Abstract
Objective
This retrospective observational study aims to evaluate the association between the extent of parametrial invasion (PMI) and disease-free survival (DFS) and cancer-specific survival (CSS) in patients with locally advanced cervical cancer (LACC).
Materials and methods
This study included patients with LACC showing parametrial invasion at Magnetic Resonance Imaging (MRI). They were treated with neoadjuvant chemo-radiotherapy (CT/RT) before undergoing radical hysterectomy. The staging MRIs were reviewed retrospectively. Measurements of maximum PMI (PMImax) and parametrial length were taken bilaterally. After that, PMIratio was calculated by dividing PMImax by parametrial length.
Analysis was conducted on homogeneous subsets of patients, grouped based on their pathological lymph nodal evaluation (N- and N+). Correlations between PMImax and PMIratio with DFS and CSS were evaluated in both the N- and N+ groups, employing univariable Cox regression analysis.
Results
Out of 221 patients, 126 (57%) had non-metastatic lymph nodes (N-), while 95 (43%) had metastatic lymph nodes (N+). The median observation period for all these patients was 73 months (95% confidence interval [CI]: 66–77). The 5-year DFS and CSS probability rates were 75% and 85.7%, respectively, for the N- group and 54.3% and 73.6%, respectively, for the N+ group. A higher PMImax (hazard ratio [HR] = 1.09) and PMIratio (HR = 1.04) correlated with worse overall survival in patients in the N- group (p = 0.025 and p = 0.042). These parameters did not show a significant statistical association in the N+ group.
Conclusions
The degree of PMI evaluated on MRI affects outcome in N- patients with LACC.
Clinical relevance statement
The degree of MRI parametrial invasion affects disease-free survival and cancer-specific survival in patients with the International Federation of Gynecology and Obstetrics (FIGO) stage IIB cervical cancer. This MRI finding can be easily incorporated into routine clinical practice.
Key Points
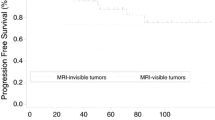
• Visual assessment of parametrial invasion on MRI was not significantly associated with prognosis in locally advanced cervical cancer (LACC).
• A greater degree of parametrial invasion is associated with poorer disease-free survival and cancer-specific survival in patients with LACC without metastatic lymph node involvement.
• The degree of parametrial invasion at MRI has no correlation with prognosis in LACC with metastatic lymph nodes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the fourth most prevalent cancer in women, causing around 600,000 new cases and 350,000 deaths annually [1]. The International Federation of Gynecology and Obstetrics (FIGO) updated the staging system in 2018 to include imaging and pathological findings, where available, to assign the stage. Moreover, for the first time, the revised staging permits the utilization of various imaging techniques, such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET), to define tumor size, lymph node (LN) status, and local or systemic spread [2].
Patients with locally advanced cervical cancer (LACC) (FIGO stage 2018 IB3-IVA) are treated with platinum-based chemotherapy associated with external beam radiotherapy and intracavitary brachytherapy (CCRT), which is the globally recognized treatment standard, showing 5-year overall survival (OS) rate ranging from 60 to 75%, depending on the stage of the disease [3, 4]. Radical surgery (RS) as replacement for intracavitary brachytherapy after chemo-radiotherapy (CT/RT) has been proposed to enhance control over local disease and decrease the amount of radiation dosage, which can potentially lower toxicity levels [5,6,7,8]. Two meta-analyses published by Shim et al [9] and Lu et al [10] reported that completion of hysterectomy after CT/RT versus CCRT did not improve overall survival in LACC patients but reduced the risk of local recurrence.
Parametrial invasion (PMI) is one of the most important prognostic factors and is defined as FIGO stage IIB. The lateral parametrium is divided into two parts, i.e., the proximal part medially to the ureter, and the distal part, laterally to the ureter [11, 12]. MRI is the best modality for cervical cancer staging, demonstrating high specificity (91–95%) and accuracy (92–96%) [13, 14]. The accuracy of MRI in the diagnosis of PMI is 94% [15].
