Abstract
Objective
To evaluate whether the change in computed tomography pulmonary angiography (CTPA) metrics after balloon pulmonary angioplasty (BPA) can predict treatment effect in chronic thromboembolic pulmonary hypertension (CTEPH) patients.
Methods
This study included 82 CTEPH patients who underwent both CTPA and right heart catheterization (RHC) before and at the scheduled time of 6 months after BPA. The diameters of the main pulmonary artery (dPA), ascending aorta (dAA), right atrium (dRA), right ventricular free wall thickness (dRVW), and right and left ventricles (dRV, dLV) were measured on CTPA. The correlation of the New York Heart Association functional class (NYHA FC), 6-minute walking distance (6MWD), brain natriuretic peptide (BNP) level, and calculated CT metrics with a decrease in mean pulmonary artery pressure (ΔmPAP) using RHC (used as the reference for BPA effect) was investigated. Using multiple regression analysis, independent variables were also identified.
Results
In univariate analysis, clinical indicators (NYHA FC, 6MWD, and BNP level) improved significantly after BPA and were significantly correlated with ΔmPAP (p < 0.01). In the univariate analysis of CTPA parameters, dPA, dRA, dPA/dAA ratio, dRVW, and dRV/dLV ratio decreased significantly and were significantly correlated with ΔmPAP (p < 0.01). Multivariate analysis demonstrated that decreased dPA (p = 0.001) and decreased dRA (p = 0.039) on CTPA were independent predictive factors of ΔmPAP.
Conclusions
Decreased dPA and dRA on CTPA could predict a decrease in mPAP after BPA, thus potentially eliminating unnecessary invasive catheterization.
Key Points
• The reduction in mean pulmonary artery pressure after balloon pulmonary angioplasty in CTEPH patients was significantly correlated with the clinical indices improvement and CTPA parameter decrease.
• The decreased diameter of the main pulmonary artery and the decreased diameter of the right atrium on CTPA were independent predictors of mean pulmonary artery pressure reduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH), defined as Group 4 in the Nice clinical classification of pulmonary hypertension [1], has a very poor prognosis except with appropriate treatment [2]. Although postoperative pulmonary thromboendarterectomy (PEA) patients have a better prognosis than non-postoperative patients, approximately 40% of the patients cannot undergo PEA because of the presence of a distal thrombus or other comorbidities [3]. Balloon pulmonary angioplasty (BPA) has emerged as an alternative treatment option for patients with inoperable CTEPH [4]. BPA reduces the pulmonary artery pressure in CTEPH patients, resulting in improved New York Heart Association functional class (NYHA FC), 6-minute walking distance (6MWD) [5], and life prognosis of CTEPH patients [6].
Right heart catheterization (RHC) with pulmonary angiography is routinely performed to confirm the diagnosis and determine the hemodynamic characteristics of CTEPH; it is the gold standard for the evaluation of CTEPH and for pre-operative assessment. Although RHC is a relatively safe examination, serious complications occur in 1.1% patients even at experienced centers, and the procedure-related mortality rate has been reported to be 0.055% [7]. Conversely, recently, computed tomography pulmonary angiography (CTPA), which is often used to identify the location and extent of a thrombus, plays an important role in the diagnosis of suspected CTEPH [8]. The advantage of CTPA is its high spatial and contrast resolution with a short scan time. In addition, the pulmonary perfusion imaging obtained by dual-energy CT (DECT) is useful because it provides both anatomical information and segmental pulmonary perfusion at the target site simultaneously [9], which are important in determining treatment.
To date, several studies have reported the value of performing CTPA before treatment [10, 11]; however, whether CT imaging could reflect the efficacy of BPA in CTEPH patients remains unclear. If post-treatment CTPA images can be used to easily estimate the effectiveness of BPA without the use of special analysis applications, unnecessary invasive catheterization and hospitalization can be avoided. Consequently, the patient’s distress can be reduced, with a positive impact on the healthcare system. Therefore, we hypothesized that changes in CTPA imaging findings could be used to predict a decrease in the mean pulmonary arterial pressure (mPAP) obtained with RHC in patients with CTEPH after BPA.
Thus, the aim of this study was to evaluate whether changes in CTPA metrics after BPA can predict a treatment effect in CTEPH patients.
Materials and method
Data collection and patients’ characteristics
The study complied with the Declaration of Helsinki, and the protocol for this retrospective study was approved by the Ethics Committee of our institution (No. 20120224). Written informed consent was obtained from all patients.
