Abstract
Objectives
To assess long-term prognosis after low-dose 64-slice coronary computed tomography angiography (CCTA) using prospective electrocardiogram-triggering.
Methods
We included 434 consecutive patients with suspected or known coronary artery disease referred for low-dose CCTA. Patients were classified as normal, with non-obstructive or obstructive lesions, or previously revascularized. Coronary artery calcium score (CACS) was assessed in 223 patients. Follow-up was obtained regarding major adverse cardiac events (MACE): cardiac death, myocardial infarction and elective revascularization. We performed Kaplan-Meier analysis and Cox regressions.
Results
Mean effective radiation dose was 1.7 ± 0.6 mSv. At baseline, 38% of patients had normal arteries, 21% non-obstructive lesions, 32% obstructive stenosis and 8% were revascularized. Twenty-nine patients (7%) were lost to follow-up. After a median follow-up of 6.1 ± 0.6 years, MACE occurred in 0% of patients with normal arteries, 6% with non-obstructive lesions, 30% with obstructive stenosis and 39% of those revascularized. MACE occurrence increased with increasing CACS (P < 0.001), but 4% of patients with CACS = 0 experienced MACE. Multivariate Cox regression identified obstructive stenosis, lesion burden in CCTA and CACS as independent MACE predictors (P ≤ 0.001).
Conclusion
Low-dose CCTA with prospective electrocardiogram-triggering has an excellent long-term prognostic performance with a warranty period >6 years for patients with normal coronary arteries.
Key Points
• Coronary CT angiography (CCTA) has an excellent long-term prognostic performance.
• CCTA can accurately stratify cardiac risk according to coronary lesion severity.
• A normal CCTA predicts freedom from cardiac events for >6 years.
• Patients with a coronary calcium score of 0 may experience cardiac events.
• CCTA allows for reclassification of cardiac risk compared with ESC SCORE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary computed tomography angiography (CCTA) is increasingly used for the non-invasive assessment of suspected or known coronary artery disease (CAD). CCTA has an excellent diagnostic accuracy with outstanding sensitivity and negative predictive value [1, 2], and new methods have been shown to further improve these parameters [3]. Moreover, several meta-analyses [4,5,6], the large CONFIRM registry [7] and more recent studies [8] demonstrated an excellent prognostic value of CCTA regarding cardiac events. However, the average follow-up duration of these prognostic studies was limited to 2–3 years, which seems short when considering the slowly progressive development of coronary atherosclerosis. Few authors have assessed the ability of CCTA to predict cardiac events in the long term. For this purpose, they used the older electron-beam CT [9] or retrospective electrocardiogram (ECG) gating with tube current modulation [10,11,12,13]. The latter technique is no longer the preferred method for CCTA, as it exposes patients to radiation doses of up to 10–20 mSv [14]. More recent long-term studies were focussed on coronary dominance [15], or on specific populations [16, 17].
Prospective ECG triggering was introduced for 64-slice CCTA in 2007 [18]. This technique combines an excellent diagnostic accuracy [19] with a low mean radiation dose of 1.8 mSv [20, 21], which may in turn reduce the potential risk associated with CCTA. We have previously reported on the excellent prognostic performance of CCTA with prospective ECG triggering after 1 year of follow-up [22].
Similarly, coronary artery calcium score (CACS) has been demonstrated to constitute a strong predictor of coronary events [23]. The high negative predictive value of CACS for cardiac events was highlighted in a recent meta-analysis [24], but several authors warned about non-calcified stenoses, which may be associated with increased cardiovascular risk but are not assessed by CACS [25]. Thus, the comparative value of CACS versus CCTA remains a matter of debate. A few studies suggested a superior prognostic value of CCTA over CACS but were mostly limited to short-term follow-up periods of 2-3 years [26, 27].
The primary aim of the present study was to assess the long-term prognostic performance of low-dose CCTA with prospective ECG triggering on major adverse cardiac events. The secondary aim was to compare the prognostic performance of CACS and low-dose CCTA. We hypothesized that low-dose CCTA can predict cardiac events in the long term and may have a higher prognostic performance than CACS.