Past evidence has shown that parametrial invasion is associated with deep stromal invasion, uterine corpus invasion, lymphovascular space invasion (LVSI), and pelvic LN metastases [16]. However, there is no data in the literature regarding the impact of the degree of PMI at MRI evaluation on the prognosis of patients with LACC. Therefore, our study aimed to measure the degree of PMI by MRI in LACC patients managed by preoperative CT/RT followed by radical surgery and verify whether different degrees of PMI at MRI evaluation correlate with clinical outcomes in patients with or without metastatic LNs.
Methods
Study design
This retrospective study was approved by the Local Ethics Committee (no. 4360). Patients with biopsy-proven cervical cancer with parametrial invasion (FIGO stage 2009 IIB-IIIB) evaluated at MRI, treated with CT/RT, and undergone surgery for LACC between January 2009 and December 2019 were retrieved from the database of the gynecologic department.
Inclusion criteria were as follows: (a) age > 18 years old; (b) available staging MRI for the review; (c) available histopathology results at diagnosis; (d) available data from clinical follow-up. Exclusion criteria: patients with concurrent malignancies at other sites, and MRI exam with artifacts limiting the evaluation.
MRI protocol
A 1.5-T scanner (Echospeed Horizon and Infinity, GE HealthCare) with a body coil and an eight-channel cardiac phased-array coil was used to perform pelvic scans. The scan consisted of axial T1-weighted fast spin echo (FSE) sequence and T2-weighted FSE sequences in various imaging planes (sagittal/axial/axial and coronal oblique according to the long axis of the cervix). Additionally, diffusion-weighted imaging (DWI) was obtained in the same plane of axial oblique FSE T2-weighted images (WI) using a single-shot SE echo-planar imaging sequence with two b values (0 and 800/1000 s/mm2). Axial T2-WI fast recovery FSE (FRFSE) was obtained to include the renal hila to assess the presence of retroperitoneal lymphadenopathies. More details of the acquisition protocol can be found in Supplementary Material.
Treatment
Pretreatment workup included pelvic clinical examination under anesthesia with biopsies, MRI, complete blood count, liver and kidney function measurement, cystoscopy, and proctoscopy if necessary. More details can be found in Supplemental Material. Patients underwent preoperative CT/RT administered as whole pelvic irradiation (total dose from 39.6–45 Gy) in combination with cisplatin (20 mg/m2, days 1–4 and 26–30 of treatment) combined with capecitabine (20 mg/m2, 2-h intravenous infusion, on days 1–4 and 26–30 of treatment) [6, 17].
Five/six weeks after completion of CT/RT, the MRI was performed to evaluate response to treatment according to the RECIST criteria [18].
In 6–8 weeks after CT/RT completion, all patients were treated with radical hysterectomy and pelvic lymphadenectomy, while retroperitoneal lymphadenectomy was performed where pelvic LNs were still involved on imaging after CT/RT, intraoperative assessment of palpable or hardened or immobile pelvic and/or aortic LNs, and intraoperative evaluated involved pelvic LNs at intraoperative pathological examination.
Data analysis
All data were retrospectively retrieved from the clinical charts and included age at diagnosis, body mass index (BMI), histotype, grading, FIGO stage, presence of metastatic LN, tumor size as measured on MRI, the status of last follow-up, and the site of recurrence. Patients were restaged according to FIGO 2018, based on the evaluation of MRI and PET/CT (when indicated) at our Institutional multidisciplinary team meeting. Vaginal recurrences were considered local, pelvic/para-aortic recurrence as regional, and upper abdominal and/or extra-abdominal as distant.
Imaging analysis
The parametrial invasion was retrospectively assessed with T2-weighted images by a radiologist with 3 years of experience in gynecologic imaging. Parametrial involvement was visually assessed, co-registering T2-WI with DWI.
The parametrial invasion was identified as the disruption of a low-SI stromal ring associated with the presence of spiculated tumor–parametrium interface or gross nodular tumor extension into parametrium or encasement of uterine vessels by tumor [19].