From November 2012 to December 2016, 117 consecutive patients aged over 20 years with no history of renal dysfunction or allergy to contrast agents, but with known or suspected CTEPH, were considered for inclusion in this study. CTEPH was diagnosed in accordance with the 2015 European Society of Cardiology and the European Respiratory Society guidelines for the diagnosis and treatment of pulmonary hypertension (Electronic Supplementary Material).
Of the 117 patients, those meeting the following criteria were excluded: did not receive contrast-enhanced CT (n = 18), did not receive RHC at the scheduled time of 6 months after treatment (n = 1), and had a history of pulmonary endarterectomy before or after treatment (n = 7). Of the remaining 91 patients, 82 met all the listed CTEPH diagnostic criteria and were ultimately enrolled for the analysis. Despite having been diagnosed with chronic thromboembolic disease, which means that they had pulmonary hypertension due to chronic pulmonary thromboembolism [12], nine patients were excluded from the study because their pre-treatment mPAP was < 25 mmHg.
Data on patients’ characteristics, BPA-related information, and RHC data were retrieved from the electronic medical records. The NYHA FC was determined by the severity of the heart failure symptoms complained of by the patient (Electronic Supplementary Material) [13]. The post-treatment NYHA FC, 6MWD, and BNP minus the pre-treatment NYHA FC, 6MWD, and BNP were defined as ΔNYHA FC, Δ6MWD, and ΔBNP, respectively. The body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Imaging protocol of CTPA
CT examinations were performed using 320 detector-row CT (AquilionONE, Canon Medical Systems), 256 detector-row CT (Revolution CT, GE Healthcare), and 64 detector-row CT scanners (Discovery CT750HD, GE Healthcare), before and at the scheduled time of 6 months after treatment. A contrast material with a concentration of 370 or 350 mg/mL was injected via the cubital vein over a period of 25 s using a weight-adapted bolus-tracking injection protocol (1.5 mL/kg body weight). Scanning to commence data acquisition was performed under one breath and started 7.4–14 s after the CT value of the region of interest (ROI) placed in the right ventricle reached a threshold of + 100 HU. Image acquisition was performed at a peak tube voltage of 100 kVp or 140/80 kVp (dual-energy CT), 0.275–0.500 s/rotation, under automatic exposure control with a noise index of 20 (for a slice thickness of 5 mm). The helical pitch factor was set to 0.828–0.992. CT images with 1.00- or 1.25-mm thickness were reconstructed using the adaptive iterative dose reduction 3D [14] or adaptive statistical iterative reconstruction. The CT dose index volume and dose length product for CT pulmonary angiography were 11.7 ± 4.7 mGy and 432.0 ± 178.8 mGy・cm, respectively. The effective dose estimates for CT were determined by using measured dose length products and the appropriate normalized coefficients reported in the literature [15] and was 6.0 ± 2.5 mSv.
Hemodynamic studies
Previous studies have reported that the pulmonary arteries treated by BPA become larger with follow-up, than at immediately after the procedure [16, 17], and several previous studies evaluating the therapeutic effect of BPA on CTEPH were conducted at 6 months, rather than immediately after BPA treatment [18, 19]. For this reason, the present study compared RHC and CTPA at the scheduled time of 6 months after BPA. All patients underwent RHC using a 6-French (Fr.) or 7-Fr. Swan-Ganz catheter (Swan-Ganz CCO CEDV; Edwards Lifesciences). The hemodynamic parameter values, including mPAP, mean right atrial pressure (mRAP), mean PAWP (mPAWP), systolic/diastolic/mean blood pressures (sBP/dBP/mBP), cardiac output (CO), and cardiac index (CIx), were measured in all patients, both before and at the scheduled time of 6 months after BPA. From the values obtained for these parameters, pulmonary vascular resistance (PVR) was calculated using the following formula:
The mPAP before minus the mPAP at the scheduled time of 6 months after BPA treatment was defined as ΔmPAP and was used as a reference for the treatment effect. Although PVR is also often used as a hemodynamic indicator, it is sensitive to both the blood flow and pressure and may not accurately reflect the pulmonary circulation. For this reason, mPAP, with a high objectivity and reliability, was determined as an indicator of the treatment effect in the present study.