Material and methods
Study protocol and patient selection
We performed an observational, retrospective, non-blinded, single-centre cohort follow-up study. We retrospectively included all consecutive patients undergoing low-dose 64-slice CCTA with prospective ECG triggering to evaluate suspected or known CAD from September 2007 to December 2008 in our centre. None of these patients had any of the routine exclusion criteria for CCTA, such as irregular heartbeat, contraindications for β-blocking drugs, failure to reach a heart rate <65 beats/min (bpm) despite intravenous β-blocking drugs, inability to follow breath-hold commands, known allergy to iodinated contrast agent or renal insufficiency (serum creatinine >150 μmol/L) [20]. Patients undergoing CCTA for other indications, including left atrial assessment before electrophysiological procedures or evaluation of congenital heart disease, were not included. The study population was shared with a previous report on short-term outcome after CCTA [22].
At baseline, we recorded demographic variables, traditional cardiovascular risk factors, cardiac symptoms (typical or atypical angina pectoris, dyspnoea) and cardiac medication. Furthermore, we extracted previous cardiac events (myocardial infarction [MI], percutaneous coronary intervention [PCI] or coronary artery bypass graft [CABG]) from clinical records.
Follow-up was performed using telephone interviews with patients and referring physicians. Additionally, electronic medical records were searched for cardiac events. Patients lost to follow-up were excluded from the study. Primary end-points were major adverse cardiac events (MACE), defined as cardiac death, non-fatal MI or elective revascularization by PCI or CABG. Cardiac death was defined as either sudden death with probable cardiac origin or death caused by acute MI, ventricular arrhythmias or refractory heart failure. Non-fatal MI was defined on the basis of symptoms, ECG and biomarkers of ischaemia [28]. Revascularization procedures within the first 6 weeks after CCTA were excluded because they may have been directly triggered by the CCTA findings per se [13]. This avoids an important confounder between the diagnostic and the prognostic value of the test. However, patients with such early revascularizations remained in the study and the next MACE was considered as the first event. The protocol was approved by the local ethics committee (KEK-ZH-No. 2013-0585) and the need for written informed consent was waived. Our study complied with the Declaration of Helsinki.
CCTA data acquisition
Patients were pre-treated with intravenous metoprolol (Beloc, AstraZeneca, London, UK) up to 30 mg if necessary to achieve a target heart rate <65 bpm, and with 2.5 mg sublingual isosorbide dinitrate (Isoket, UCB Pharma, Brussels, Belgium). Scanning was performed on a LightSpeed VCT XT scanner (GE Healthcare, Milwaukee, WI, USA) using prospective ECG triggering with validated scanning parameters [18, 20, 29]: slice acquisition 64 x 0.625 mm, smallest x-ray window at 75% of the RR cycle, z-coverage 40 mm with an increment of 35 mm, gantry rotation time 350 ms, body mass index-adapted tube voltage and current, and an overall z-coverage of 11–25 cm. Image acquisition with 75 ± 12 ml iodixanol (Visipaque 320, GE Healthcare) was initiated 4 s after the signal density reached a visually detectable threshold in the ascending aorta (visual bolus tracking). The effective radiation dose for CCTA was estimated as the dose-length product multiplied by a conversion coefficient for the chest (0.014 mSv∙mGy-1∙cm-1) [14].
CACS data acquisition and measurement
Unenhanced CT for CACS was performed with a 64-slice CT scanner (LightSpeed VCT XT, GE Healthcare) in patients ≥45 years old [30] and those undergoing hybrid imaging with either single photon emission computed tomography (SPECT) or positron emission tomography (PET) using CT-based attenuation correction [31]. Scan parameters for CACS were as follows: prospective ECG triggering, 2.5-mm slice thickness, 120 kV tube voltage and 200 mA tube current [30, 31]. Calcium scoring was performed on a dedicated workstation using a commercially available semi-automatic software package (SmartScore, GE Healthcare). All voxels with attenuation above a threshold of 130 Hounsfield units (HU) were automatically colour marked and lesions were manually selected. The software then calculated the CACS (i.e. Agatston score) [31].
