Abstract
Purpose
To evaluate if background parenchymal enhancement (BPE) on contrast-enhanced mammography (CEM), graded according to the 2022 CEM-dedicated Breast Imaging Reporting and Data System (BI-RADS) lexicon, is associated with breast density, menopausal status, and age.
Methods
This bicentric retrospective analysis included CEM examinations performed for the work-up of suspicious mammographic findings. Three readers independently and blindly evaluated BPE on recombined CEM images and breast density on low-energy CEM images. Inter-reader reliability was estimated using Fleiss κ. Multivariable binary logistic regression was performed, dichotomising breast density and BPE as low (a/b BI-RADS categories, minimal/mild BPE) and high (c/d BI-RADS categories, moderate/marked BPE).
Results
A total of 200 women (median age 56.8 years, interquartile range 50.5−65.6, 140/200 in menopause) were included. Breast density was classified as a in 27/200 patients (13.5%), as b in 110/200 (55.0%), as c in 52/200 (26.0%), and as d in 11/200 (5.5%), with moderate inter-reader reliability (κ = 0.536; 95% confidence interval [CI] 0.482–0.590). BPE was minimal in 95/200 patients (47.5%), mild in 64/200 (32.0%), moderate in 25/200 (12.5%), marked in 16/200 (8.0%), with substantial inter-reader reliability (κ = 0.634; 95% CI 0.581–0.686). At multivariable logistic regression, premenopausal status and breast density were significant positive predictors of high BPE, with adjusted odds ratios of 6.120 (95% CI 1.847–20.281, p = 0.003) and 2.416 (95% CI 1.095–5.332, p = 0.029) respectively.
Conclusion
BPE on CEM is associated with well-established breast cancer risk factors, being higher in women with higher breast density and premenopausal status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Background parenchymal enhancement (BPE) is defined as the physiological contrast agent uptake of breast parenchyma on contrast-enhanced imaging [1, 2]. BPE seems to be related to the amount of fibroglandular breast tissue and to the aging process and was initially described as an ancillary finding on contrast-enhanced breast magnetic resonance imaging (MRI), then widely recognized as a highly variable phenomenon over time and among women [3].
BPE is influenced by hormonal fluctuations throughout a woman’s life related to the menstrual cycle, endocrine therapy, and other factors. This enhancing tissue may indicate areas with increased proliferative potential that are sensitive to hormones, potentially explaining BPE association with well-known breast cancer risk factors that stem from hormonal changes, including mammographic breast density [4,5,6]. Furthermore, a recent meta-analysis confirmed that BPE on breast MRI is an independent indicator of breast cancer risk, with a higher degree of BPE being associated with a higher breast cancer risk [7]. Of note, high BPE might also influence the interpretation of breast MRI, potentially by masking underlying breast lesions (increasing false negatives) or mimicking malignancy (increasing false positives) [3, 8,9,10].
Contrast-enhanced mammography (CEM) has recently emerged as an alternative to MRI for several contrast-enhanced breast imaging indications [11,12,13,14,15]. CEM exploits the same physio-pathological principles underlying the morpho-functional approach of contrast-enhanced breast MRI [16, 17]. As for MRI, BPE is also recognizable at CEM, albeit with different appearance and patterns [18]; these different characteristics warrant specific investigation, especially considering that high BPE degree can potentially have a more pronounced impact on CEM interpretation compared to breast MRI due to the overlapping fibroglandular tissue caused by the bidimensional nature of CEM images [19, 20]. Of note, a systematization of BPE description and reporting at CEM has been achieved only very recently, with the CEM-dedicated supplement to the American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS) Atlas 2013 published in 2022 [21].
However, aside from BPE description and reporting, the factors which might play a role in determining variations of CEM BPE remain poorly investigated [22,23,24], whereas factors influencing BPE on contrast-enhanced breast MRI are well established [2, 25]. Indeed, very few studies have reported an association between CEM BPE and women’s age, breast density, or menopausal status [22, 24, 26, 27]: a clear and unambiguous interpretation of the correlation between CEM BPE and breast cancer risk and/or menopausal status is still lacking, as well as the use in these investigations of the new ACR BI-RADS lexicon for the reporting of CEM BPE [21].