BPA procedure
The approach to the pulmonary artery was performed through the internal right jugular vein or common femoral vein. First, an 8-Fr. sheath was placed and then a 6-Fr. guiding sheath was advanced to the main pulmonary artery trunk using a 0.035-in. hydrophilic guidewire. The 6-Fr. guiding catheter was then used to select the target vessel. After engaging the guiding catheter to the target vessel, the target lesion was crossed with a 0.014-in. guidewire. If the wire successfully passed through the lesion, a dilatation balloon was used to inflate the lesion. Appropriate balloon size was selected based on target vessel diameter measurements by angiography, intravascular ultrasound, or optimal coherence tomography, and after inflating the balloon, a similar device was used to confirm that the vessel was successfully dilated. A successful procedure was defined as having improved blood flow after treatment, or having dilation on images obtained by angiography, intravascular ultrasound, or optimal coherence tomography.
Image analysis
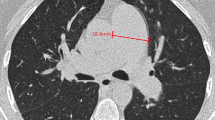
All CTPA images were evaluated by two board-certified radiologists with 11 and 14 years of experience, respectively. Blinding the clinical data was ensured by using a dedicated commercially available diagnostic workstation (Centricity RA 1000, GE Healthcare). The widest short-axis diameter of the main pulmonary artery (dPA) was measured, and the mean of two perpendicular measurements of the ascending aortic diameter (the aortic diameter [dAA]) was calculated using transverse images at the level of the PA bifurcation (Fig. 1a, b); the dPA/dAA ratio was calculated [20, 21]. The widest long-axis diameter of the right atrium (dRA) and the widest short-axis diameters of the right (dRV) and left ventricles (dLV) were also measured on the transverse images (Fig. 1c, d); the dPA/dRA ratio and dRV/dLV ratio were calculated [21]. Diameter of the right ventricular free wall thickness (dRVW) was also measured (Fig. 1c, d) according to previous literature [22]. The measurements of the blood vessel, atrium, and ventricle diameters were performed using the outer limits of the filled contrast medium.
Vascular and cardiac chamber diameter measurements on computed tomography pulmonary angiography images. The widest short-axis diameter of the main pulmonary artery (dPA) is measured, and the mean of two perpendicular measurements of the ascending aortic diameter to represent the aortic diameter (dAA) is calculated using transverse images at the level of the PA bifurcation (orange, blue, and green lines in Fig. 1a, b, respectively). The dPA/dAA ratio is calculated. The widest long-axis diameter of the right atrium (dRA), the widest short-axis diameters of the right and left ventricles (dRV, dLV), and the short-axis diameters of right ventricular free wall thickness (dRVW) are also measured on transverse images (orange, blue, green, and yellow lines in Figs. 1c, d, respectively). The dPA/dRA ratio and dRV/dLV ratio are calculated.
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation (SD) for continuous variables. Pre- and post-treatment differences were compared using the paired t test for continuous variables: NYHA-FC, 6MWD, BNP, hemodynamic characteristics, and CTPA parameters. The correlations between changes in NYHA-FC, 6MWD, BNP, or imaging parameters obtained from CTPA and changes in hemodynamics obtained from RHC were evaluated using the Pearson correlation coefficients. Multiple regression analysis was also used to identify the independent variables of CTPA parameters related to hemodynamic changes. Inter- and intra-observer agreements were assessed by calculating the intraclass correlation coefficient (ICC) for the first 30 consecutive patients. All statistical analyses were performed using IBM SPSS Statistics for Macintosh, Version 26.0 (IBM Corp.). Two-sided p values of less than 0.05 were considered to be statistically significant.
Results
The characteristics of the CTEPH patients are described in Table 1. The study population consisted of 32 male and 50 female patients (mean age ± SD, 61.3 ± 14.3 years; range 22–83 years). The mean intervals between CTPA and RHC before BPA initiation and at the scheduled time of 6 months after the final BPA procedure were 26.9 ± 55.7 days and 0.1 ± 2.1 days, respectively. The duration between the last BPA and the CT for determining the treatment effect was 196.8 ± 34.0 days. BPA treatment resulted in procedural success in all cases. A total of 6.4 ± 1.9 BPA sessions were performed per patient, with 14.2 ± 2.2 lesions treated in each session. After BPA, there was a significant improvement in BNP, NYHA FC, and 6MWD (Fig. 2). Decreases in BNP (r = − 0.339, p < 0.01) and NYHA FC (r = 0.396, p < 0.01) and an increase in 6MWD (r = 0.536, p < 0.01) were significantly correlated with a decrease in mPAP (Fig. 2).