Assessment of coronary lesions
Coronary arteries were divided into 16 segments according to a modified version of the American Heart Association model [32] with the intermediate branch defined as segment 16, if present. CCTA image interpretation was performed by two independent readers with at least 2 years of experience in CCTA derived from 64-slice CT scanners from axial source images, multi-oblique and multi-planar curved reformations, maximum intensity projections and volume-rendered images, as recommended [33]. Disagreement between readers was solved by consensus. Segments with doubling or discontinuity of the vessel, or non-differentiable structures (no clear delineation between vessel and surrounding tissue) were classified as non-evaluable. For lesion severity, non-evaluable segments were scored as the more proximal evaluable segment of the respective vessel [7]. Thus, all segments were scored in all patients. Coronary lesions were defined as plaques of ≥1 mm2 in orthogonal reconstructions within and/or adjacent to the vessel lumen, not belonging to surrounding tissue. An obstructive lesion was defined as a stenosis with a visual luminal diameter narrowing ≥50%. Patients were stratified according to coronary lesions documented by CCTA: normal coronary arteries, non-obstructive lesions (luminal narrowing <50% or eccentric wall calcifications), obstructive stenosis (luminal narrowing ≥50%) or revascularized patients (previous PCI or CABG). Additionally, a segment involvement score (SIS: 1 point for each coronary segment with any luminal narrowing) and segment severity score (SSS: total of all segments scored according to lesion severity with 0 = no lesion, 1 = narrowing <50%, 2 = stenosis 50–69%, 3 = stenosis ≥70%) was calculated for each patient [11]. Readers interpreting CCTA had access to patient data, such as symptoms, age, risk factors or previous events. However, the reading was performed immediately after scanning. Thus, readers were unaware of any subsequent invasive coronary angiography or MACE.
Statistical analysis
We used SPSS Statistics 22 (IBM, Armonk, NY, USA) and MedCalc Statistical Software 15.8 (MedCalc Software, Ostend, Belgium) for statistical analyses. Categorical variables are presented as frequencies with percentages and continuous variables as mean ± standard deviation (SD) or median ± interquartile range (IQR), as appropriate. Categorical variables were compared using the χ2 or Fisher’s exact test and continuous variables by Student’s t-test or the Mann-Whitney U-test, as appropriate. We performed Kaplan-Meier event-free survival analysis using the time to the first MACE of each patient. Event-free survival curves were compared between groups based on coronary lesions and CACS levels using the log-rank test. Moreover, the annual MACE rate was compared using the Mann-Whitney U-test and Kruskall-Wallis test. In patients without previous revascularization, we assessed the influence of baseline characteristics and CCTA findings on MACE occurrence using univariate Cox proportional hazard regression. Then, multivariate Cox regression was performed in a forward stepwise conditional manner with entry at P ≤ 0.05 and removal at P ≥ 0.10 to identify independent predictors of MACE. Cox regression results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). Furthermore, receiver operating characteristic (ROC) curves were generated to compare the prognostic performance of CCTA and CACS regarding MACE in patients who underwent both examinations. Areas under the curves (AUCs) with 95% CI were compared using the method of DeLong et al. [34]. Finally, we calculated the cardiovascular risk according to the SCORE algorithm of the European Society of Cardiology (ESC) [35] in a subset of patients with appropriate data to assess the reclassification rate after CCTA. Two-sided P values <0.05 were considered statistically significant. We reported our results according to the STROBE guidelines for observational studies [36].
Results
Patient population
We enrolled 434 patients who underwent low-dose CCTA with prospective ECG triggering. Among them, 29 (7%) were lost to follow-up due to invalid contact information or migration to a foreign country. Thus, 405 patients were included into the final statistical analysis. Baseline characteristics are presented in Table 1.
