Abstract
Objectives
To investigate the diagnostic performance of attenuation imaging (ATI) for the assessment of low-grade hepatic steatosis using liver biopsy as the reference standard.
Methods
The study included 57 potential donor candidates for living liver transplantation who underwent ATI, transient elastography (TE), and liver biopsy for evaluation of hepatic steatosis between February 2020 and April 2020. The attenuation coefficient (AC) from ATI and the controlled attenuation parameter (CAP) from TE were measured for each participant in a random and blind manner. The histologic hepatic fat fraction (HFF) was graded (S0, < 5%; S1, 5–33%; S2, 33–66%; S3, > 66%). The accuracy of ATI for diagnosing hepatic steatosis was compared with that of CAP using ROC analysis. Correlations between AC and HFF were evaluated, and factors affecting AC were determined by linear regression analysis.
Results
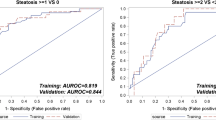
The median HFF was 3% (range: 0–35%), with 31 (54.4%), 24 (42.0%), and 2 (3.5%) participants being graded as S0, S1, and S2, respectively. The AUCs for the ROCs of AC and CAP for the detection of hepatic steatosis were 0.808 (95% CI: 0.682–0.900) and 0.829 (95% CI: 0.706–0.916), respectively, with the difference not being statistically significant (p = 0.762). AC showed 61.5% of sensitivity and 90.3% of specificity. AC was positively correlated with HFF (p < 0.001). HFF was the only factor significantly affecting AC.
Conclusions
ATI showed moderate sensitivity and high specificity in the diagnosis and quantification of hepatic steatosis in low-grade steatosis without fibrosis. Only HFF significantly affected AC.
Key Points
• Attenuation imaging showed moderate sensitivity and high specificity performance in the diagnosis and quantification of hepatic steatosis in low-grade steatosis without fibrosis.
• The diagnostic performance of the attenuation coefficient by attenuation imaging did not significantly differ from that of the controlled attenuation parameter by transient elastography in quantifying low-grade steatosis.
• The histopathologically determined hepatic fat fraction was the only factor significantly affecting the attenuation coefficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) is rapidly growing worldwide because of the increased incidence of obesity and insulin resistance [1, 2], and is currently up to 30–40% [3, 4]. As hepatic steatosis may progress to cirrhosis and can be associated with adverse liver-related outcomes in patients with underlying liver disease [5,6,7], the detection and quantification of hepatic fat are crucial for patient management. In living donor liver transplantation, accurate fat quantification in the liver graft is important for both donor safety and successful outcomes in recipients, as steatosis affects hepatocyte function and impairs regeneration following major hepatic resection [8].
Liver biopsy is considered the gold standard for assessment of hepatic steatosis. However, besides being an invasive procedure, it is prone to sampling error and interobserver variability in the histopathology analysis [9,10,11]. Though in recent years, noninvasive quantification of hepatic steatosis using MRI has shown comparable accuracy to liver biopsy [3, 12, 13], the MRI approaches have drawbacks such as high cost and limited availability.
Ultrasonography (US) has been widely used as a screening tool for the general population at risk of hepatic steatosis. Of the various US-based parameters that can be used to quantify hepatic steatosis, the controlled attenuation parameter (CAP) obtained from transient elastography (TE) has been used in a number of observational studies and clinical trials [14,15,16,17]. A recent meta-analysis of individual patient data revealed the CAP to have high accuracy (AUCs, 0.83–0.89) for quantification of hepatic steatosis in comparison to histopathology [18]. However, TE is not integrated with B-mode US, and it can measure hepatic fat only at a pre-determined depth. To overcome this limitation, recently, attenuation imaging (ATI) incorporated with B-mode US has been developed [19, 20]. ATI provides measurement of the attenuation coefficient (AC) corresponding to the slope of the attenuation profile of the transmitted beam, which is determined by the media in the region of interest (ROI) after the exclusion of acoustic transmission characteristics (i.e., the influence of beam focusing and gain) unique to the probe [19, 21]. Even though several studies have demonstrated ATI to have high accuracy for the assessment of steatosis in patients with chronic liver disease [20, 22, 23], its diagnostic performance in healthy adult population with low-grade steatosis is yet to be evaluated. It would also be meaningful to investigate the diagnostic value of AC in the absence of confounding factors such as fibrosis.
