Abstract
Background
The standard treatment for cervical cancer is chemoradiation although some patients showed treatment resistance. The purpose of this study was to investigate the clinical efficacy of surgery after chemoradiation for cervical cancer.
Methods
Patients with FIGO stage IB2 to IIB cervical cancer were included in the study between 2005 and 2015. A total of 50 patients who underwent surgery after neoadjuvant chemoradiation and 76 patients who received only chemoradiation were compared. Baseline differences between the two groups were adjusted with inverse probability of treatment weighting method using propensity scores composed of the following independent variables: age, stage, tumor size, lymph node metastasis, and histological subtypes.
Results
Median follow-up was 64.8 (range 4.8–143.9) months. After adjustment with inverse probability of treatment weighting, Kaplan–Meier curves showing adjusted progression-free survival and overall survival were significantly longer in the neoadjuvant chemoradiation compared with the chemoradiation-only group (p = 0.027 and p = 0.017, respectively). Moreover, in patients with squamous cell carcinoma, recurrence in previously irradiated field and recurrence both in and out of previously irradiated field were significantly decreased in the neoadjuvant chemoradiation compared with the chemoradiation-only group (3.1% and 18.4%, respectively; OR 0.142, p = 0.001]. Adverse events of surgery after chemoradiation were acceptable, although temporary hydronephrosis was frequently observed (23.1%).
Conclusions
Surgery after chemoradiation reduced pelvic recurrence, and as a result, patients who underwent neoadjuvant chemoradiation showed more favorable survival outcomes compared with those who only underwent chemoradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is one of the most common malignancies in females worldwide. According to cancer statistics, an estimated 527,600 new cases and 265,700 deaths associated with cervical cancer were reported in 2012 [1]. Treatment strategy is dictated by the International Federation of Gynecology and Obstetrics (FIGO) staging system, with two major treatment options for patients with this type of tumor: radical hysterectomy and concurrent chemoradiation (CRT) [2, 3]. For patients with locally advanced cervical cancer (LACC, FIGO stage IB2–IVA), CRT is considered the standard treatment [2, 4]. Surgery is the mainstay of treatment for early-stage disease (FIGO stage IA–IB1) and is also performed for patients with FIGO stage ≤ IIB LACC [5, 6]. Despite of the clinical efficacy of CRT and surgery, some patients develop treatment resistance, and the prognosis of patients with disease recurrence remains poor [2]. Prognosis is particularly poor for patients who experience recurrence in a previously irradiated field [7,8,9]. Therefore, new treatment modalities have been investigated to maximize local control and eventually improve survival of LACC patients.
The advantage of neoadjuvant therapy is its ability to shrink bulky tumors and, therefore, improve resection rate success compared with surgery without neoadjuvant therapy. Neoadjuvant chemotherapy followed by surgery has been widely performed. However, its impact on survival has been inconsistent [10,11,12]. Similar to other malignancies, such as head and neck cancer and rectal cancer, neoadjuvant CRT (NACRT) was proposed for use in cervical cancer, showing efficacy and feasibility [13,14,15]. However, few reports exist comparing the clinical efficacy of NACRT and CRT [16, 17]. Moreover, some studies reported high complication rates associated with NACRT [18, 19]. Therefore, NACRT is not widely performed in most countries, with the clinical benefit and feasibility of additional surgery after CRT remaining controversial.
The present study compared the outcomes of patients treated with NACRT and those treated with CRT only using inverse probability of treatment weighting (IPTW), increasing the body of evidence supporting the clinical efficacy of NACRT.
Patients and methods
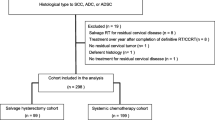
All the clinical records of patients treated in our hospital were retrospectively reviewed from 2005 to 2015, and 174 patients with FIGO stage IB2 to IIB LACC were identified. Patient selection flowchart is presented in Fig. 1. Patients who underwent surgery with or without adjuvant chemotherapy were excluded. As a result, 50 patients who received NACRT and after underwent hysterectomy (NACRT group) and 76 patients who received CRT only (CRT group) were included. The study was approved by the ethics committee of the institution (Approval no.: 2017-0053), and approval for an opt-out consent method was given. Informed consent was obtained by the opt-out consent method on the website of Nagoya University.