In this scenario, we aimed to evaluate the relationship of BPE on CEM—assessed according to the new ACR BI-RADS 4-category scale—with breast density, patients’ age, and menopausal status.
Methods
Study design and population
This is a secondary retrospective analysis of CEM examinations acquired in a prospective study on the use of CEM in the work-up of screening recalls [28] between January 2019 and July 2021. Approval for this bicentric retrospective study was obtained by the Ethics Committee of IRCCS Ospedale San Raffaele, Milan, Italy (protocol code CESM; approved May 10th, 2018) and by the Ethics Committee of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy (protocol code P-20190076950, approved September 25th, 2019).
Examinations were performed at the Radiology Unit of IRCCS Policlinico San Donato, San Donato Milanese, Italy (Center 1) and at the Department of Breast Radiology of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy (Center 2). In the aforementioned prospective study [28], CEM was proposed to women aged 40–80 years for the work-up of suspicious findings detected at screening mammography, after collection of personal data including age, body mass index (BMI), family history of breast cancer, menstrual cycle timing, and menopausal status.
For the purpose of this secondary analysis, we first excluded all incomplete or low-quality CEM examinations and patients with incomplete clinical data. Then, all included subjects were categorized as pre- or post-menopausal: women in peri-menopausal status were included in the post-menopausal group.
Imaging technique
All CEM examinations were performed on Senographe Pristina mammography systems (General Electric Healthcare, Buc, France). Two minutes after the intravenous administration of a 1.5 mL/kg dose of a non-ionic monomeric, low-osmolar contrast agent (Iohexol 350 mgI/mL; General Electric Healthcare, Buc, France), the standard mediolateral oblique and craniocaudal views were obtained in a maximum timeframe of 10 min.
Imaging interpretation
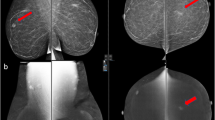
BPE and breast density were visually assessed by three readers (G.M., A.B., F.S.) with 2, 14, and 37 years of experience in breast imaging, respectively. Breast density was assessed on low-energy CEM images and classified in almost entirely fatty (category a), scattered areas of fibroglandular density (category b), heterogeneously dense (category c), extremely dense (category d). BPE was evaluated on recombined CEM images and classified as minimal (Fig. 1), mild (Fig. 2), moderate (Fig. 3) and marked (Fig. 4), according to the 2022 ACR BI-RADS lexicon for CEM interpretation [21]. Readers were blinded to each other and to the original breast density and BPE assessments.
For both BPE and breast density, the most frequent category (mode) assigned by the three independent readers for each examination was used for the analyses (considering both projections for both sides).
Statistical analysis
Categorical variables were reported as absolute numbers and percentages, continuous variables as mean ± standard deviation (SD) or as median and interquartile range (IQR) according to their distribution, assessed with the Shapiro–Wilk test.
Inter-reader reliability for breast density and BPE interpretation was assessed using Fleiss κ statistics, interpreted according to the Landis and Koch scale [29].
The correlation of BPE with breast density and menopausal status was calculated using the Spearman’s rank order coefficient (ρ) for ordinal data, while the correlation between BPE and patients’ age was calculated using Kendall’s τb. Correlations were interpreted as: very strong correlation (at least 0.90); strong correlation (0.70 up to 0.89); moderate correlation (0.40 up to 0.69); weak correlation (0.10 up to 0.39); negligible correlation (less than 0.10) [30].
To find potential associations between clinical characteristics and breast cancer risk factors in predicting the degree of BPE dichotomized as low and high (minimal/mild vs. moderate/marked), we first used univariate binary logistic regression to calculate unadjusted odds ratios (ORs) with their 95% confidence intervals (95% CIs) for the following independent variables: age, BMI, family history of breast cancer, breast density (dichotomized as low and high, i.e., category a/b vs. category c/d), and menopausal status. Multivariable linear regression (with backward elimination at p < 0.010) was then used to select variables that entered multivariable binary logistic regression, with calculation of adjusted ORs and their 95% CIs.