CCTA and CACS findings
In 405 patients, a total of 5,781 coronary segments were evaluated. We noted 699 missing segments due to anatomical variations, such as the often missing intermediate branch. Normal coronary arteries were observed in 153 patients (38%), non-obstructive lesions in 87 patients (21%) and obstructive stenosis in 131 patients (32%), whereas 34 patients (8%) were previously revascularized. Mean dose-length product of CCTA scanning was 123 ± 42 mGy∙cm, resulting in an effective radiation dose of 1.72 ± 0.59 mSv.
Mean SIS and SSS were 0 for patients with normal coronary arteries, but both 0.91 ± 1.21 with non-obstructive lesions. (This value is slightly <1, because of minimal eccentric wall calcifications without luminal narrowing.) In patients with obstructive stenosis, mean SIS was 3.62 ± 2.23 and mean SSS 7.35 ± 5.28.
CACS was performed in a subpopulation of 223 patients (56%). Median CACS was 61 ± 508.
Follow-up results
During a median follow-up of 6.1 ± 0.6 years, 116 MACE occurred in 101 patients (25%). Of these events, we excluded 50 elective revascularizations within 6 weeks of CCTA. Thus, we studied 66 MACE occurring in 55 patients (14%). These events were 7 cardiac deaths, 9 non-fatal myocardial infarctions, 38 elective PCI and 12 elective CABG. Furthermore, 16 patients died of non-cardiac causes: 4 of infection, 4 of cancer, three of suicide, two of haemorrhage, two after surgery and one of multiple diseases. These results are detailed by group in Table 2.
Survival analysis
Event-free survival was excellent in patients with normal coronary arteries, but decreased among patients with non-obstructive lesions, and decreased further with obstructive stenosis as diagnosed by CCTA and in previously revascularized patients (all pairwise P ≤ 0.003, except not significant for obstructive vs. revascularized; Fig. 1). Similarly, event-free survival decreased with increasing CACS (P for trend < 0.001) (Fig. 2). Of note, no MACE occurred among patients with normal coronary arteries according to CCTA, whereas 4% of patients with a CACS of 0 experienced MACE. Annual MACE rates stratified according to CCTA findings and to CACS levels are given in Figs. 3 and 4.
Kaplan-Meier cumulative event-free survival according to CCTA diagnosis. Log-rank test showed significant differences in cardiac events for all pairwise comparisons between groups (all P ≤ 0.003), except for obstructive stenosis versus previous revascularization (P = 0.82). CCTA coronary CT angiography
Mean annual MACE rate according to CCTA diagnosis. P-values for pairwise comparisons using Mann-Whitney U-test: *P < 0.001 vs. normal; #P = 0.003 vs. normal; §P < 0,001 vs. non-obstructive. Global comparison using Kruskall-Wallis test showed P < 0.001. MACE major adverse cardiac event, CCTA coronary computed tomography angiography
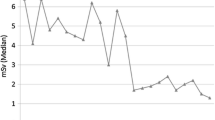
Mean annual MACE rate according to CACS. P-values for pairwise comparisons using Mann-Whitney U-test: *P < 0.05 vs. CACS 0; #P < 0.001 vs. CACS 0; § P ≤ 0.001 vs. CACS 1-100; †P < 0.05 vs. CACS 101-400. Global comparison using Kruskall-Wallis test and testing for increasing trend both showed P < 0.001. MACE major adverse cardiac event
Predictors of MACE
Results of univariate Cox regression analysis for demographics, cardiovascular risk factors, symptoms, previous events, CACS and CCTA findings are given in Table 3. In multivariate Cox regression analysis adjusted for demographics, cardiovascular risk factors, symptoms and previous cardiac events, CCTA findings of obstructive stenosis, SIS and SSS remained strong independent MACE predictors (all P ≤ 0.001) (Table 4).
CCTA versus CACS
In the subgroup of patients who underwent a CACS scan (n = 223), CACS was a strong independent MACE predictor in multivariate Cox regression analysis (P < 0.001). However, CCTA findings of obstructive stenosis, SIS and SSS remained independent MACE predictors even after adding CACS to the multivariate Cox regression model (all P ≤ 0.01; Table 5). A head-to-head comparison of the prognostic performance of CCTA and CACS for MACE prediction using ROC analysis in this subpopulation of patients who underwent both CCTA and CACS revealed a slightly larger AUC for CCTA findings than for CACS, particularly for SSS, but the differences fell short of statistical significance (Fig. 5).