Therefore, the purpose of this study was to evaluate the diagnostic performance of ATI for detection and quantification of low-grade hepatic steatosis in healthy adults, using liver biopsy as the reference standard.
Materials and methods
Participants
This work formed a sub-study of a prospective multicenter trial to determine the usefulness of 2D-shear wave elastography and attenuation imaging for the diagnosis of nonalcoholic steatohepatitis, for which the study protocol is registered at the Clinical Research Information Service (KCT0004326). This study was approved by our institutional review board, and written informed consent was obtained from all participants. Potential donor candidates for living liver transplantation who were scheduled for liver biopsy for the assessment of hepatic steatosis prior to surgery at Asan Medical Center were consecutively enrolled from February 2020 to April 2020. According to our institution’s predefined protocol for liver transplantation, all donor candidates underwent pre-operative imaging workup for measurement of liver volume and evaluation of hepatic vascular and biliary anatomy, as well as a percutaneous liver biopsy to evaluate hepatic steatosis. All participants were asked to fast for at least 6 h prior to liver biopsy. On the same day as the liver biopsy, participants underwent an anthropometric examination for recording details such as body mass index (BMI), history of alcohol consumption (amount and drinking pattern), and serological analysis of parameters including liver function tests (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), lipid panel (total cholesterol, high-density lipoprotein cholesterol [HDL], and triglycerides), level of platelets, and coagulation parameters (prothrombin time/international normalized ratio). A significant bleeding risk (platelets < 80 000/μL, prothrombin time international normalized ratio > 1.5) was considered a contraindication for percutaneous liver biopsy.
US examinations
Dedicated US scanners were used to perform ATI and TE examinations on the same day to acquire AC and CAP, respectively. These US examinations were performed just before the liver biopsy. One of two board-certified abdominal radiologists with expertise in US elastography measurement (J.K.J. and S.Y.K. with approximately 100 and 500 cases) performed the ATI. The TE was performed by one of the two radiologic technicians who had experience in TE (I.W.Y. and Y.B.C. with approximately 3500 cases). All operators were blinded to the results of the other US examinations and clinical information, except for the knowledge that the subjects were scheduled for a liver biopsy for liver donation. For a subset of 15 participants, the two radiologists alternately and independently performed ATI to allow evaluation of inter-operator reproducibility. The AC values obtained by the first operator were used for the main analysis.
ATI was performed using a dedicated US scanner (Aplio i800, Canon Medical System) with a 1–8 MHz convex transducer (i8CX1, Canon Medical System). ATI was obtained from the right anterior liver through an intercostal window with the subject in the supine position. For evaluation of the AC (dB/cm/MHz) within a breath-hold, a 2 × 4-cm fan-shaped ROI was placed in the sampling box (4 × 8 cm; Fig. 1). A detailed technical information on the ATI procedure is described elsewhere [19, 20]. AC measurements were repeated until five valid values (R2 ≥ 80, displayed value on ATI) were obtained, and the median of these five measurements was then used as the representative AC value. After completion of the ATI, the skin-capsular distance, defined as the distance from the skin to the liver capsule, was measured on B-mode imaging.
Ultrasound attenuation imaging presenting the attenuation coefficient (AC, 0.62 dB/cm/MHz) in a 43-year-old female participant. A pair of B-mode images (left) and a color-coded attenuation image (right) with sampling box (green box) and region of interest (yellow box) are provided simultaneously. An R2 value (arrow) indicating reliability of the AC measurement is displayed in the left lower corner next to the attenuation coefficient. The histopathologic hepatic fat fraction and steatosis grade are 5% and S1, respectively
The CAP was measured on TE (FibroScan-502, Echosens) using an M probe (3.5 MHz) based on the recommendations of the World Federation for Ultrasound in Medicine and Biology (WFUMB) [24, 25]. The median of ten valid measurements was considered the representative value for each participant.