All statistical analyses were performed using SPSS v.28.0 (IBM SPSS Inc., Chicago, IL, USA), p values < 0.05 being considered statistically significant.
Results
Out of 207 CEM examinations performed for the work-up of screening recalls, 7 (3.5%) were excluded from this secondary analysis due to unavailable images or patients’ clinical data. A total of 200 CEM examinations from 200 women with a median age of 56.8 years (IQR 50.5–65.6 years) were included in this study, 116/200 (58%) from Center 1 and 84/200 (42%) from Center 2. Among these 200 women, 60 (30.0%) were premenopausal and 140 (70.0%) were post-menopausal.
Overall, BPE was assessed as minimal in 95/200 examinations (47.5%), as mild in 64/200 examinations (32.0%), as moderate in 25/200 examinations (12.5%), and as marked in 16/200 (8.0%) examinations. Breast density was classified as BI-RADS category a in 27/200 examinations (13.5%), BI-RADS category b in 110/200 examinations (55.0%), BI-RADS category c in 52/200 examinations (26.0%), and BI-RADS category d in 11/200 examinations (5.5%).
Inter-reader agreement and reliability
For the 4-category classification of breast density, the raw inter-reader agreement was 59.0% (118/200 examinations), while reliability analysis showed a moderate agreement with a Fleiss κ of 0.536 (95% CI 0.482–0.590). For the 2-category classification of breast density (low vs. high), the raw inter-reader agreement was 76.5% (153/200 examinations), while reliability analysis showed a substantial agreement with a Fleiss κ of 0.614 (95% CI 0.534–0.694).
For the 4-category classification of CEM BPE, the raw inter-reader agreement was 64.0% (128/200 examinations), while reliability analysis showed a substantial agreement with a Fleiss κ of 0.634 (95% CI 0.581–0.686). For the 2-category classification of BPE (low vs. high), the raw inter-reader agreement was 85.0% (170/200 examinations), while reliability analysis showed a substantial agreement with a Fleiss κ of 0.707 (95% CI 0.627–0.787).
Correlation analysis
BPE showed a weak positive correlation with breast density (ρ = 0.342, p < 0.001), a moderate negative correlation with menopausal status (ρ = − 0.462, p < 0.001), and a weak negative correlation with age (τb = −0.330, p < 0.001). The boxplot in Fig. 5 shows the distribution of patients’ age according to each BPE category.
Regression analysis
Multivariable linear regression selected age, breast density, and menopausal status as independent predictors of high BPE, with unadjusted ORs of 0.893 (95% C.I. 0.851–0.937, p < 0.001), 3.332 (95% C.I. 1.640–6.773, p = 0.001), and 9.978 (95% C.I. 4.580–21.472, p < 0.001), respectively (Table 1).
At multivariable logistic regression, only premenopausal status and breast density remained statistically significant positive predictors of high BPE, with adjusted ORs of 6.120 (95% CI 1.847–20.281, p = 0.003) and 2.416 (95% CI 1.095–5.332, p = 0.029) respectively, whereas single-unit increase in patients’ age did not show any statistically significant association with BPE degree (OR 0.975, 95% C.I. 0.911–1.042, p = 0.453).
Discussion
CEM is a promising morpho-functional imaging technique with similar sensitivity and specificity compared to breast MRI [15], being more affordable and more available [12, 31]. Furthermore, CEM has been reported to be preferred by patients (up to 70% vs. 30% for MRI), particularly because of shorter examination times, greater overall comfort, and lower stress/anxiety/claustrophobia [32,33,34]. However, previous studies have suggested that the interpretation of CEM may be hindered by BPE, which could mask underlying breast lesions [35]. Indeed, as reported by Yuen et al. [35] in a study evaluating CEM versus MRI for the preoperative assessment of the extent of newly diagnosed breast cancer, CEM diagnostic performance in women with low BPE was reported to be higher than in women with high BPE: in women with low BPE, CEM even surpassed the diagnostic performance of breast MRI, whereas MRI retained a higher performance among women with high BPE.