Lateral parametria were divided into a proximal part, extending from the lateral border of the cervix to the ureter, and a distal part, extending from the ureter to the pelvic fascia. Quantitative evaluation was performed on the coronal oblique T2-WI: first, a longitudinal line along the uterine lateral border at the isthmus site at the entrance of the uterine vessels was drawn on both sides; second, the transverse diameter of the lateral parametrium was measured in millimeters (mm), between the lateral border of the cervix and the pelvic fascia, at the site of the maximum lateral parametrium extension. The axial oblique plane was used in conjunction with the coronal one to better evaluate the site of the maximum lateral parametrial extension.
Maximum parametrial invasion (PMImax) was measured in millimeters. Afterward, the percentage of infiltration (PMIratio), designed as 100 × (PMImax)/(parametrium total length in mm), was calculated (Fig. 1) and three classes of parametrial invasion were identified (PMI degree). PMI degree was assessed as < 1/3, between 1/3 and 2/3, > 2/3, in case of PMIratio < 33.3%; ≥ 33.3% and ≤ 66.6% and greater than 66.6% respectively.
Coronal oblique (A) T2-weighted image shows a large T2 intermediate signal intensity lesion disrupting cervical stroma circumferentially. Tumor invades both parametria (FIGO stage IIB). Right parametrium was greater invaded than left. First, the length of the lateral parametrium (dotted arrow) was measured from the lateral border of the cervical canal (dotted line) to the pelvic wall. Then, PMImax (continuous arrow) was measured from the lateral border of the cervical to the lateral border of the tumor. Schematic illustration of measurements (B). PMImax: maximum parametrial invasion
In the case of bilateral parametrial involvement at MRI, the side with the larger extent of invasion was evaluated.
In order to assess the inter-rater reliability PMImax, parametrial total length, and PMIratio were also evaluated by a second independent reader with a greater degree of expertise (7 years of experience in gynecologic imaging) in 30 randomly selected cases [20].
Statistical analysis
Qualitative variables were summarized with absolute and percentage frequencies; quantitative variables were presented as median (min-max) (not normally distributed). The normality of continuous data was verified with the Shapiro-Francia test. Parametrial infiltration was evaluated according to both qualitative (distal versus proximal, PMI degree) and quantitative (mm of infiltration and % of infiltration) variables as appropriate. According to previous recommendations, inter-rater reliability was assessed using the intraclass correlation coefficient (ICC) [20]. In detail, a two-way random effects, two-raters, absolute agreement model was used. ICC values less than 0.5 indicated poor reliability, values between 0.5 and 0.75 indicated moderate reliability, values between 0.75 and 0.9 indicated good reliability, and values greater than 0.90 indicated excellent reliability.
All the analyses were performed in homogeneous subset grouping patients according to LNs assessment, i.e., patients without metastatic LNs (N-) and patients with metastatic LNs (N+) at histological evaluation.
In both groups (N- and N+), the association of parametrial infiltration (PMImax, PMIratio, and PMI degree) with clinical outcomes was analyzed by univariable Cox regression analysis provided both in terms of disease-free survival (DFS) and cancer-specific survival (CSS) [21]. DFS was defined as the time interval between the date of diagnosis (biopsy taken at the time of examination under anesthesia) and the date of the first clinical or imaging detection of recurrence/progression or last follow-up. CSS was defined as the time interval between the date of diagnosis and the time when cancer-specific death occurred or the last follow-up/death for other causes.
PMImax was evaluated both as a continuous (measurement in mm) and dichotomous variable (under or equal to best cut-off and over best cut-off). The best cut-off value for PMImax was identified through a receiver operating characteristic (ROC) analysis according to the maximization of Youden’s index method.
Median follow-up was calculated according to the inverted Kaplan-Meier technique [22]. DFS and CSS curves were estimated by the Kaplan-Meier product limit method and compared using the Mantel-Cox (log-rank) statistics [21, 23].
In all cases, as reported in the literature, the role of age, BMI, FIGO stage, histotype (squamous vs non-squamous), and grading (G1-G2 vs G3) as possible confounder parameters was also assessed. Multivariable analysis was planned in order to adjust MRI results according to pathological confounder parameters if a p value < 0.05 at univariable analysis was found.