Liver biopsy and histopathologic analysis
After completion of a pair of US examinations, US-guided percutaneous liver biopsy was performed in the right anterior section of the liver by the same operators who conducted the ATI. Two liver specimens, each being approximately 1.5 cm in length, were obtained with 18-gauge needles (Stericut 18G Coaxial, TSK Laboratory). Histopathologic analysis of the liver specimens was performed by an experienced board-certified hepatic pathologist who was blinded to all clinical and radiologic information. Hepatic steatosis was visually quantified as the hepatic fat fraction (HFF) according to a percentage scale of the amount of liver parenchyma replaced by steatotic droplets on hematoxylin–eosin-stained specimens. The HFF was considered the reference standard for hepatic steatosis and was further categorized according to the histological scoring system for NAFLD as follows: S0 (< 5%, none), S1 (5–33%, mild), S2 (33–66%, moderate), and S3 (> 66%, severe) [26]. Fibrosis was categorized according to the METAVIR scoring system as follows: F0 (no fibrosis), F1 (portal fibrosis without septa), F2 (portal fibrosis with few septa), F3 (numerous septa without cirrhosis), and F4 (cirrhosis) [27].
Statistical analysis
The Mann–Whitney test was used to compare the median ACs based on the results of histopathology (S0 vs. S1–S3). Spearman correlation was employed to evaluate correlations between AC and HFF, and between AC and CAP. Coefficients of variation (CVs) were calculated to assess the reproducibility between the two operators for subgroups of 15 participants. CVs of ≤ 10%, 10–25%, and ≥ 25% were regarded as good, moderate, and poor reproducibility, respectively [28]. ROC curves of AC and CAP were calculated for diagnosis of hepatic steatosis (S1–S3), and the corresponding AUCs were compared using DeLong’s test [29]. Sensitivity and specificity were estimated using the highest Youden index. All variables with a value of p < 0.2 on univariate analysis following logarithmic transformation of the measured AC were subjected to a multivariate linear regression analysis to identify factors significantly affecting the AC. p-values < 0.05 were considered to indicate a statistically significant difference. All statistical analyses were performed using the MedCalc statistical software package (version 16.8, MedCalc Software).
Results
Participant characteristics
A total of 60 participants who were potential liver donor candidates were initially included in this study. Among them, three participants were excluded from further analysis because of an inappropriate use of an M probe during CAP measurement (skin-capsular distance ≥ 25 mm) [30,31,32]. The technical feasibility of CAP measurement of TE was 95% (57/60). In all the participants, ATI successfully measured AC with R2 values ≥ 80, and the technical feasibility of CAP was 100% (60/60). The characteristics of the 57 participants are shown in Table 1. Based on the histopathologic analysis, the median HFF of the 57 participants was 3%, ranging from 0 to 35%. Twenty-six (45.6%) participants were diagnosed with hepatic steatosis of grades S1–S2, with the majority of the cases being S1 (92.3%, 24/26). Only one (1.8%) participant was diagnosed as having portal fibrosis without septa (F1), with the others had no fibrosis. Among the 57 participants, 15 (26.3%) and 14 (24.6%) were classified as overweight, and 15 (26.3%) and one (1.8%) as obese according to the Asia–Pacific and World Health Organization guidelines, respectively [33, 34].
AC and CAP measurements
In all 57 participants, five repetitive AC measurements were made for each individual, resulting in a total of 285 valid AC values without any technical failure. In the acquisition of ten valid CAP measurements, no invalid measurement was noted in 46 participants (81%), whereas one invalid measurement was observed in six participants, and three in five participants.
The median AC was 0.59 (dB/cm/MHz), ranging from 0.46 to 0.93. The ACs in participants with histopathologically proven hepatic steatosis were significantly higher than in those without hepatic steatosis (0.665 [0.510 − 0.93] vs. 0.550 [0.460 − 0.680]; p < 0.001; Fig. 2). There was a significant positive correlation between AC and HFF (ρ = 0.619, 95% confidence interval [CI]: 0.428–0.758; p < 0.001). The median CAP value was 222.0 (dB/m), ranging from 139 to 346, and there was a significant positive correlation between AC and CAP (ρ = 0.509, 95% CI: 0.286–0.679; p < 0.001).
The CV of the median AC values obtained from the two operators was 9.6% (95% CI, 7.2–11.6%), and thus, the reproducibility between them was considered as good.
Factors affecting AC
The factors affecting AC are summarized in Table 2. In the univariate linear regression analysis, HFF (ρ = 0.013, p < 0.001), skin-capsular distance (ρ = 0.016, p = 0.020), cholesterol/HDL (ρ = 0.058, p = 0.020), and triglyceride/HDL (ρ = 0.052, p = 0.001) were significantly associated with AC. However, in the multivariate linear regression, only HFF was positively associated with AC (ρ = 0.011, p < 0.001).