BPE has been firstly investigated on breast MRI: a number of studies have emphasized the potential influence of BPE on images interpretation, as BPE leads to an increase in false-positive findings—by mimicking malignancy—and to an increase in false-negative findings through a masking effect [2]. Furthermore, BPE on breast MRI can be influenced by several factors, including endogenous hormonal status, menstrual cycle, and the amount of fibroglandular tissue [3]. In this scenario, the fact that CEM and MRI rely on the same physiopathological principles of contrast-enhancement led to the assumption that factors affecting BPE on MRI may also play a role in determining BPE on CEM.
Thus, for this analysis on the association of CEM BPE with women’s clinical data and breast cancer risk factors, we evaluated 200 women that had undergone CEM for the work-up of mammography-detected suspicious findings as part of another study [28]. Regression analysis revealed that premenopausal status and breast density were significant positive predictors of BPE, the premenopausal status (OR 6.120) and breast density categories c and d (OR 2.416) being associated with increased BPE. Our results are in accordance with previous studies that found a positive association between increased BPE and younger age [18, 22,23,24, 26], increased breast density [22, 24, 26, 27, 36], and premenopausal status [18, 22,23,24, 27].
In particular, considering the influence of menopausal status on BPE, our findings are in line with the well-established assumption that BPE in premenopausal women may be considered as a measure of microvessel density within the fibroglandular tissue of the breast, resulting from the increased expression of vascular endothelial growth factor in the terminal ductal lobular units, which is a stimulator of endothelial cells proliferation and of vascular permeability [37].
According to the univariate analysis, a one-unit increase in age emerged as a significant negative predictor of high BPE, reflecting the changes happening in women's breasts during their lifespan, particularly during the transition to menopause. This is confirmed by the observation that, at multivariable analysis, dichotomous variables (i.e., menopausal status and breast density) that represent strong proxies of age had even stronger significant associations with BPE.
Of note, the positive association between BPE, breast density, and premenopausal status is an expected result, considering that breast density and menopausal status are closely related aspects: the observable decrease of breast density occurring with aging, and in particular over the menopause transition, corresponds to the decline of circulating reproductive hormones during the mid-life of women [38, 39]. As previously reported in studies performed on breast MRI [4, 40], BPE fluctuates with variations in hormones, particularly with estrogen levels, thus representing physiologically active breast tissue that has proliferative potential and is hormonally responsive.
Moreover, under this last assumption, areas of breast parenchyma with higher BPE might be more susceptible to estrogen-induced malignant transformation, as investigated by several MRI studies [9, 41,42,43], while lower BPE may be attributed to fibrotic changes as the primary contributors to the observed breast density. However, this aspect has been only tangentially investigated on CEM, with a few studies showing that increased CEM BPE levels are associated with an increased risk of breast cancer [26, 27, 44], thus highlighting the need for further research to better understand the underlying pathophysiological mechanism that links BPE and breast cancer risk and assess if CEM and MRI BPE are equally linked to increased breast cancer risk.
In the absence of a recognized CEM lexicon for BPE interpretation, earlier studies variably adopted the BI-RADS classification system for breast MRI [45], thus leading to a lack of consistency and comparability in the evaluation of BPE [22,23,24, 26, 27, 46]. Of note, in our study, BPE evaluation showed substantial inter-reader reliability, matching those achieved in previous studies [18, 24, 27, 46]. However, in these studies, CEM had already been introduced in clinical practice for some time and readers examined a standardized training set of examinations that included all BPE categories prior to CEM images review. This was not the case in our study: thus, the application of the standardized ACR BI-RADS lexicon for BPE reporting seems to immediately translate in high inter-reader agreement and reliability even without specific pre-training of the readers and without specific clinical CEM experience.