Statistical analysis was performed by an experienced biostatistician using STATA software (STATA/BE 17.0 for Windows, StataCorp LP). Two-sided tests were used, and the significance level was set at p < 0.05. No imputation was carried out for missing data, and all estimates were presented with two-sided 95% confidence intervals (CIs).
Results
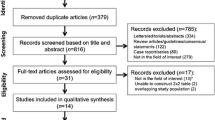
Figure 2 shows the flow diagram of the study population. Between 2009 and 2019, there were 429 patients with parametrial invasion (FIGO 2009 stage IIB-IIIB) at diagnosis; in 197 patients the MRI was not available, thus resulting in 232 patients with available MRI. No evidence of PMI at MRI was found in 11 patients, resulting in 221 patients included in the analysis.
Table 1 summarizes the clinico-pathological features of the 221 patients: the median age was 52 years (range: 20-79), and median BMI was 24.1 (range: 15.6–44.5). At histology, 197 (89.1%) patients had squamous cell carcinoma, 23 (10.4%) patients had adenocarcinoma, and 1 (0.5%) patient had adenosquamous carcinoma. Tumor grading was available for 189/221 patients. In 189 patients, grade 1 was shown in 5 patients (2.6%), grade 2 in 116 patients (61.4%), and grade 3 in 68 patients (36.0%), respectively.
Following FIGO stage 2018, 106 (48.0%) patients had FIGO stage IIB disease, 6 (2.7%) patients had stage IIIA, and 14 (6.3%) patients had stage IIIB. Patients with positive pelvic LNs were considered as stage IIIC1 (N = 88, 39.8%), while patients with positive aortic LNs were considered as stage IIIC2 (N = 7, 3.2%). One hundred and twenty-six patients out of 221 had negative lymph nodes (N-) and 95/221 had metastatic lymph nodes (N+).
Tumor characteristics at MRI
Table 2 summarizes the tumor characteristics on MRI. Overall, the median maximum tumor diameter was 50 mm (range: 10–120); PMI was unilateral in 88 (39.8%) and bilateral in 133 cases (60.2%). The proximal part of the parametrium was involved in 203 (91.9%) and the distal part was involved in 18 cases (8.1%). Median PMImax was 10 mm (range: 3–35); median PMImax was 9 mm (range: 3–35) in N- group, and PMImax was 10 mm (range: 3–29) in the N+ group. The median PMIratio was 22.2% (range: 5.1–117.4%), and the median length of parametrium was 45 mm (range: 23–69). PMIratio was 21.9% (range: 5.1–63.0) in the N- group and 25.0% in the N+ group (8.9–117.4%). There was no statistical difference between N- patients vs. N+ patients.
ICC analysis
The ICC in order to evaluate the agreement of a single measure of PMImax and parametrial length between the two radiologists were 0.75 (95% CI: 0.53–0.87) and 0.81 (95% CI: 0.64–0.91), respectively.
The impact of the degree of parametrial invasion on clinical outcomes
Table 3 summarizes the clinical outcomes: overall, median follow-up was 72.9 months (95% CI 65.9–77.1). At the last follow-up, 32/221 (14.5%) patients had developed loco-regional recurrence, 23/221 (10.4%) patients developed distant metastasis; 10/221 patients (4.5%) experienced both loco-regional and distant metastasis. Forty-three patients out of 221 (19.5%) died of cancer-specific disease. Two patients died from other causes. The first one died from osteosarcoma and the second one from a myocardial infarction 81 and 31 months after cervical cancer diagnosis, respectively. Figure 3 provides Kaplan-Meyer curves of the probability of DFS and CSS according to lymph node status: for all 221 patients, the probability of 5-year DFS was 75.0% (95%CI 66.0–82.0) in N- group and 54.3% (95%CI 42.8–64.3) in N+ group. The probability of a 5-year CSS rate was 80.7% (95%CI 74.2–85.7%). The probability of 5-year CSS was 85.7% (95%CI 77.6–91.0%) in the N- group and 73.6% (95%CI 62.1–82.2%) in the N+ group.