The treatment strategy for each patient had been previously determined by several gynecologic oncologists based on patient age, performance status, disease spread, and histology. In the NACRT group, external beam radiotherapy (EBRT) and two courses of chemotherapy were performed. EBRT was performed at 1.8 Gy once per day (total dose of 39.6 Gy in 22 fractions), and cisplatin (70 mg/m2 on day 1) and 5‑fluorouracil (700 mg/m2, 24 h continuous intravenous infusion on days 1–4) combination chemotherapy was usually administered. Treatment response was evaluated, and if possible, radical hysterectomy plus pelvic lymph node dissection was performed. Additional chemotherapy was considered depending on pathological findings. In the CRT group, combination radiotherapy—which included EBRT and intracavity brachytherapy (ICBT)—and five courses of chemotherapy were performed. EBRT was performed at 1.8 Gy once per day (total dose of 50.4 Gy in 28 fractions), and ICBT was remotely performed after loading the system with a Co 60 source. The total dose to point A (a reference location 2 cm laterally and 2 cm above the cervical os) was 15 Gy.
Post-treatment follow-up was performed monthly at the beginning, with the interval subsequently extended. Recurrence was determined via physical examination, transvaginal ultrasound, vaginal stump cytology, laboratory tests, and computed tomography.
The primary outcomes were progression-free survival (PFS) and overall survival (OS). PFS was defined as the time elapsed between treatment initiation and death caused by tumor progression and OS as the time elapsed between treatment initiation and death from any cause. The secondary outcomes were recurrent pattern, residual lesion, and adverse events. Common Terminology Criteria for Adverse Events Version 4.0 was used to grade observed adverse events.
Statistical analyses for IPTW using propensity scores were performed with SPSS version 25 (IBM Corp., Armonk, NY). Baseline differences between the NACRT and CRT groups were adjusted with IPTW using propensity scores with the following independent variables: age, FIGO stage, tumor size, lymph node metastases, and histological subtypes. Differences between the two groups were assessed by Mann–Whitney U test and t test. Kaplan–Meier curves were generated and compared with the log-rank test using SAS version 8 (SAS Institute Inc., Cary, NC). To evaluate differences in recurrence sites, odds ratio (OR) and 95% confidence interval (CI) were calculated. Differences at p < 0.05 were considered significant.
Results
To evaluate the clinical efficacy of NACRT, 50 patients who received NACRT and 74 patients who received CRT were compared retrospectively. In the NACRT group, all patients had been submitted to surgery, and the median period from the end of radiation to the surgery was 23.5 (range 8–42) days. Most patients (n = 43) underwent radical hysterectomy, and six patients underwent modified radical hysterectomy. These patients also underwent pelvic lymph node dissection, and one patient underwent tumor debulking surgery due to cancer progression. For 43 patients who underwent radical hysterectomy, the median operation time was 330 (range 211–501) min. Moreover, the median blood loss was 784 (231–3332) mL, and 19 patients (44%) received blood transfusions. In the CRT group, two patients with squamous cell carcinoma (SCC) had radiological residual lesion after CRT and underwent salvage hysterectomy. Baseline patients’ characteristics are shown in Table 1. Age, FIGO stage, tumor size, and clinical lymph node metastases were well balanced between the two groups, but histological subtypes were significantly different between both. While non-SCC patients comprised more than half of the NACRT group (52%), only four non-SCC patients were included in the CRT group (5.3%). Most of the non-SCC cases were adenocarcinoma (n = 25), three cases were undifferentiated carcinoma, one case was adenosquamous carcinoma, and one case was small-cell carcinoma. Baseline differences between the two groups were subsequently adjusted with IPTW using propensity scores previously described. After adjustment, no significant differences existed between the two groups (Table 1).