This study has some limitations. First, its retrospective design prevented a longitudinal appraisal of the relationship between BPE and breast cancer risk: serial evaluation of BPE changes over time could provide further information about its influence on breast cancer risk. Second, as clinical information was retrospectively collected from medical records, there was an inherent risk of incorrect or incomplete documentation. In this regard, the evaluation of further relationships between additional clinical data (e.g., menstrual cycle timing, personal history of breast cancer, previous endocrine therapy) and BPE degree was not possible, due to the unavailability of these data for all the included patients. Third, the population included in this analysis comes from a study focused on a specific population of women who underwent CEM for the work-up of suspicious findings detected at screening mammography and consequently includes a high rate of post-menopausal women: these two aspects might limit the generalizability of our findings to broader populations.
In conclusion, this study provides valuable insights into the association between BPE on CEM and clinical factors, confirming the influence of breast density and menopausal status on BPE. Understanding these associations can contribute to individualized screening strategies for women undergoing CEM, even though further research is warranted to validate these findings, to investigate the relationship between CEM and MRI BPE, and to explore the clinical implications of BPE assessment in the context of breast cancer risk assessment and management.
References
Bauer E, Levy MS, Domachevsky L et al (2022) Background parenchymal enhancement and uptake as breast cancer imaging biomarkers: a state-of-the-art review. Clin Imaging 83:41–50. https://doi.org/10.1016/j.clinimag.2021.11.021
Liao GJ, Henze Bancroft LC, Strigel RM et al (2020) Background parenchymal enhancement on breast MRI: a comprehensive review. J Magn Reson Imaging 51:43–61. https://doi.org/10.1002/jmri.26762
Giess CS, Yeh ED, Raza S, Birdwell RL (2014) Background parenchymal enhancement at breast MR imaging: normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 34:234–247. https://doi.org/10.1148/rg.341135034
Arslan G, Celik L, Cubuk R et al (2017) Background parenchymal enhancement: is it just an innocent effect of estrogen on the breast? Diagnostic Interv Radiol 23:414–419. https://doi.org/10.5152/dir.2017.17048
Pike MC, Pearce CL (2013) Mammographic density, MRI background parenchymal enhancement and breast cancer risk. Ann Oncol 24:37–41. https://doi.org/10.1093/annonc/mdt310
Hellgren R, Saracco A, Strand F et al (2020) The association between breast cancer risk factors and background parenchymal enhancement at dynamic contrast-enhanced breast MRI. Acta radiol 61:1600–1607. https://doi.org/10.1177/0284185120911583
Zhang H, Guo L, Tao W et al (2021) Possible breast cancer risk related to background parenchymal enhancement at breast MRI: a meta-analysis study. Nutr Cancer 73:1371–1377. https://doi.org/10.1080/01635581.2020.1795211
Telegrafo M, Rella L, Stabile Ianora AA et al (2016) Effect of background parenchymal enhancement on breast cancer detection with magnetic resonance imaging. Diagn Interv Imaging 97:315–320. https://doi.org/10.1016/j.diii.2015.12.006
King V, Brooks JD, Bernstein JL et al (2011) Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 260:50–60. https://doi.org/10.1148/radiol.11102156
Sippo DA, Burk KS, Mercaldo SF et al (2019) Performance of screening breast MRI across women with different elevated breast cancer risk indications. Radiology. https://doi.org/10.1148/radiol.2019181136
Jochelson MS, Lobbes MBI (2021) Contrast-enhanced mammography: state of the art. Radiology 299:36–48. https://doi.org/10.1148/radiol.2021201948
Cozzi A, Schiaffino S, Sardanelli F (2019) The emerging role of contrast-enhanced mammography. Quant Imaging Med Surg 9:2012–2018. https://doi.org/10.21037/qims.2019.11.09
Cozzi A, Magni V, Zanardo M et al (2022) Contrast-enhanced Mammography: a systematic review and meta-analysis of diagnostic performance. Radiology 302:568–581. https://doi.org/10.1148/radiol.211412