Correlation between hemodynamic changes and the New York Heart Association functional class (NYHA FC), 6-minute walking distance (6MWD), brain natriuretic peptide (BNP) level, and computed tomography pulmonary angiography parameter changes before and after balloon pulmonary angioplasty (BPA). The decrease in mPAP after BPA is significantly associated with a decrease in the NYHA FC, an increase in the 6MWD, a decrease in BNP level, and a decrease in dPA, dRA, dRV/dLV ratio, and dPA/dAA ratio (all p < 0.01).
The hemodynamic changes obtained from RHC and the changes in the imaging parameters obtained from CTPA are shown in Table 2. The hemodynamic parameters improved following BPA, with a statistically significant decrease in mPAP, mRAP, mPAWP, dBP, mBP, CO, CIx, and PVR. In terms of CTPA parameters, there were statistically significant reductions in dPA, dRA, dRVW, dPA/dAA ratio, dRV/dLV ratio, and dRA/dPA ratio, and the interobserver ICC for these results ranged from 0.829 to 0.993 while the intraobserver ICC ranged from 0.844 to 0.997 (p < 0.01, Table 2).
The results of the Pearson correlation analysis between the hemodynamic changes obtained from RHC and the changes in imaging parameters obtained from CTPA are also shown in Fig. 2. The decrease in mPAP was significantly correlated with decreased dPA (ΔdPA, r = 0.590), dRA (ΔdRA, r = 0.516), dRV/dLV ratio (ΔdRV/dLV ratio, r = 0.452), and dPA/dAA ratio (ΔdPA/dAA ratio, r = − 0.435) (all p < 0.01).
The results of the multiple regression analysis for the predictors of mPAP reduction after BPA are shown in Table 3. In this multiple linear regression model, age, sex, BMI, ΔdPA, ΔdRA, and ΔdRV/dLV ratio were included as factors, considering the collinearity of the variables (e.g., BMI, height, and weight; ΔdPA and ΔdPA/dAA ratio; and ΔdRA and ΔdRA/dPA ratio). The multivariate analysis demonstrated that decreased ΔdPA (p < 0.001) and ΔdRA (p = 0.039) on CTPA were independent predictors of decreased mPAP after adjusting for other variables.
Forty-six patients (56%) received either mono- or combination pulmonary vasodilator medication therapy (endothelin receptor antagonist, phosphodiesterase type 5 inhibitor, or a soluble guanylyl cyclase stimulant) prior to BPA, and 61 patients (74%) received it at 6 months of BPA. At least one antiplatelet or anticoagulant was used continuously in all the patients. Fifty-two patients (63%) received either mono- or combination medication for right heart failure (diuretics, vasopressin receptor antagonists, or long-acting bronchodilators) prior to BPA, and 61 patients (74%) received them after 6 months of BPA.
Discussion
In this study, decreased dPA and dRA on CTPA were independent predictors of mPAP decrease, as demonstrated through multiple regression analysis. These results suggest that the measurements of ΔdPA and ΔdRA on CTPA, before and after BPA, may predict a decrease in mPAP, thereby leading to a reduction in the frequency of post-treatment RHC. The decrease in dPA and dPA/dAA on CTPA may be a direct finding reflecting the reduction of mPAP after BPA. Similarly, the reduction in atrial and ventricular-related parameters such as PVR and mRAP (Table 2) after BPA may indicate an improvement in the right cardiac load, which may be reflected as a reduction in dRA and dRV/dLV ratio in CTPA.
The treatment efficacy of CTEPH can be comprehensively predicted by the combination of a noninvasive assessment of NYHA-FC [23], 6MWD, and biomarkers such as BNP [24], and by an invasive assessment using RHC [25]. In particular, the relationship between the mPAP obtained in RHC and the prognosis is emphasized in some studies, and remarkable improvement in prognosis is observed using mPAP as a treatment index [26,27,28]. Since the NYHA FC is based on the subjective judgement of the patient, it does not have a high degree of objectivity. Similarly, although baseline 6MWD is closely associated with the hemodynamic parameters and has been used as the primary endpoint in many clinical trials [29], clear conclusions have yet to be reached regarding the relationship between improved 6MWD and hemodynamic status. This may be due to the fact that if the pre-treatment 6MWD is already optimal, therapy may not lead to an improved 6MWD. Additionally, 6MWD is known to be influenced by the patients’ desire to learn, the patients’ characteristics, and the presence of comorbidities; thus, its reliability as an objective index may not be sufficiently high. In this regard, because CTPA is a simple, objective, and less invasive method for assessing treatment efficacy, it is clinically meaningful that changes in CTPA parameters are independent predictors of ΔmPAP.
