin V, Yagil Y, Shalmon A et al (2020) Background parenchymal enhancement at contrast-enhanced spectral mammography (CESM) as a breast cancer risk factor. Acad Radiol 27:1234–1240
Rao AA, Feneis J, Lalonde C, Ojeda-Fournier H (2016) A pictorial review of changes in the BI-RADS fifth edition. Radiographics 36:623–639
Lee CH, Phillips J, Sung JS et al (2022) Contrast enhanced mammography (A supplement to ACR BI-RADS mammography) 2013 1–64. https://www.acr.org/-/media/ACR/Files/RADS/BI-RADS/BIRADS_CEM_2022.pdf
Chikarmane SA, Michaels AY, Giess CS (2017) Revisiting nonmass enhancement in breast MRI: analysis of outcomes and follow-up using the updated BI-RADS atlas. AJR Am J Roentgenol 209:1178–1184
Neeter LMFH, Raat HPJF, Alcantara R et al (2021) Contrast-enhanced mammography: what the radiologist needs to know. BJR Open 3:20210034. 24
Panicek DM, Hricak H (2016) How sure are you, doctor? A standardized lexicon to describe the radiologists level of certainty. AJR Am J Roentgenol 207:2–3
Bauer E, Levy MS, Domachevsky L et al (2022) Background parenchymal enhancement and uptake as breast cancer imaging biomarkers: a state-of-the-art review. Clin Imaging 83:41–50
Jones LI, Klimczak K, Geach R (2023) Breast MRI: an illustration of benign findings. Br J Radiol 96:20220280
Sung JS, Lebron L, Keating D et al (2019) Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology 293:81–88
Gluskin J, Saccarelli CR, Avendano D et al (2020) Contrast-enhanced mammography for screening women after breast conserving surgery. Cancers (Basel) 12:3495. 24
Nissan N, Ochoa-Albiztegui RE, Fruchtman H et al (2023) Breast MRI in patients with implantable loop recorder: initial experience. Eur Radiol 34:155–164
Rella R, Bufi E, Belli P et al (2018) Background parenchymal enhancement in breast magnetic resonance imaging: a review of current evidences and future trends. Diagn Interv Imaging 99:815–826
King V, Gu Y, Kaplan JB et al (2012) Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol 22:2641–2647
Nissan N, Bauer E, Moss Massasa EE, Sklair-Levy M (2022) Breast MRI during pregnancy and lactation: clinical challenges and technical advances. Insights Imaging 9 13:71
Huck LC, Truhn D, Wilpert C et al (2022) Background parenchymal enhancement in contrast-enhanced MR imaging suggests systemic effects of intrauterine contraceptive devices. Eur Radiol 32:7430–7438
Pfleiderer SOR, Sachse S, Sauner D et al (2004) Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res 6:R232–R238
King V, Goldfarb SB, Brooks JD et al (2012) Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology 264:670–678
Ray KM, Kerlikowske K, Lobach IV et al (2018) Effect of background parenchymal enhancement on breast mr imaging interpretive performance in community-based practices. Radiology 286:822–829
DeMartini WB, Liu F, Peacock S et al (2012) Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol 198:W373–W380
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316
Dontchos BN, Rahbar H, Partridge SC et al (2019) Influence of menstrual cycle timing on screening breast MRI background parenchymal enhancement and diagnostic performance in premenopausal women. J Breast Imaging 1:205–211
Lee CH, Bryce Y, Zheng J et al (2020) Outcome of screening MRI in premenopausal women as a function of the week of the menstrual cycle. AJR Am J Roentgenol 214:1175–1181
Kornecki A (2022) Current status of contrast enhanced mammography: a comprehensive review. Can Assoc Radiol J 73:141–156
Niell BL, Abdalah M, Stringfield O et al (2021) Quantitative measures of background parenchymal enhancement predict breast cancer risk. AJR Am J Roentgenol 217:64–75
Kim YJ, Kim SH, Choi BG et al (2014) Impact of radiotherapy on background parenchymal enhancement in breast magnetic resonance imaging. Asian Pac J Cancer Prev 15:2939–2943
Ben-David MA, Corn BW, Evron E et al (2020) Prophylactic breast irradiation reduces background parenchymal enhancement (BPE) on MRI: a secondary analysis. Breast 49:70–73
Li J, Dershaw DD, Lee CF et al (2010) Breast MRI after conservation therapy: usual findings in routine follow-up examinations. AJR Am J Roentgenol 195:799–807