van Nijnatten TJA, Lobbes MBI, Cozzi A et al (2023) Barriers to implementation of contrast-enhanced mammography in clinical practice: AJR expert panel narrative review. Am J Roentgenol 221:3–6. https://doi.org/10.2214/AJR.22.28567
Neeter LMFH, Robbe MMQ, van Nijnatten TJA et al (2023) Comparing the diagnostic performance of contrast-enhanced mammography and breast MRI: a systematic review and meta-analysis. J Cancer 14:174–182. https://doi.org/10.7150/jca.79747
Zanardo M, Cozzi A, Trimboli RM et al (2019) Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review. Insights Imaging 10:76. https://doi.org/10.1186/s13244-019-0756-0
Sensakovic WF, Carnahan MB, Czaplicki CD et al (2021) Contrast-enhanced Mammography: how does it work? Radiographics 41:829–839. https://doi.org/10.1148/rg.2021200167
Sogani J, Morris EA, Kaplan JB et al (2017) Comparison of background parenchymal enhancement at contrast-enhanced spectral mammography and breast MR imaging. Radiology 282:63–73. https://doi.org/10.1148/radiol.2016160284
Sorin V, Yagil Y, Yosepovich A et al (2018) Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. Am J Roentgenol 211:W267–W274. https://doi.org/10.2214/AJR.17.19355
Wessling D, Männlin S, Schwarz R et al (2022) Background enhancement in contrast-enhanced spectral mammography (CESM): are there qualitative and quantitative differences between imaging systems? Eur Radiol 33:2945–2953. https://doi.org/10.1007/s00330-022-09238-9
Lee CH, Phillips J, Sung JS, et al (2022) ACR BI-RADS® Contrast Enhanced Mammography (CEM) (A supplement to ACR BI-RADS® Mammography 2013). In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. American College of Radiology, Reston (VA)
Karimi Z, Phillips J, Slanetz P et al (2021) Factors associated with background parenchymal enhancement on contrast-enhanced mammography. Am J Roentgenol 216:340–348. https://doi.org/10.2214/AJR.19.22353
Zhao S, Zhang X, Zhong H et al (2020) Background parenchymal enhancement on contrast-enhanced spectral mammography: influence of age, breast density, menstruation status, and menstrual cycle timing. Sci Rep 10:8608. https://doi.org/10.1038/s41598-020-65526-8
Wang S, Sun Y, You C et al (2023) Association of clinical factors and degree of early background parenchymal enhancement on contrast-enhanced mammography. Am J Roentgenol. https://doi.org/10.2214/AJR.22.28769
Brooks JD, Christensen RAG, Sung JS et al (2022) MRI background parenchymal enhancement, breast density and breast cancer risk factors: A cross-sectional study in pre- and post-menopausal women. npj Breast Cancer. https://doi.org/10.1038/s41523-022-00458-2
Sorin V, Yagil Y, Shalmon A et al (2020) Background parenchymal enhancement at contrast-enhanced spectral mammography (CESM) as a breast cancer risk factor. Acad Radiol 27:1234–1240. https://doi.org/10.1016/j.acra.2019.10.034
Savaridas SL, Taylor DB, Gunawardana D, Phillips M (2017) Could parenchymal enhancement on contrast-enhanced spectral mammography (CESM) represent a new breast cancer risk factor? Correlation with known radiology risk factors. Clin Radiol 72:1085.e1-1085.e9. https://doi.org/10.1016/j.crad.2017.07.017
Cozzi A, Schiaffino S, Fanizza M et al (2022) Contrast-enhanced mammography for the assessment of screening recalls: a two-centre study. Eur Radiol 32:7388–7399. https://doi.org/10.1007/s00330-022-08868-3
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174. https://doi.org/10.2307/2529310
Schober P, Boer C, Schwarte LA (2018) Correlation coefficients. Anesth Analg 126:1763–1768. https://doi.org/10.1213/ANE.0000000000002864
Coffey K, Jochelson MS (2022) Contrast-enhanced mammography in breast cancer screening. Eur J Radiol 156:110513. https://doi.org/10.1016/j.ejrad.2022.110513
Hobbs MM, Taylor DB, Buzynski S, Peake RE (2015) Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): Patient preferences and tolerance. J Med Imaging Radiat Oncol 59:300–305. https://doi.org/10.1111/1754-9485.12296
Pires-Gonçalves L, Henriques Abreu M, Ferrão A et al (2023) Patient perspectives on repeated contrast-enhanced mammography and magnetic resonance during neoadjuvant chemotherapy of breast cancer. Acta radiol 64:1816–1822. https://doi.org/10.1177/02841851221144021