The measurement method for the pulmonary artery diameter on CT used in this study has been previously investigated as a screening and diagnostic method for pulmonary hypertension [20]. Truong et al proposed a severity classification system using dPA and dPA/dAA ratio for the diagnosis of and prognostication in pulmonary hypertension using CT images; groups classified as either moderate or severe in this classification had increased mortality compared to those classified as normal [20]. Several other advanced CT techniques using dual-energy CT [30, 31] have been reported to be useful for the evaluation of segmental pulmonary perfusion in patients with CTEPH. However, whether CT imaging could reflect the efficacy of BPA remains unclear. Koike et al demonstrated improvements in segmental lung perfusion after BPA using DECT in 17 CTEPH patients [30]. Another study attempted to estimate the pulmonary perfusion change using CTPA with special analytical applications in 14 CTEPH patients, and density change in the vascular and parenchymal areas correlated significantly with the hemodynamic change [32]. These previous methods of determining treatment efficacy using DECT or special applications to compare the lung perfusion before and after treatment are complicated and, therefore, difficult for all hospitals to employ. In contrast, our study confirmed the efficacy of using CTPA to predict the treatment efficacy in 82 patients, the largest number ever reported, without the use of any special analytical applications. The method used in this study to measure the vessel, atrial, and ventricular diameters using cross-sectional CTPA images is simple and can be immediately applied clinically in almost all hospitals.
A previous study reported the correlation between changes in postoperative CTPA findings and changes in mPAP in CTEPH patients who underwent PEA, but not BPA [22]. The study targeted mPAP or PVR and evaluated the association with CT scores generated by the four categories of lesion distribution, dPA, mosaic perfusion, and tricuspid regurgitation. As a result, in the 69 CTEPH patients treated with PEA, though there was a correlation with lesion distribution when targeting PVR, but the strongest correlation with dPA was seen when targeting mPAP [22]. Although our study confirmed that ΔdPA was significantly associated with ΔmPAP after BPA, when ΔdPA is used to predict post-treatment mPAP reduction in clinical application, the reduction in dPA associated with a reduction in mPAP of approximately 20 mmHg (19.4 ± 9.9 mmHg, Table 2) is small, that is approximately 2 mm (1.8 ± 2.9 mm, Table 2), and thus, there is a risk of error in measurement. On the other hand, when ΔdRA is used to predict post-treatment mPAP reduction, a reduction of approximately 7 mm is associated with a reduction in mPAP of approximately 20 mmHg, which may result in less error from measurement. To predict how much the mPAP will decrease, the equation in Fig. 2 (ΔmPAP = 0.4694ΔdRA − 16.094) could be used to estimate the approximate value, to some extent.
In our study, patients with known allergies to contrast materials or with renal dysfunction were excluded for the purpose of ensuring accuracy; however, several authors have reported the validity of mPAP prediction using dPA and dPA/dAA ratio on non-contrast CT [20, 33]. Although the frequency of adverse events associated with nonionic, low-osmolarity iodinated contrast is not high, ranging from 0.2 to 3.1%, they should not be used, especially in patients with a history of allergies, as one in every 170,000 injections can be a potentially fatal adverse event [34, 35]. Although there are no reports comparing the accuracy of dPA measurements in patients with pulmonary hypertension between contrast- and non-contrast CT, non-contrast CT may be a substitute for the estimation of mPAP in patients with renal failure.
Our study had several limitations. First, this study included only 82 patients at a single institution; further studies in a larger patient population are needed to confirm our observations. Second, due to the high number of CT scans taken per day in clinical practice at our institution where the study was conducted, it was difficult to ensure that the same CT scan was used before and after BPA and that the concentration of contrast agent was accurately matched. However, as this study only measured vessel, ventricle, and wall diameters without special applications, we believe that the differences in these imaging conditions did not affect the results of this study. In addition, the CT taken in this study was a non-electrocardiogram-gated CT. Thus, the measurement method used in this study was highly versatile and can have good clinical applicability. Third, in the present study, the mean interval between CTPA and RHC at the scheduled time of 6 months after the end of BPA was very short (0.1 day), while the mean interval between CTPA and RHC before the initiation of BPA was 27 days. Although there is a risk of error over time when CTPA and RHC are performed at larger intervals after treatment, the impact on imaging parameters and hemodynamics before BPA is thought to be small as the patients do not undergo any intervention in the interim. Thus, we believe that the interval between CTPA and RHC before the initiation of BPA would not have affected the results. Finally, for simplicity, we evaluated only changes in the vessel, ventricular, and atrial diameters on CTPA transverse images as imaging parameters and did not perform other quantitative measurements using special applications. This is because we believe that this simple method is practical and easy to apply clinically.