Healy NA, Benson JR, Sinnatamby R (2021) Role of early post-operative breast MRI: how helpful is it in deciding the next step for women who may have residual disease? BJR Open 29 3:20210024
Nissan, Massasa EEM N, Bauer E et al (2023) MRI can accurately diagnose breast cancer during lactation. Eur Radiol 33:2935–2944
Nissan N, Allweis T, Menes T et al (2020) Breast MRI during lactation: effects on tumor conspicuity using dynamic contrast-enhanced (DCE) in comparison with diffusion tensor imaging (DTI) parametric maps. Eur Radiol 30:767–777
Nissan N, Anaby D, Mahameed G et al (2023) Ultrafast DCE-MRI for discriminating pregnancy-associated breast cancer lesions from lactation related background parenchymal enhancement. Eur Radiol 33:8122–8131
Nissan N, Sorin V, Bauer E et al (2022) MRI of the lactating breast: computer-aided diagnosis false positive rates and background parenchymal enhancement kinetic features. Acad Radiol 29:1332–1341
Chadashvili T, Ghosh E, Fein-Zachary V et al (2015) Nonmass enhancement on breast MRI: review of patterns with radiologic-pathologic correlation and discussion of management. AJR Am J Roentgenol 204:219–227
Lunkiewicz M, Forte S, Freiwald B et al (2020) Interobserver variability and likelihood of malignancy for fifth edition BI-RADS MRI descriptors in non-mass breast lesions. Eur Radiol 30:77–86
Mori N, Sheth D, Abe H (2020) Nonmass enhancement breast lesions: diagnostic performance of kinetic assessment on ultrafast and standard dynamic contrast-enhanced MRI in comparison with morphologic evaluation. AJR Am J Roentgenol 215:511–518
Klang E, Krosser A, Amitai MM et al (2018) Utility of routine use of breast ultrasound following contrast-enhanced spectral mammography. Clin Radiol 73:908.e11–908.e16
Ohashi A, Kataoka M, Iima M et al (2023) A multiparametric approach to predict triple-negative breast cancer including parameters derived from ultrafast dynamic contrast-enhanced MRI. Eur Radiol 33:8132–8141
Coffey K, Dixon LB, Sevilimedu V et al (2023) Short-term follow-up of contrast-enhanced mammography lesions after negative breast MRI in women with elevated breast cancer risk. Eur J Radiol 168:111097
Weaver OO, Yang WT, Scoggins ME et al (2023) Challenging contrast-enhanced mammography–guided biopsies: practical approach using real-time multimodality imaging and a proposed procedural algorithm. AJR Am J Roentgenol 220:512–523
Alcantara R, Posso M, Pitarch M et al (2023) Contrast-enhanced mammography-guided biopsy: technical feasibility and first outcomes. Eur Radiol 33:417–428
Uematsu T, Kasami M, Watanabe J (2011) Does the degree of background enhancement in breast MRI affect the detection and staging of breast cancer? Eur Radiol 21:2261–2267
Boca I, Ciurea AI, Ciortea CA et al (2021) Differentiating breast tumors from background parenchymal enhancement at contrast-enhanced mammography: the role of radiomics—a pilot reader study. Diagnostics 11:1248. 13
Acknowledgements
The authors thank Joanne Chin for editing the manuscript.
Funding
This work was supported in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Maxine Jochelson.
Conflict of interest
M.J.’s disclosure includes a previously paid lecture for GE, whose mammograms were utilized as part of the routine clinical work in our institute, regardless of this current study. The rest of the authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (N.N.) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board (Memorial Sloan Kettering Cancer Center) approval was obtained.
Study subjects or cohorts overlap
No overlap.
Methodology
-
Retrospective
-
Observational
-
Performed at one institution
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nissan, N., Gluskin, J., Ochoa-Albiztegui, R.E. et al. Asymmetric background parenchymal enhancement on contrast-enhanced mammography: associated factors, diagnostic workup, and clinical outcome. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10856-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10856-8