ROC curves for MACE prediction in patients with CCTA and CACS (n = 223). CCTA diagnosis: AUC 0.755 (0.678–0.831) CCTA SSS: AUC 0.791 (0.727–0.855) CACS: AUC 0.745 (0.647–0.842) All pairwise comparisons of ROC curves showed non-significant P-values. Each curve vs. reference line: P < 0.001. ROC: receiver operating characteristic. MACE major adverse cardiac event, CCTA coronary computed tomography angiography, CACS coronary artery calcium score, SSS summed severity score, AUC area under the curve (with 95% confidence interval)
Reclassification after CCTA
In the subgroup of patients with appropriate data for cardiovascular risk stratification using the ESC SCORE (n = 142), reclassification analysis showed that 50% of the patients were reclassified after CCTA, particularly those at moderate risk (72%, see Table 6). Comparison of ROC curves regarding MACE prediction demonstrated a significantly higher AUC for CCTA diagnosis versus ESC SCORE (0.82 [0.75–0.88] vs. 0.65 [0.57–0.73]; P = 0.005).
Discussion
Our results highlight the excellent long-term prognostic performance of low-dose CCTA with prospective ECG triggering regarding cardiac events. All patients with normal coronary arteries in CCTA had a completely event-free survival during the median follow-up of 6.1 years. Patients with non-obstructive coronary lesions had a low risk with an annual event rate of 1% during the same period. By contrast, patients with obstructive stenosis were at much higher risk with an annual event rate of 14%. Similarly, CACS allowed risk stratification with a progressive increase of annual event rates up to 15% for patients with CACS >1,000. Multivariate Cox regression analysis revealed that CCTA findings as well as CACS were strong independent predictors of MACE. Contrary to a normal CCTA scan, however, a CACS of 0 could not predict freedom from MACE during the follow-up. This confirms that non-calcified coronary lesions are associated with a cardiovascular risk not assessed by CACS. Moreover, CCTA findings of obstructive stenosis and lesion scores remained independent MACE predictors even after adjustment for CACS. This underlines the additional predictive value of stenosis assessment using CCTA over CACS measurement. In direct comparison, CCTA yielded a slightly better prognostic performance than CACS. The difference, however, did not reach statistical significance. Finally, reclassification analysis showed the ability of CCTA to reclassify 50% of the patients compared with ESC SCORE, particularly in patients at moderate risk, with a significant improvement of MACE prediction.
Our findings are in line with previous results on the short-term prognostic value after low-dose CCTA with prospective ECG triggering, already suggesting an accurate stratification of patients into risk categories based on stenosis severity [22]. Similarly, several meta-analyses [4,5,6] and the large CONFIRM registry [7] concluded that the absence of coronary lesion on CCTA was associated with a very low risk of events, whereas obstructive stenosis predicted a much poorer prognosis after follow-up periods of 2–3 years. However, these studies did not assess the prognostic performance of CCTA on the long term. To our knowledge, only few studies have assessed a longer follow-up after CCTA than in the present study. Among them, Ostrom et al. showed that the presence of atherosclerosis and an increasing number of coronary lesions were associated with an increase in all-cause mortality in 2,538 symptomatic patients with a mean follow-up of 6.5 years after electron-beam CT [9]. In multivariate analysis, obstructive stenosis and three-vessel non-obstructive lesions remained the only independent predictors of mortality. In this cohort, however, most cases of death occurred in patients without obstructive CAD and the cause of death was unknown, rendering the results difficult to interpret. In a recent study, Dougoud et al. confirmed the excellent incremental prognostic value of CCTA diagnosis using retrospective ECG gating in 218 patients with a median follow-up of 6.9 years [13]. The present study extends previous knowledge by demonstrating the excellent prognostic long-term performance of CCTA with state-of-the-art low-dose prospective ECG triggering technique, resulting in a mean radiation dose exposure of 1.7 mSv. The latter highlights an improved risk-to-benefit ratio, and, thus, further corroborates the clinical value of low-dose CCTA with prospective ECG triggering.