In the 64.8 (range 4.8–143.9) months of median follow-up, 43 patients (34.1%) experienced recurrence, and 26 patients (20.6%) died. PFS and OS Kaplan–Meier curves showed no significant differences between groups (p = 0.219 and 0.217, respectively). After IPTW, adjusted PFS and OS Kaplan–Meier curves were significantly longer in the NACRT than in the CRT group (p = 0.027 and p = 0.017, respectively) (Fig. 2a, b). In the NACRT and CRT groups, adjusted 5-year PFS rates were 78.3% and 56.9%, and adjusted 5-year OS rates were 87.7% and 66.2%, respectively.
To investigate the better survival outcomes observed in the NACRT group, recurrence sites were stratified by histological subtype. In the patients with SCC, remarkedly less patients experienced recurrence in previously irradiated field (in-field recurrence) in the NACRT [n = 1 (4.2%)] compared with the CRT group [n = 10 (13.9%)]. Moreover, all the four patients experiencing recurrence both in and out of previously irradiated field (mixed recurrence) were in the CRT group. After IPTW adjustment, NACRT significantly decreased local failure rate [the NACRT and CRT groups; 3.1% and 18.4%, respectively; OR 0.142 (95% CI 0.040–0.505), p = 0.001] (Table 2). However, there were no significant differences in recurrence out of previously irradiated field (out-field recurrence). Then we evaluated the recurrence pattern of non-SCC patients. While one patient (25.0%) in the CRT group experienced mixed recurrence, four patients (15.4%) in the NACRT group experienced in-field recurrence. After IPTW adjustment, NACRT tended to decrease local failure rate, but there was no significant difference [the NACRT and CRT groups; 15.4% and 37.1%, respectively; OR 0.308 (95% CI 0.087–1.092), p = 0.085] (Table 2). Whereas out-field recurrence rates were not significantly different between the two groups.
The proportion of residual lesions in surgical samples was also evaluated. In stages IB2 and IIA, no patients with SCC had macroscopic residual lesions, and 45.5% of non-SCC patients had macroscopic lesions (Table 3). On the other hand, in stage IIB, 47.4% and 66.7% of patients with and without SCC had macroscopic residual lesions, respectively. This suggests that the pathological efficacy of chemoradiotherapy tends to decrease in stage IIB compared with stages IB2 and IIA, although this result was not statistically significant (p = 0.120 and 0.330 in patients with and without SCC, respectively) (Table 3). The two patients in the CRT group who underwent salvage hysterectomy showed apparent pathological residual lesions.
Adverse events of the 52 patients who underwent surgery after CRT were evaluated. As intraoperative injury, one patient experienced ureter injury, and another patient experienced left obturator nerve injury. Postoperative adverse events in the NACRT group were shown in Table 4. No patients reported grade 4 or 5 adverse events. Fourteen grade 3 acute adverse events were reported in 12 patients (23.1%), and all the patients had hydronephrosis. The patient who experienced intraoperative ureter injury presented hydronephrosis and ureterovaginal fistula (grade 3) postoperatively, and vesicoureteric anastomosis was performed approximately 3 years after the initial treatment. Another patient experienced subcutaneous surgical site infection (grade 3). One patient underwent surgery for right lower limb lymphedema approximately 5 years after the initial treatment. Mild urinary toxicity and lymphocele were observed frequently (Table 4). Whereas there were 22 patients (44%) who experienced no adverse events.
Discussion
Since CRT was established, the prognosis of patients with LACC has improved. However, some patients still develop treatment-resistant tumors and have poor prognosis. NACRT has been proposed to improve patient outcomes, but the advantages of this treatment compared with CRT have not been demonstrated yet. Therefore, the present study proposed to investigate the clinical efficacy of NACRT using IPTW.