Berg WA, Bandos AI, Sava MG (2023) Analytic hierarchy process analysis of patient preferences for contrast-enhanced mammography versus MRI as supplemental screening options for breast cancer. J Am Coll Radiol 20:758–768. https://doi.org/10.1016/j.jacr.2023.05.014
Yuen S, Monzawa S, Gose A et al (2022) Impact of background parenchymal enhancement levels on the diagnosis of contrast-enhanced digital mammography in evaluations of breast cancer: comparison with contrast-enhanced breast MRI. Breast Cancer 29:677–687. https://doi.org/10.1007/s12282-022-01345-1
Sogani J, Mango VL, Keating D et al (2021) Contrast-enhanced mammography: past, present, and future. Clin Imaging 69:269–279. https://doi.org/10.1016/j.clinimag.2020.09.003
Sung JS, Corben AD, Brooks JD et al (2018) Histopathologic characteristics of background parenchymal enhancement (BPE) on breast MRI. Breast Cancer Res Treat 172:487–496. https://doi.org/10.1007/s10549-018-4916-6
Burton A, Maskarinec G, Perez-Gomez B et al (2017) Mammographic density and ageing: a collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLOS Med 14:e1002335. https://doi.org/10.1371/journal.pmed.1002335
Magni V, Capra D, Cozzi A et al (2023) Mammography biomarkers of cardiovascular and musculoskeletal health: a review. Maturitas 167:75–81. https://doi.org/10.1016/j.maturitas.2022.10.001
Kuhl CK, Bieling HB, Gieseke J et al (1997) Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 203:137–144. https://doi.org/10.1148/radiology.203.1.9122382
Dontchos BN, Rahbar H, Partridge SC et al (2015) Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 276:371–380. https://doi.org/10.1148/radiol.2015142304
Arasu VA, Miglioretti DL, Sprague BL et al (2019) Population-based assessment of the association between magnetic resonance imaging background parenchymal enhancement and future primary breast cancer risk. J Clin Oncol 37:954–963. https://doi.org/10.1200/JCO.18.00378
Watt GP, Sung J, Morris EA et al (2020) Association of breast cancer with MRI background parenchymal enhancement: the IMAGINE case-control study. Breast Cancer Res. https://doi.org/10.1186/S13058-020-01375-7
Xu C, Jiang M, Lin F et al (2023) Qualitative assessments of density and background parenchymal enhancement on contrast-enhanced spectral mammography associated with breast cancer risk in high-risk women. Br J Radiol. https://doi.org/10.1259/bjr.20220051
Morris EA, Comstock CE, Lee CH (2013) ACR BI-RADS® Magnetic Resonance Imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System, 5th ed. American College of Radiology, Reston, VA, USA
Meucci R, Pistolese CA, Perretta T et al (2022) Background parenchymal enhancement in contrast-enhanced spectral mammography: a retrospective analysis and a pictorial review of clinical cases. In Vivo (Brooklyn) 36:853–858. https://doi.org/10.21873/invivo.12773
Funding
This study was supported by an unconditional research grant from GE Healthcare. This company did not have any influence on the study protocol planning, did not have any access to the study database, and was not involved in any way in the manuscript writing or submission phases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Francesco Sardanelli received research grants from GE Healthcare, Bayer, and Bracco; he is a member of the advisory board of Bayer, Bracco, and GE Healthcare; he also had talks sponsored by Bayer and Siemens Healthineers. All other authors declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical approval
This study was approved by the Ethics Committee of IRCCS Ospedale San Raffaele, Milano, Italy (protocol code CESM; approved on May 10, 2018), and by the Ethics Committee of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy (protocol code P-20190076950, approved on September 25, 2019).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Magni, V., Cozzi, A., Muscogiuri, G. et al. Background parenchymal enhancement on contrast-enhanced mammography: associations with breast density and patient’s characteristics. Radiol med 129, 1303–1312 (2024). https://doi.org/10.1007/s11547-024-01860-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-024-01860-5