In conclusion, the changes in CTPA findings after BPA reflected well the decrease in mPAP achieved with RHC. In particular, the decrease in dPA and dRA appeared to be independent predictors of the reduction in mPAP after BPA. Therefore, the prediction of mPAP reduction by CTPA, which is a simple, objective, and less invasive method for assessing treatment, is of high clinical utility, thus potentially eliminating unnecessary invasive assessment using RHC.
Abbreviations
- 6MWD:
-
6-minute walking distance
- BMI:
-
Body mass index
- BNP:
-
Brain natriuretic peptide
- BPA:
-
Balloon pulmonary angioplasty
- CIx:
-
Cardiac index
- CO:
-
Cardiac output
- CTEPH:
-
Chronic thromboembolic pulmonary hypertension
- CTPA:
-
Computed tomography pulmonary angiography
- dAA:
-
Diameter of the ascending aorta
- DECT:
-
Dual-energy computed tomography
- dLV:
-
Diameter of the left ventricle
- dPA:
-
Diameter of the main pulmonary artery
- dRA:
-
Diameter of the right atrium
- dRV:
-
Diameter of the right ventricle
- ICC:
-
Intraclass correlation coefficient
- mPAP:
-
Mean pulmonary arterial pressure
- mRAP:
-
Mean right atrial pressure
- NYHA FC:
-
New York Heart Association functional class
- PAH:
-
Pulmonary arterial hypertension
- PAWP:
-
Pulmonary artery wedge pressure
- PEA:
-
Pulmonary thromboendarterectomy
- PVR:
-
Pulmonary vascular resistance
- RHC:
-
Right heart catheterization
- ROI:
-
Region of interest
- sBP/dBP/mBP:
-
Systolic/diastolic/mean blood pressures
- SD:
-
Standard deviation
References
Simonneau G, Gatzoulis MA, Adatia I et al (2013) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62:D34–D41
Riedel M, Stanek V, Widimsky J, Prerovsky I (1982) Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 81:151–158
Pepke-Zaba J, Delcroix M, Lang I et al (2011) Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 124:1973–1981
Kataoka M, Inami T, Kawakami T, Fukuda K, Satoh T (2019) Balloon pulmonary angioplasty (percutaneous transluminal pulmonary angioplasty) for chronic thromboembolic pulmonary hypertension: a Japanese perspective. JACC Cardiovasc Interv 12:1382–1388
Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ (2001) Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 103:10–13
Ogawa A, Satoh T, Fukuda T et al (2017) Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes 10(11):e004029
Hoeper MM, Lee SH, Voswinckel R et al (2006) Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol 48:2546–2552
Renapurkar RD, Shrikanthan S, Heresi GA, Lau CT, Gopalan D (2017) Imaging in chronic thromboembolic pulmonary hypertension. J Thorac Imaging 32:71–88
Dournes G, Verdier D, Montaudon M et al (2014) Dual-energy CT perfusion and angiography in chronic thromboembolic pulmonary hypertension: diagnostic accuracy and concordance with radionuclide scintigraphy. Eur Radiol 24:42–51
Ley S, Ley-Zaporozhan J, Pitton MB et al (2012) Diagnostic performance of state-of-the-art imaging techniques for morphological assessment of vascular abnormalities in patients with chronic thromboembolic pulmonary hypertension (CTEPH). Eur Radiol 22:607–616
Liu M, Ma Z, Guo X, Zhang H, Yang Y, Wang C (2011) Computed tomographic pulmonary angiography in the assessment of severity of chronic thromboembolic pulmonary hypertension and right ventricular dysfunction. Eur J Radiol 80:e462–e469
Held M, Kolb P, Grun M et al (2016) Functional characterization of patients with chronic thromboembolic disease. Respiration 91:503–509
Ponikowski P, Voors AA, Anker SD et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200
Yamada Y, Jinzaki M, Hosokawa T et al (2012) Dose reduction in chest CT: comparison of the adaptive iterative dose reduction 3D, adaptive iterative dose reduction, and filtered back projection reconstruction techniques. Eur J Radiol 81:4185–4195
Cynthia McCollough DC, Edyvean S, Geise R et al (2008) The Measurement, Reporting, and Management of Radiation Dose in CT (2008). American association of physicists in medicine AAPM REPORT NO. 96
Ikeda N (2020) Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Cardiovasc Interv Ther 35:130–141
Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H (2012) Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 5:748–755