Degree of parametrial invasion and clinical outcomes
Table 4 shows the univariate analysis by the Cox proportion hazard model according to patients’ characteristics as prognostic parameters for DFS and CSS in N- patients; there were no statistically significant associations with DFS. Mean lower BMI (hazard ratio [HR] = 0.88; 95%CI 0.77–0.99) was associated with better overall survival in the N- group (p = 0.046).
Greater PMImax (HR = 1.09; 95%CI 1.01–1.17) and PMIratio (HR = 1.04; 95%CI 1.00–1.08) were associated with poorer overall survival in N- group (p = 0.025 and p = 0.042).
PMImax in the N- group was dichotomized according to 11.3 mm, which was the best cut-off value retrieved from ROC analysis according to Youden’s index. PMImax greater than or equal to 11.3 mm (HR = 2.53; 95%CI 1.03–6.25) was associated with poorer overall survival (p = 0.043).
Greater PMI degree (HR = 2.91; 95%CI 1.11–7.67) was associated with poorer overall survival in N- group (p = 0.031)
Table 5 shows the univariate analysis by the Cox proportion hazard model according to patient’s characteristics as prognostic parameters for DFS and CSS in N+ patients; no statistically significant association was observed between these parameters and DFS and CSS in the N+ group.
Figure 4 provides Kaplan-Meyer of the probability of DFS and CSS by dichotomized PMImax.
As reported in Tables 4 and 5 no clinical and pathological characteristics achieved a statistical significance in survival prediction in terms of DFS and CSS and both in N- and N+ group, so no adjustments were required.
Discussion
Our study focused on the assessment of PMI in LACC. We found a significant association between greater PMI -in terms of both PMImax and PMIratio- and poorer CSS in the N- group. Furthermore, given the median length of the parametrium in our population (45 mm), PMImax lower than 11.3 mm indicated significantly better CSS. As derived from Fig. 4, the 5-year CSS probability was 90% in the subgroup with PMImax < 11.3 mm and 75% in the subgroup with PMImax ≥ 11.3 mm. Conversely, the association between the degree of PMI in terms of proximal vs. distal and DFS and CSS was not statistically significant.
Our study confirms that lymph node metastases represent a greater prognostic factor than PMI. Indeed, no statistically significant association between the degree of PMI and DFS/CSS was found in patients with nodal metastases (N+ group). The probability of both DFS and CSS in the N+ group was significantly worse than that of the N- group, indicating that nodal metastases are an important prognostic factor in LACC.
PMImax can depict an early and localized invasion of the parametria in the absence of lymph node spread, which is of higher prognostic significance. In these cases, PMImax can be used as an early indicator of the aggressiveness of the disease. Our results indicate that quantitative evaluation of PMI is more impactful than visual evaluation. This difference is due to the improved accuracy and objectivity of PMImax and PMIratio as quantitative measurements. In fact, qualitative analysis often depends on subjective interpretations and varies depending on the experience and expertise of the review radiologists. Good inter-rater reliability on a sample cohort supported the reproducibility of the method proposed for the quantitative measurement of PMI (PMImax and PMIratio).
To our knowledge, our study is the only one in the literature evaluating the relationship between the degree of PMI at MRI evaluation and outcome, whereas all previous studies have performed a qualitative binary evaluation (absent vs. present PMI). Advanced MRI techniques in detecting PMI, such as diffusion tensor imaging (DTI) and fused T2-WI and DWI, have reported good results in the visual detection of PMI [24, 25].
From a clinical point of view, demonstration of the significance of PMImax can refine the pathway of disease progression and prognosis in LACC. Clinicians may consider PMImax while planning their therapeutic strategies, particularly for patients without metastatic LNs. CCRT is the standard treatment for LACC with parametrial invasion [26]. In this setting, PMImax could signal a need for modulating the radiation dose according to it, leading to fully personalized radiation therapy. Moreover, concerning the better prognosis of patients with initial PMI, further studies could investigate the role of PMImax in guiding the decision between surgery and chemoradiation. A recent meta-analysis with a large number of stages IIB undergoing primary hysterectomy supported adjuvant chemotherapy as a safe option for women desiring to protect their ovaries from radiation injury [27]. In this specific cohort, PMImax could help in the selection of patients with cervical cancer willing to preserve their ovarian function.