Diagnostic performance of AC for detection of hepatic
Figure 3 shows the ROCs of AC and CAP for detection of hepatic steatosis (S1) in all 57 participants. The AUCs of AC and CAP for the detection of hepatic steatosis (S1) were 0.808 (95% CI: 0.682–0.900) and 0.829 (95% CI: 0.706–0.916), respectively, which were not significantly different (p = 0.762; Table 3). The sensitivity (61.5% vs. 61.5%) and specificity (90.3% vs. 93.6%) of AC and CAP were similar at the estimated cut-off values with the highest Youden index.
Discussion
In this study, ATI showed moderate sensitivity and high specificity for the diagnosis and quantification of hepatic steatosis in low-grade hepatic steatosis without fibrosis. ATI accurately identified hepatic steatosis (≥ S1) with a high AUC (0.808). In a head-to-head comparison using the same participants, the diagnostic performance of AC for diagnosing steatosis was not significantly different from that of CAP (AUC, 0.808 vs. 0.829, p = 0.762). In a multivariate analysis, AC was associated only with HFF (ρ = 0.011, p < 0.001), and was not significantly affected by anthropometric or laboratory findings. The ATI measurement procedure showed a high technical success rate along with good reproducibility.
Previous studies with biopsy-proven cohorts have shown that ATI can accurately detect hepatic steatosis (≥ S1; AUC values, 0.81–0.88) [22, 23, 35]. At the AC cut-off values of 0.64–0.69 determined in previous studies, the sensitivity and specificity were 75–76% and 77–100%, respectively. In our study, the AUC (0.81) and sensitivity (62% at an AC cut-off of 0.62) were slightly lower than those in the earlier studies. Given the differences in the distribution of the degree of hepatic steatosis, we conjecture that the differences between the studies can be attributed to spectrum bias [36]. Difference in patient characteristics across cohorts could result in different cut-off values and diagnostic accuracies. The prevalence of advanced steatosis (≥ S2) in previous studies was much higher (20–50%) than in our study (3.5%); hence both the cut-off value and overall accuracy might have been overestimated in the earlier studies. The recent WFUMB guidelines suggest a large overlap between adjacent grades and no consensus for the CAP value cut-off [25]. Thus, the cut-off values and their diagnostic performance in our study also need further validation. Our study results also suggest a limitation in the detection of low-grade steatosis using US-based methods, as CAP values also indicated a similar sensitivity. A meta-analysis of CAP data reported 68.8% sensitivity for the detection of steatosis ≥ S1 [18]. In addition, the proportion of participants with significant fibrosis (≥ F2) was 33–50% in previous studies, as they included patients with various chronic liver diseases, while on the contrary, our study included healthy participants without significant fibrosis. Therefore, the results of this study are particularly relevant for living donor liver transplantation, where the participants under evaluation come from a healthy population with low-grade hepatic steatosis. Hepatic steatosis impairs hepatocyte function and regeneration after major hepatic resection, including liver donation. Some studies suggest that even the mildest form of steatosis can increase the incidence of primary nonfunction and decrease the chances of patient survival after liver transplantation [8]. In addition, in patients with NAFLD, early identification of hepatic steatosis may have a role in preventing disease progression [37, 38]. The performance of ATI for diagnosing a HFF ≥ S1 in healthy participants was similar to that of CAP (AUC, 0.81 vs. 0.83, respectively; p = 0.762). This finding is in accord with previous studies reporting the AUCs of AC (0.90–0.91) and CAP (0.85) for HFF ≥ S1 without significant difference (p > 0.05) [39, 40]. In addition, AC positively correlated with CAP (ρ = 0.509, p < 0.001) in our study, which is in line with a previous observation that showed a strong correlation (ρ = 0.81, p < 0.001) between the two measurements [41]. However, ATI offers the advantages of simultaneous B-mode US and indifference to the acoustic transmission characteristics of the probes, whereas CAP measurement on TE is performed without direct B-mode image guidance, and its accuracy varies according to the type of probe (M vs. XL probes) [32, 42]. ATI also allows the operator to visualize the tissue in which the measurement is performed and avoid artifacts, such as reverberation, and structures other than hepatic parenchyma, such as vessels.