IPTW is a propensity score method, and the number of studies using this analysis is rapidly increasing in recent years [20]. In observational studies, treatment assignment is influenced by patients’ baseline characteristics, and propensity score is often used to reduce or minimize the effects of confounders due to measured baseline covariates. The advantage of using a propensity score method is that it allows observational studies to have a similar design for randomized interventions [20, 21]. In this study, most patients without SCC underwent NACRT. Subtypes without SCC are generally considered radioresistant, and the prognosis of these patients is poorer than of those with SCC [22]. In this study, after IPTW adjustment, patients’ baseline characteristics were well balanced between the two groups.
After IPTW adjustment, both OS and PFS in the NACRT group were significantly improved compared with the CRT group. Although several reports in the literature show the clinical efficacy of NACRT [23,24,25,26,27,28,29], only few reports compared the outcomes of patients treated with NACRT with those of patients treated with CRT alone [16, 17]. According to one previous study, OS and disease-free survival rates of patients who underwent NACRT were significantly improved compared with those of patients who underwent CRT only [16]. Moreover, another report showed that recurrence rate was significantly decreased in patients treated with NACRT compared with CRT alone [17]. This suggests that additional surgery may confer a clinical benefit. Conversely, in a meta-analysis, additional surgery reduced the risk of recurrence but did not improve OS [30], but the study did not take into account the differences in treatment protocols between institutions and differences in patients’ characteristics, such as tumor size and stage and nodal metastasis. To maximize the efficacy of NACRT, patients’ eligibility criteria and treatment protocols should, therefore, be considered.
Regarding recurrence sites, NACRT significantly reduced in-field recurrence in SCC patients. Previous reports also showed good local control associated with NACRT [16, 17, 23,24,25,26,27], with results attributed to the removal of residual cancer in the uterus and/or pelvic lymph nodes by additional surgery. In this study, several patients (mainly stage IIB and non-SCC histological subtype) had residual regional pathology after EBRT (39.6 Gy) plus chemotherapy. Moreover, two SCC patients in the CRT group had residual cancer after radical irradiation (EBRT 50.4 Gy, ICBT 15 Gy). Previous reports also suggested that residual disease was present in nearly half of patients who underwent surgery after CRT and that it was an important prognostic factor [19, 25,26,27,28, 31]. Therefore, even after definitive CRT, some patients show treatment resistance. In addition, particular attention should be paid to minimal residual disease not detected by clinical examinations, including ultrasound and computed tomography. Reduction of in-field recurrence by additional surgery is beneficial, since the prognosis of patients with in-field recurrence remains very poor due to limited treatment options [2]. Overall, in this study, surgery after CRT was considered to provide better local control, and as a result, survival rate in the NACRT group is higher than in the CRT group.
Out-field recurrence rates were no different between the two groups, as observed in around 15% of SCC and 25% of non-SCC patients. For highly metastatic tumors, local surgery is considered to have limited effect on survival and is unable to prevent metastases. Therefore, development of new therapeutic options is desirable to improve patient prognosis.
In this study, adverse events of NACRT were considered tolerable, with temporary hydronephrosis most frequently observed. A review previously reported a complication rate of 15–46% associated with NACRT; it has also been reported that most grade 2–3 complications were urological or bowel complications [32]. Other reports also showed an acceptable morbidity associated with NACRT [24,25,26,27]. Nevertheless, attention should be paid to adverse events, as one study reported two deaths due to complications of this treatment modality (although it included 11 patients with FIGO stage III or IV) [19]. An important point is that postoperative complications may happen, not only due to the surgical procedure itself but also due to the spread of the disease. Moreover, total radiotherapy dosing and chemotherapy regimens were different between groups. Therefore, to better evaluate adverse events, NACRT eligibility criteria and treatment protocols should be taken into consideration, and prospective studies should be performed.