Our study has certain limitations. First, it is a retrospective study. Thus, subgroup analysis was conducted to obtain homogeneous cohorts. A selection bias can be represented by the fact that patients previously enrolled in two phase II clinical trials have been included. However, these patients were similar to those of the remaining cohort and received the same treatment. Second, patients did not undergo standard-of-care treatment (CCRT and brachytherapy). However, in the last decades, several randomized trials reported that radical hysterectomy after neoadjuvant chemo-radiotherapy does not impair clinical outcomes compared with CCRT and brachytherapy in LACC [5,6,7,8]. Third, our subgroups were small.
In conclusion, our study establishes that MRI is a valid and reproducible tool for quantitative assessment of PMI in LACC. The measurement of PMImax can be particularly useful for risk stratification and a more personalized treatment approach, especially for patients without lymph node metastases. However, larger prospective studies are necessary to validate our data.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence Interval
- CCRT:
-
Concurrent chemo-radiotherapy
- CSS:
-
Cancer-specific survival
- CT:
-
Chemotherapy
- CT/RT:
-
Chemo-radiotherapy
- DFS:
-
Disease-free survival
- DWI:
-
Diffusion-weighted imaging
- FIGO:
-
International Federation of Gynecology and Obstetrics
- FSE:
-
Fast spin echo
- ICC:
-
Intraclass correlation coefficient
- LACC:
-
Locally advanced cervical cancer
- LN:
-
Lymph node
- OS:
-
Overall survival
- PMI:
-
Parametrial invasion
- RS:
-
Radical surgery
- RT:
-
Radiotherapy
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249
Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R (2018) Cancer of the cervix uteri. Int J Gynaecol Obstet 143(Suppl 2):22–36
Chemoradiotherapy for Cervical Cancer Meta-analysis C (2010) Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev CD008285
Shrivastava S, Mahantshetty U, Engineer R et al (2018) Cisplatin chemoradiotherapy vs radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix: a randomized clinical trial. JAMA Oncol 4:506–513
Ferrandina G, Ercoli A, Fagotti A et al (2014) Completion surgery after concomitant chemoradiation in locally advanced cervical cancer: a comprehensive analysis of pattern of postoperative complications. Ann Surg Oncol 21:1692–9
Ferrandina G, Gambacorta A, Gallotta V et al (2014) Chemoradiation with concomitant boosts followed by radical surgery in locally advanced cervical cancer: long-term results of the ROMA-2 prospective phase 2 study. Int J Radiat Oncol Biol Phys 90:778–85
Pervin S, Ruma FI, Rahman K et al (2019) Adjuvant hysterectomy in patients with residual disease after radiation for locally advanced cervical cancer: a prospective longitudinal study. J Glob Oncol 5:1–7
Yoshida K, Kajiyama H, Yoshihara M et al (2020) The role of additional hysterectomy after concurrent chemoradiation for patients with locally advanced cervical cancer. Int J Clin Oncol 25:384–390
Shim SH, Kim SN, Chae SH, Kim JE, Lee SJ (2018) Impact of adjuvant hysterectomy on prognosis in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy: a meta-analysis. J Gynecol Oncol 29:e25
Lu W, Lu C, Yu Z and Gao L (2021) Chemoradiotherapy alone vs. chemoradiotherapy and hysterectomy for locally advanced cervical cancer: a systematic review and updated meta-analysis. Oncol Lett 21: 160
Ercoli A, Delmas V, Fanfani F et al (2005) Terminologia Anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: a dissection-based comparative study. Am J Obstet Gynecol 193:1565–73
Valentini AL, Gui B, Micco M et al (2016) MRI anatomy of parametrial extension to better identify local pathways of disease spread in cervical cancer. Diagn Interv Radiol 22:319–25
Manganaro L, Lakhman Y, Bharwani N et al (2021) Staging, recurrence and follow-up of uterine cervical cancer using MRI: updated guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. Eur Radiol 31(10):7802–7816
Woo S, Atun R, Ward ZJ, Scott AM, Hricak H, Vargas HA (2020) Diagnostic performance of conventional and advanced imaging modalities for assessing newly diagnosed cervical cancer: systematic review and meta-analysis. Eur Radiol. 30(10):5560–5577