Of note, we found that low-dose CCTA with prospective ECG-triggering has the potential to identify patients at very low cardiac risk, namely those with normal coronary arteries who did not suffer from any MACE during the 6.1-year follow-up period. The ‘warranty period’ of a normal CCTA, thus, seems to be very long, rendering repeat testing within this period unnecessary. Other non-invasive imaging tests based on myocardial perfusion are limited to detecting only flow-limiting lesions, i.e. obstructive stenosis. Thus, patients with normal perfusion tests may have several non-obstructive lesions that go undetected and are therefore at higher risk than patients with normal coronary arteries in CCTA. This may explain to some extent the shorter ‘warranty periods’ of about 2–4 years reported after stress echocardiography [37], SPECT [38], PET [39] and magnetic resonance imaging [40], depending on baseline patient risk.
Regarding the comparison between CCTA and CACS, both tests were previously shown to offer a robust prognostic performance. An additional value of CCTA over CACS is a matter of debate in the literature. The predictive value of CACS is supported by strong data [24]. However, short-term [26, 27] and long-term studies [11, 13] reported a possible superior predictive value of CCTA over CACS for cardiac events. In line with these data, our results suggest a tendency towards a higher predictive value from CCTA over CACS, but without significant difference in direct comparison. Of note, our low-risk patient sample was not primarily powered for this analysis. Nevertheless, the fact that patients with a CACS of 0 may experience MACE highlights the ability of CCTA to detect non-calcified lesions, which are associated with a non-negligible cardiac risk [25]. Moreover, although CACS is strongly associated with cardiac events at the cohort level, it does not provide a clear decisional cut-off on an individual basis. By contrast, CCTA does provide a practical cut-off value of ≥50% stenosis to support the decision towards further diagnostic and therapeutic work-up for the individual patient in everyday clinical practice.
It may be perceived as a potential limitation of this study that elective coronary revascularizations were included in our composite end-point. This, however, ensures comparability with similar previous studies also including these events [10,11,12,13]. Moreover, we used cardiac death in our end-point instead of all-cause death, as previously reported [10, 11], because we were able to retrieve information on the cause of death and did not expect CCTA to accurately predict non-cardiac death. Furthermore, our decision to exclude early revascularizations from the analyses, but not the patients undergoing them, may be considered as a further limitation of our study. Since many patients with obstructive stenosis diagnosed by CCTA underwent early revascularisation, excluding all these patients would have substantially reduced our sample of high-risk patients and thus hampered our ability to demonstrate the accurate risk stratification by CCTA and CACS, as reported in recent studies [12, 13]. Besides, CACS was only performed in a subgroup of patients, limiting the power of our analyses involving this parameter. However, our CACS sample remains larger than in recent similar reports [13]. Finally, the MACE rate of 25% reported in our study may appear high. This may be due to the long follow-up and to the inclusion of elective revascularisations, of patients with known CAD, and of patients with intermediate to high risk. In line with the results of the present study, Dougoud et al. found a MACE rate of 21% during a follow-up of 6.9 years after CCTA [13]. Our study also has some mentionable strengths, such as the long follow-up of 6.1 years and the extensive data collected, allowing multivariate modelling and detailed analysis of outcome predictors. Moreover, the use of prospective ECG triggering led to an important reduction of radiation dose compared to previous methods [14], but not as low as the latest technical refinements [41,42,43]. Finally, our study design closely reflecting the everyday practice of CCTA ensured a high generalisability of our results.
Conclusion
Low-dose CCTA with prospective ECG triggering has an excellent prognostic performance with a warranty period of at least 6 years for patients with normal coronary arteries.