This study has several limitations. First, it was a small-scale retrospective study, with only four non-SCC patients receiving CRT. Although it is reported that randomized controlled trials and propensity score analyses likely yield similar results, bias may persist even after IPTW [33]. Therefore, the clinical benefit of NACRT should be validated in large-scale randomized controlled trials. Second, eligibility criteria of NACRT compared with CRT has not been decided, and the treatment strategy for each patient had been previously determined by several gynecologic oncologists in this retrospective study. Moreover, optimal timing of surgery after CRT has not yet been investigated. Thus, to maximize the efficacy of NACRT, further investigations about detailed treatment protocol are needed. Third, no comparison was performed between patients who received NACRT and those who received neoadjuvant chemotherapy. Consequently, the possible advantage of NACRT over neoadjuvant chemotherapy should be evaluated in the future. Lastly, the detailed treatment strategy for LACC, especially FIGO stage IIB, is different between countries and institutions, as well as the total radiation dosing and chemotherapy regimens.
In conclusion, the present study suggests the clinical efficacy of NACRT using IPTW. Surgery after CRT reduced pelvic recurrence and, as a result, provided favorable PFS and OS. Therefore, NACRT may be a potential treatment option for patients with LACC, and further investigation is worth pursuing.
References
Torre LA, Bray F, Siegel RL et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Waggoner SE (2003) Cervical cancer. Lancet 361(9376):2217–2225
Small W Jr, Bacon MA, Bajaj A et al (2017) Cervical cancer: a global health crisis. Cancer 123(13):2404–2412
Rose PG, Bundy BN, Watkins EB et al (1999) Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 340(15):1144–1153
Mikami M, Aoki Y, Sakamoto M et al (2014) Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (Bulky Tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer 24(7):1333–1340
Yuan L, Guo J, Zhang X et al (2018) Feasibility of radical hysterectomy in women with FIGO stage IIB cervical cancer: an observation study of 10-year experience in a tertiary center. Onco Targets Ther 11:5527–5533
Legge F, Chiantera V, Macchia G et al (2015) Clinical outcome of recurrent locally advanced cervical cancer (LACC) submitted to primary multimodality therapies. Gynecol Oncol 138(1):83–88
Kim TH, Kim MH, Kim BJ et al (2017) Prognostic importance of the site of recurrence in patients with metastatic recurrent cervical cancer. Int J Radiat Oncol Biol Phys 98(5):1124–1131
Yoshida K, Kajiyama H, Utsumi F et al (2018) A post-recurrence survival-predicting indicator for cervical cancer from the analysis of 165 patients who developed recurrence. Mol Clin Oncol 8(2):281–285
Osman M (2014) The role of neoadjuvant chemotherapy in the management of locally advanced cervix cancer: a systematic review. Oncol Rev 8(2):250
Lee J, Kim TH, Kim GE et al (2016) Neoadjuvant chemotherapy followed by surgery has no therapeutic advantages over concurrent chemoradiotherapy in International Federation of Gynecology and Obstetrics stage IB–IIB cervical cancer. J Gynecol Oncol 27(5):e52
Gadducci A, Landoni F, Cosio S et al (2018) Neoadjuvant platinum-based chemotherapy followed by radical hysterectomy for stage IB2–IIB adenocarcinoma of the uterine cervix—an Italian Multicenter Retrospective Study. Anticancer Res 38(6):3627–3634
Keys HM, Bundy BN, Stehman FB et al (1999) Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 340(15):1154–1161
Mancuso S, Smaniotto D, Benedetti Panici P et al (2000) Phase I–II trial of preoperative chemoradiation in locally advanced cervical carcinoma. Gynecol Oncol 78(3 Pt 1):324–328
Shibata K, Kikkawa F, Suzuki Y et al (2004) Usefulness of preoperative chemoradiation in locally advanced cervical carcinoma. Gynecol Obstet Invest 57(2):93–99