Woo S, Suh CH, Kim SY, Cho JY, Kim SH (2018) Magnetic resonance imaging for detection of parametrial invasion in cervical cancer: an updated systematic review and meta-analysis of the literature between 2012 and 2016. Eur Radiol 28:530–541
Matsuo K, Shimada M, Nakamura K et al (2019) Predictors for pathological parametrial invasion in clinical stage IIB cervical cancer. Eur J Surg Oncol 45:1417–1424
Testa AC, Moro F, Pasciuto T et al (2018) PRospective Imaging of CErvical cancer and neoadjuvant treatment (PRICE) study: role of ultrasound to assess residual tumor in locally advanced cervical cancer patients undergoing chemoradiation and radical surgery. Ultrasound Obstet Gynecol 52:110–118
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–47
Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C (2013) The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology 266:717–40
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–63
Cox D (1972) 197–220. J R Stat Soc 34:197–220
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–6
Kaplan FLMP (1958) Non parametric estimation from incomplete observations. Am J Stat Assoc 53:457–481
Di Paola V, Perillo F, Gui B et al (2022) Detection of parametrial invasion in women with uterine cervical cancer using diffusion tensor imaging at 1.5T MRI. Diagn Interv Imaging 103:472–478
Park JJ, Kim CK, Park SY, Park BK (2015) Parametrial invasion in cervical cancer: fused T2-weighted imaging and high-b-value diffusion-weighted imaging with background body signal suppression at 3 T. Radiology 274:734–41
Pujade-Lauraine E, Tan DSP, Leary A et al (2022) Comparison of global treatment guidelines for locally advanced cervical cancer to optimize best care practices: a systematic and scoping review. Gynecol Oncol. 167(2):360–372
Zhang YF, Fan Y, Zhang P, Ruan JY, Mu Y, Li JK (2022) Cervical cancer recurrence and patient survival after radical hysterectomy followed by either adjuvant chemotherapy or adjuvant radiotherapy with optional concurrent chemotherapy: a systematic review and meta-analysis. Front Oncol 12:823064
ECR 2022 Book of Abstract Insights Imaging 13 (Suppl 4): 205 (2022)
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Benedetta Gui.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (T. P.) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in the following manuscripts:
• Chemoradiation with concomitant boosts followed by radical surgery in locally advanced cervical cancer: long-term results of the ROMA-2 prospective phase 2 study (https://doi.org/10.1016/j.ijrobp.2014.07.033).
• PRospective Imaging of CErvical cancer and neoadjuvant treatment (PRICE) study: role of ultrasound to assess residual tumor in locally advanced cervical cancer patients undergoing chemoradiation and radical surgery (https://doi.org/10.1002/uog.17551).
• The PRICE study: the role of conventional and diffusion-weighted magnetic resonance imaging in assessment of locally advanced cervical cancer patients administered by chemoradiation followed by radical surgery (https://doi.org/10.1007/s00330-017-5233-x).
• Prospective multimodal imaging assessment of locally advanced cervical cancer patients administered by chemoradiation followed by radical surgery-the “PRICE” study 2: role of conventional and DW-MRI (https://doi.org/10.1007/s00330-018-5768-5).
However, we focused on a novel aspect not explored previously: parametrial invasion. This study provides new insights on the qualitative and quantitative assessment of it and uniquely contributes to the knowledge base of this patient cohort.
Preliminary results of the present were presented at European Congress of Radiology (ECR 2022) and reported in the ECR 2022 Book of Abstracts [28].
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Russo, L., Pasciuto, T., Lupinelli, M. et al. The value of MRI in quantification of parametrial invasion and association with prognosis in locally advanced cervical cancer: the “PLACE” study. Eur Radiol 34, 4003–4013 (2024). https://doi.org/10.1007/s00330-023-10443-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10443-3