Roller FC, Kriechbaum S, Breithecker A et al (2019) Correlation of native T1 mapping with right ventricular function and pulmonary haemodynamics in patients with chronic thromboembolic pulmonary hypertension before and after balloon pulmonary angioplasty. Eur Radiol 29:1565–1573
Aoki T, Sugimura K, Tatebe S et al (2017) Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo-embolic pulmonary hypertension: long-term effects and procedure-related complications. Eur Heart J 38:3152–3159
Truong QA, Bhatia HS, Szymonifka J et al (2018) A four-tier classification system of pulmonary artery metrics on computed tomography for the diagnosis and prognosis of pulmonary hypertension. J Cardiovasc Comput Tomogr 12:60–66
Grosse A, Grosse C, Lang I (2018) Evaluation of the CT imaging findings in patients newly diagnosed with chronic thromboembolic pulmonary hypertension. PLoS One 13:e0201468
Leone MB, Giannotta M, Palazzini M et al (2017) A new CT-score as index of hemodynamic changes in patients with chronic thromboembolic pulmonary hypertension. Radiol Med 122:495–504
Delcroix M, Lang I, Pepke-Zaba J et al (2016) Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation 133:859–871
Simonneau G, D'Armini AM, Ghofrani HA et al (2016) Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 4:372–380
Saouti N, de Man F, Westerhof N et al (2009) Predictors of mortality in inoperable chronic thromboembolic pulmonary hypertension. Respir Med 103:1013–1019
Ogawa A, Ejiri K, Matsubara H (2014) Long-term patient survival with idiopathic/heritable pulmonary arterial hypertension treated at a single center in Japan. Life Sci 118:414–419
Madani MM, Auger WR, Pretorius V et al (2012) Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 94:97–103 discussion 103
Lewczuk J, Piszko P, Jagas J et al (2001) Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest 119:818–823
Miyamoto S, Nagaya N, Satoh T et al (2000) Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 161:487–492
Koike H, Sueyoshi E, Sakamoto I, Uetani M, Nakata T, Maemura K (2018) Comparative clinical and predictive value of lung perfusion blood volume CT, lung perfusion SPECT and catheter pulmonary angiography images in patients with chronic thromboembolic pulmonary hypertension before and after balloon pulmonary angioplasty. Eur Radiol 28:5091–5099
Tamura M, Yamada Y, Kawakami T et al (2017) Diagnostic accuracy of lung subtraction iodine mapping CT for the evaluation of pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: correlation with perfusion SPECT/CT. Int J Cardiol 243:538–543
Zhai Z, Ota H, Staring M et al (2018) Treatment effect of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension quantified by automatic comparative imaging in computed tomography pulmonary angiography. Invest Radiol 53:286–292
Ng CS, Wells AU, Padley SP (1999) A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging 14:270–278
Cochran ST, Bomyea K, Sayre JW (2001) Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol 176:1385–1388
Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K (1990) Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 175:621–628
Acknowledgements
The authors would like to acknowledge all of the patients for their willingness to participate in the study. The authors also thank Naomi Tamaki and Kyoko Komatsu for their valuable assistance.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yoshitake Yamada.
Conflict of interest
Masahiro Jinzaki is a recipient of a grant from Canon Medical Systems. However, Canon Medical Systems was not involved in the design and implementation of this study, the collection, analysis, and interpretation of the data, or the preparation, review, and approval of the manuscript.
All other authors are not associated with companies or other entities.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
The study complied with the Declaration of Helsinki, and the protocol for this prospective observational study was approved by the Ethics Committee of our institution (No. 20120224).
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Tsukada, J., Yamada, Y., Kawakami, T. et al. Treatment effect prediction using CT after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Radiol 31, 5524–5532 (2021). https://doi.org/10.1007/s00330-021-07711-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07711-5