Our study found that the only factor significantly affecting AC was HFF (ρ = 0.011, 95% CI: 0.007 − 0.015; p < 0.001), with no other clinical or anthropometric factors having a substantial effect (p > 0.099). This result corresponds well with the findings reported in literature documenting that AC was only associated with steatosis grade [23, 35].
The results of this study also demonstrated the technical robustness of ATI, showing high interobserver agreement and low technical failure rates. The CV of ATI was 9.6% in the present study, indicating good reproducibility between the two operators. Yoo et al. had also demonstrated high intra-observer (ICC, 0.929) and interobserver agreement (ICC, 0.792) for ATI in healthy volunteers [43]. Invalid measurement, which is more common in obese patients, is another issue with the FibroScan system [16]. Because the reliability index (R2) is displayed during the acquisition of the ATI, the operator can easily determine whether the measurement is valid or not. There was no invalid measurement in any of the total of 285 AC measurements made across 57 participants in our study, which is consistent with previous findings of very low rates of invalid measurements (0 − 4%) [20, 23, 35].
This study has several limitations. First, because this is a sub-study of a prospective trial, a relatively small number of healthy adults recruited from a single center were included. Therefore, a further prospective multicenter trial with a larger population is required to verify our results. Second, the use of ROIs in ATI, and the use of liver biopsy as a reference standard, results in inherent limitations related to sampling errors. However, we used a large ROI (2 × 4-cm fan-shaped ROI) and tried to target the liver portion where the AC measurement was performed during the liver biopsy. Third, the diagnostic accuracy for advanced steatosis (≥ S2) was not evaluated in this study. There were only two participants with S2, and none of the participants had S3. Fourth, the proportion of participants who were overweight to obese was relatively small (26.3%). Thus, the findings of this study may not be extended to those with larger body habitus.
In conclusion, ATI showed moderate sensitivity and high specificity for the diagnosis and quantification of hepatic steatosis in healthy adults, with high reproducibility and success rates. Furthermore, the ATI-derived AC was only significantly affected by the HFF.
Abbreviations
- AC:
-
Attenuation coefficient
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ATI:
-
Attenuation imaging
- BMI:
-
Body mass index
- CAP:
-
Controlled attenuation parameter
- HDL:
-
High-density lipoprotein cholesterol
- HFF:
-
Hepatic fat fraction
- NAFLD:
-
Nonalcoholic fatty liver disease
- TE:
-
Transient elastography
References
Chalasani N, Younossi Z, Lavine JE et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67:328–357
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) (2016) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 59:1121–1140
Kühn JP, Meffert P, Heske C et al (2017) Prevalence of fatty liver disease and hepatic iron overload in a Northeastern German population by using quantitative MR Imaging. Radiology 284:706–716
Szczepaniak LS, Nurenberg P, Leonard D et al (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288:E462-468
Angulo P, Kleiner DE, Dam-Larsen S et al (2015) Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149:389-397.e310
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64:73–84
Castéra L, Hézode C, Roudot-Thoraval F et al (2003) Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut 52:288–292
Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM (2007) Steatosis as a risk factor in liver surgery. Ann Surg 245:20–30
Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344:495–500
Ratziu V, Charlotte F, Heurtier A et al (2005) Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128:1898–1906
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD (2009) Liver biopsy. Hepatology 49:1017–1044
Raptis DA, Fischer MA, Graf R et al (2012) MRI: the new reference standard in quantifying hepatic steatosis? Gut 61:117–127
Noureddin M, Lam J, Peterson MR et al (2013) Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 58:1930–1940
Abeysekera KWM, Fernandes GS, Hammerton G et al (2020) Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol Hepatol 5:295–305
Caussy C, Alquiraish MH, Nguyen P et al (2018) Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 67:1348–1359
de Lédinghen V, Vergniol J, Capdepont M et al (2014) Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol 60:1026–1031
Thiele M, Rausch V, Fluhr G et al (2018) Controlled attenuation parameter and alcoholic hepatic steatosis: diagnostic accuracy and role of alcohol detoxification. J Hepatol 68:1025–1032