Abbreviations
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass graft
- CACS:
-
Coronary artery calcium score
- CAD:
-
Coronary artery disease
- CCTA:
-
Coronary computed tomography angiography
- CI:
-
Confidence interval
- ECG:
-
Electrocardiogram
- ESC:
-
European Society of Cardiology
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- LVEF:
-
Left ventricular ejection fraction
- MACE:
-
Major adverse cardiac event
- MI:
-
Myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- PET:
-
Positron emission tomography
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- SIS:
-
Summed involvement score
- SPECT:
-
Single photon emission computed tomography
- SSS:
-
Summed severity score
References
Stein PD, Yaekoub AY, Matta F, Sostman HD (2008) 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med 121:715–725
Mowatt G, Cook JA, Hillis GS et al (2008) 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart 94:1386–1393
Plank F, Burghard P, Friedrich G et al (2016) Quantitative coronary CT angiography: absolute lumen sizing rather than %stenosis predicts hemodynamically relevant stenosis. Eur Radiol 26:3781–3789
Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC (2011) Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol 57:1237–1247
Bamberg F, Sommer WH, Hoffmann V et al (2011) Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J Am Coll Cardiol 57:2426–2436
Abdulla J, Asferg C, Kofoed KF (2011) Prognostic value of absence or presence of coronary artery disease determined by 64-slice computed tomography coronary angiography a systematic review and meta-analysis. Int J Cardiovasc Imaging 27:413–420
Min JK, Dunning A, Lin FY et al (2011) Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 58:849–860
Suh YJ, Hong YJ, Lee HJ et al (2015) Prognostic value of SYNTAX score based on coronary computed tomography angiography. Int J Cardiol 199:460–466
Ostrom MP, Gopal A, Ahmadi N et al (2008) Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol 52:1335–1343
Sozzi FB, Civaia F, Rossi P et al (2011) Long-term follow-up of patients with first-time chest pain having 64-slice computed tomography. Am J Cardiol 107:516–521
Andreini D, Pontone G, Mushtaq S et al (2012) A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging 5:690–701
Hadamitzky M, Taubert S, Deseive S et al (2013) Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J 34:3277–3285
Dougoud S, Fuchs TA, Stehli J et al (2014) Prognostic value of coronary CT angiography on long-term follow-up of 6.9 years. Int J Cardiovasc Imaging 30:969–976
Hausleiter J, Meyer T, Hermann F et al (2009) Estimated radiation dose associated with cardiac CT angiography. JAMA 301:500–507
Gebhard C, Fuchs TA, Stehli J et al (2015) Coronary dominance and prognosis in patients undergoing coronary computed tomographic angiography: results from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) registry. Eur Heart J Cardiovasc Imaging 16:853–862
Cheruvu C, Precious B, Naoum C et al (2016) Long term prognostic utility of coronary CT angiography in patients with no modifiable coronary artery disease risk factors: Results from the 5 year follow-up of the CONFIRM International Multicenter Registry. J Cardiovasc Comput Tomogr 10:22–27
Nadjiri J, Hausleiter J, Deseive S et al (2016) Prognostic value of coronary CT angiography in diabetic patients: a 5-year follow up study. Int J Cardiovasc Imaging 32:483–491
Husmann L, Valenta I, Gaemperli O et al (2008) Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J 29:191–197
Herzog BA, Husmann L, Burkhard N et al (2008) Accuracy of low-dose computed tomography coronary angiography using prospective electrocardiogram-triggering: first clinical experience. Eur Heart J 29:3037–3042
Buechel RR, Husmann L, Herzog BA et al (2011) Low-dose computed tomography coronary angiography with prospective electrocardiogram triggering: feasibility in a large population. J Am Coll Cardiol 57:332–336
Husmann L, Herzog BA, Gaemperli O et al (2009) Diagnostic accuracy of computed tomography coronary angiography and evaluation of stress-only single-photon emission computed tomography/computed tomography hybrid imaging: comparison of prospective electrocardiogram-triggering vs. retrospective gating. Eur Heart J 30:600–607
Buechel RR, Pazhenkottil AP, Herzog BA et al (2011) Prognostic performance of low-dose coronary CT angiography with prospective ECG triggering. Heart 97:1385–1390