Sun L, Sheng X, Jiang J et al (2014) Surgical morbidity and oncologic results after concurrent chemoradiation therapy for advanced cervical cancer. Int J Gynaecol Obstet 125(2):111–115
Leguevaque P, Motton S, Delannes M et al (2011) Completion surgery or not after concurrent chemoradiotherapy for locally advanced cervical cancer? Eur J Obstet Gynecol Reprod Biol 155(2):188–192
Azria E, Morice P, Haie-Meder C et al (2005) Results of hysterectomy in patients with bulky residual disease at the end of chemoradiotherapy for stage IB2/II cervical carcinoma. Ann Surg Oncol 12(4):332–337
Touboul C, Uzan C, Mauguen A et al (2010) Prognostic factors and morbidities after completion surgery in patients undergoing initial chemoradiation therapy for locally advanced cervical cancer. Oncologist 15(4):405–415
Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34(28):3661–3679
Rubin DB (2001) Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Method 2:169–188
Zhou J, Wu SG, Sun JY et al (2017) Comparison of clinical outcomes of squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix after definitive radiotherapy: a population-based analysis. J Cancer Res Clin Oncol 143(1):115–122
Houvenaeghel G, Lelievre L, Buttarelli M et al (2007) Contribution of surgery in patients with bulky residual disease after chemoradiation for advanced cervical carcinoma. Eur J Surg Oncol 33(4):498–503
Frobe A, Jones G, Bokulic T et al (2014) High-dose-rate brachytherapy and concurrent chemoradiotherapy followed by surgery for stage Ib-IIb cervical cancer: single institution experience. Anticancer Res 34(7):3861–3866
Mariagrazia D, Anna F, Gabriella F et al (2005) Preoperative chemoradiotherapy in locally advanced cervical cancer: long-term outcome and complications. Gynecol Oncol 99(3 Suppl 1):S166–S170
Classe JM, Rauch P, Rodier JF et al (2006) Surgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: morbidity and outcome: results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer). Gynecol Oncol 102(3):523–529
Ferrandina G, Lucidi A, Paglia A et al (2012) Role of comorbidities in locally advanced cervical cancer patients administered preoperative chemoradiation: impact on outcome and treatment-related complications. Eur J Surg Oncol 38(3):238–244
Chereau E, De la Hosseraye C, Ballester M et al (2013) The role of completion surgery after concurrent radiochemotherapy in locally advanced stages IB2–IIB cervical cancer. Anticancer Res 33(4):1661–1666
Pervin S, Ruma FI, Rahman K et al (2019) Adjuvant hysterectomy in patients with residual disease after radiation for locally advanced cervical cancer: a prospective longitudinal study. J Glob Oncol 5:1–7
Shim SH, Kim SN, Chae SH et al (2018) Impact of adjuvant hysterectomy on prognosis in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy: a meta-analysis. J Gynecol Oncol 29(2):e25
Hequet D, Marchand E, Place V et al (2013) Evaluation and impact of residual disease in locally advanced cervical cancer after concurrent chemoradiation therapy: results of a multicenter study. Eur J Surg Oncol 39(12):1428–1434
Morice P, Uzan C, Zafrani Y et al (2007) The role of surgery after chemoradiation therapy and brachytherapy for stage IB2/II cervical cancer. Gynecol Oncol 107(1 Suppl 1):S122–124
Anglemyer A, Horvath HT, Bero L (2014) Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 4:Mr000034
Acknowledgements
We would like to thank Enago (https://www.enago.jp/) for English editing of this manuscript.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest to disclose.
Ethical approval
The study was approved by the ethics committee of the institution (Approval no.: 2017-0053).
Informed consent
Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoshida, K., Kajiyama, H., Yoshihara, M. et al. The role of additional hysterectomy after concurrent chemoradiation for patients with locally advanced cervical cancer. Int J Clin Oncol 25, 384–390 (2020). https://doi.org/10.1007/s10147-019-01551-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01551-6