Karlas T, Petroff D, Sasso M et al (2017) Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 66:1022–1030
Tada T, Iijima H, Kobayashi N et al (2019) Usefulness of attenuation imaging with an ultrasound scanner for the evaluation of hepatic steatosis. Ultrasound Med Biol 45:2679–2687
Jeon SK, Lee JM, Joo I et al (2019) Prospective evaluation of hepatic steatosis using ultrasound attenuation imaging in patients with chronic liver disease with magnetic resonance imaging proton density fat fraction as the reference standard. Ultrasound Med Biol 45:1407–1416
Yoneda M, Honda Y, Nogami A, Imajo K, Nakajima A (2020) Advances in ultrasound elastography for nonalcoholic fatty liver disease. J Med Ultrason (2001) 47:521–533
Sugimoto K, Moriyasu F, Oshiro H et al (2020) The role of multiparametric US of the liver for the evaluation of nonalcoholic steatohepatitis. Radiology 296:532–540
Bae JS, Lee DH, Lee JY et al (2019) Assessment of hepatic steatosis by using attenuation imaging: a quantitative, easy-to-perform ultrasound technique. Eur Radiol 29:6499–6507
Ferraioli G, Filice C, Castera L et al (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol 41:1161–1179
Ferraioli G, Wong VW, Castera L et al (2018) Liver ultrasound elastography: an update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med Biol 44:2419–2440
Kleiner DE, Brunt EM, Van Natta M et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group Hepatology 24:289–293
Alajbeg IZ, Lapić I, Rogić D et al (2017) Within-subject reliability and between-subject variability of oxidative stress markers in saliva of healthy subjects: a longitudinal pilot study. Dis Markers 2017:2697464
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Sasso M, Audière S, Kemgang A et al (2016) Liver steatosis assessed by controlled attenuation parameter (CAP) measured with the XL probe of the FibroScan: a pilot study assessing diagnostic accuracy. Ultrasound Med Biol 42:92–103
Shen F, Zheng RD, Shi JP et al (2015) Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int 35:2392–2400
Petroff D, Blank V, Newsome PN et al (2021) Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol 6:185–198
Lim JU, Lee JH, Kim JS et al (2017) Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis 12:2465–2475
Deurenberg P, Deurenberg-Yap M, Guricci S (2002) Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 3:141–146
Dioguardi Burgio M, Ronot M, Reizine E et al (2020) Quantification of hepatic steatosis with ultrasound: promising role of attenuation imaging coefficient in a biopsy-proven cohort. Eur Radiol 30:2293–2301
Ransohoff DF, Feinstein AR (1978) Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med 299:926–930
Fielding CM, Angulo P (2014) Hepatic steatosis and steatohepatitis: are they really two distinct entities? Curr Hepatol Rep 13:151–158
Wong VW, Wong GL, Choi PC et al (2010) Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 59:969–974
Ferraioli G, Maiocchi L, Raciti MV et al (2019) Detection of liver steatosis with a novel ultrasound-based technique: a pilot study using MRI-derived proton density fat fraction as the gold standard. Clin Transl Gastroenterol. 10:e00081
Ferraioli G, Maiocchi L, Savietto G et al (2020) Performance of the attenuation imaging technology in the detection of liver steatosis. J Ultrasound Med. https://doi.org/10.1002/jum.15512
Sporea I, Bâldea V, Lupușoru R et al (2020) Quantification of steatosis and fibrosis using a new system implemented in an ultrasound machine. Med Ultrason 22:265–271
Caussy C, Brissot J, Singh S et al (2020) Prospective, same-day, direct comparison of controlled attenuation parameter with the M vs the XL probe in patients with nonalcoholic fatty liver disease, using magnetic resonance imaging-proton density fat fraction as the standard. Clin Gastroenterol Hepatol 18:1842-1850.e1846
Yoo J, Lee JM, Joo I et al (2020) Reproducibility of ultrasound attenuation imaging for the noninvasive evaluation of hepatic steatosis. Ultrasonography 39:121–129
Acknowledgements
The attenuation imaging was provided by Canon Medical Systems, and this study was technically supported by Canon Medical Systems.
Funding
This study was supported by a grant from Canon Medical Systems Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. So Yeon Kim.
Conflict of interest
Dr. Jang received grants from Canon Medical Systems Korea and personal fees and financial supports.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jang, J.K., Kim, S.Y., Yoo, I.W. et al. Diagnostic performance of ultrasound attenuation imaging for assessing low-grade hepatic steatosis. Eur Radiol 32, 2070–2077 (2022). https://doi.org/10.1007/s00330-021-08269-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08269-y