Polonsky TS, McClelland RL, Jorgensen NW et al (2010) Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 303:1610–1616
Sarwar A, Shaw LJ, Shapiro MD et al (2009) Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2:675–688
Villines TC, Hulten EA, Shaw LJ et al (2011) Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol 58:2533–2540
van Werkhoven JM, Schuijf JD, Gaemperli O et al (2009) Incremental prognostic value of multi-slice computed tomography coronary angiography over coronary artery calcium scoring in patients with suspected coronary artery disease. Eur Heart J 30:2622–2629
Hou ZH, Lu B, Gao Y et al (2012) Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging 5:990–999
Thygesen K, Alpert JS, Jaffe AS et al (2012) Third universal definition of myocardial infarction. Eur Heart J 33:2551–2567
Tatsugami F, Husmann L, Herzog BA et al (2009) Evaluation of a body mass index-adapted protocol for low-dose 64-MDCT coronary angiography with prospective ECG triggering. AJR Am J Roentgenol 192:635–638
Husmann L, Herzog BA, Burger IA et al (2010) Usefulness of additional coronary calcium scoring in low-dose CT coronary angiography with prospective ECG-triggering impact on total effective radiation dose and diagnostic accuracy. Acad Radiol 17:201–206
Schepis T, Gaemperli O, Koepfli P et al (2007) Use of coronary calcium score scans from stand-alone multislice computed tomography for attenuation correction of myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging 34:11–19
Austen WG, Edwards JE, Frye RL et al (1975) A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 51:5–40
Leipsic J, Abbara S, Achenbach S et al (2014) SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 8:342–358
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Piepoli MF, Hoes AW, Agewall S et al (2016) 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 37:2315–2381
von Elm E, Altman DG, Egger M et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 4, e296
Marwick TH, Case C, Sawada S et al (2001) Prediction of mortality using dobutamine echocardiography. J Am Coll Cardiol 37:754–760
Hachamovitch R, Hayes S, Friedman JD et al (2003) Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: what is the warranty period of a normal scan? J Am Coll Cardiol 41:1329–1340
Herzog BA, Husmann L, Valenta I et al (2009) Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 54:150–156
Jahnke C, Furundzija V, Gebker R et al (2012) Gender-based prognostic value of pharmacological cardiac magnetic resonance stress testing: head-to-head comparison of adenosine perfusion and dobutamine wall motion imaging. Int J Cardiovasc Imaging 28:1087–1098
Fuchs TA, Stehli J, Bull S et al (2014) Coronary computed tomography angiography with model-based iterative reconstruction using a radiation exposure similar to chest X-ray examination. Eur Heart J 35:1131–1136
Gordic S, Desbiolles L, Sedlmair M et al (2016) Optimizing radiation dose by using advanced modelled iterative reconstruction in high-pitch coronary CT angiography. Eur Radiol 26:459–468
Zhang LJ, Wang Y, Schoepf UJ et al (2016) Image quality, radiation dose, and diagnostic accuracy of prospectively ECG-triggered high-pitch coronary CT angiography at 70 kVp in a clinical setting: comparison with invasive coronary angiography. Eur Radiol 26:797–806
Acknowledgments
The University Hospital of Zurich holds an institutional research agreement with GE Healthcare. However, this work has received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ronny R. Buechel.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: GE Healthcare. The University Hospital Zurich holds a research agreement with GE Healthcare
Funding
The authors state that this work has not received any funding.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained. Study subjects or cohorts overlap:
The study population was shared with a previous report on short-term outcome after CCTA (Buechel et al., Heart 2011; 97(17):1385-90).
Methodology
-
retrospective
-
prognostic study, observational
-
performed at one institution
Additional information
Olivier F. Clerc and Basil P. Kaufmann share first authorship.
Rights and permissions
About this article
Cite this article
Clerc, O.F., Kaufmann, B.P., Possner, M. et al. Long-term prognostic performance of low-dose coronary computed tomography angiography with prospective electrocardiogram triggering. Eur Radiol 27, 4650–4660 (2017). https://doi.org/10.1007/s00330-017-4849-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4849-1