Abstract
Objective
This tertiary care experience examines the utility of magnetic resonance neurography (MRN) in the management of peripheral trigeminal neuropathies.
Materials and methods
Seventeen patients with clinically suspected peripheral trigeminal neuropathies (inferior alveolar nerve and lingual nerve) were imaged uniformly with 1.5-T examinations. MRN results were correlated with clinical and surgical findings in operated patients and the impact on clinical management was assessed.
Results
Clinical findings included pain (14/17), sensory changes (15/17), motor changes (2/17) and palpable masses (3/17). Inciting events included prior dental surgery (12/17), trauma (1/17) and idiopathic incidents (4/17). Non-affected side nerves and trigeminal nerves in the intracranial and skull base course were normal in all cases. Final diagnoses on affected sides were nerve inflammation (4/17), neuroma in continuity (2/17), LN transection (1/17), scar entrapment (3/17), infectious granuloma (1/17), low-grade injuries (3/17) and no abnormality (3/17). Associated submandibular gland and sublingual gland oedema-like changes were seen in 3/17 cases because of parasympathetic effects. Moderate-to-excellent MRN-surgical correlation was seen in operated (8/17) patients, and neuroma and nerve transection were prospectively identified in all cases.
Conclusion
MRN is useful for the diagnostic work-up of suspected peripheral trigeminal neuropathy patients with significant impact on clinical management and moderate-to-excellent correlation with intra-operative findings.
Key Points
• MRN substantially impacts diagnostic thinking and management in peripheral trigeminal neuropathy.
• MRN has moderate-to-excellent correlation with intra-operative findings.
• MRN should be considered in pre-surgical planning of peripheral trigeminal neuropathy subjects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The peripheral trigeminal nerve distal to the skull base can be injured during infratemporal, dental and oral surgery procedures or facial fractures, with more common involvement of the inferior alveolar nerve (IAN) and lingual nerve (LN). Iatrogenic injuries may be related to traumatic anaesthetic injections, dental implant osteotomy or placement, and molar extraction (often the 3rd molar). Some studies have noted a complication rate as high as 13% [1–5]. Less commonly, these nerves can be affected by inflammatory disease or mass lesions [6]. Trigeminal nerve injuries have a profound effect on patients due to resulting functional issues such as drooling while eating or drinking, unintentional chewing of the tongue and lips, pain, heat and cold sensitivity, and difficulty speaking. The symptoms of IAN injury can include altered sensation including spontaneous or evoked paresthesia, dysesthesia, analgesia and anaesthesia [7]. Injury to the lingual nerve (LN) may in addition affect taste perception on the affected side of the tongue along with loss of fungiform papillae and parasympathetic changes of the ipsilateral salivary glands. The vast majority (about 90%) of these injuries are temporary in nature and resolve within 8 weeks. However, if the injury persists, it is deemed likely to be permanent [3]. Proper assessment and surgical planning and additionally the decision for surgery and when to employ trigeminal nerve microsurgery techniques warrant in-depth clinical assessment, since the timing of the intervention can impact outcome [1]. Pre-operative imaging of the nerve has previously focussed on identifying the expected location based on known anatomic landmarks and panoramic radiographs; however, these modalities poorly evaluate the nerve or perineural tissues [8]. Magnetic resonance neurography (MRN) is now available for high-resolution demonstration of the peripheral trigeminal nerve and its branches [9–12]. Prior work by Terumitsu et al. showed the importance of the T1-weighted MR sequence in patients with suspected IAN pathologies [13] and the authors showed good correlation of perineural scarring in the mandibular canal with surgery. With currently available 3D (dimensional), steady-state and diffusion-based techniques, better T2W evaluation of different branches of the peripheral trigeminal nerve is possible, which enhances the detection of the alterations in the endoneurial fluid and presence of intra-neural oedema and mass lesions [9, 12, 14, 15]. The purpose was to evaluate a consecutive series of patients suspected of having peripheral trigeminal neuropathy with MRN using a uniform imaging protocol and assess the correlation with surgical findings and the impact of imaging in the clinical management of such patients.
Material and methods
The study was a retrospective chart review and was performed in an HIPAA-compliant manner following approval of the local IRB. A consecutive series of 17 patients suspected of peripheral trigeminal neuropathies referred from two surgeons (JRZ and JC) for pre-surgical evaluation were included. All imaging studies were uniformly performed using a multichannel head coil on a 1.5-T scanner (Siemens, Avanto, Erlangen, Germany) over a 20-month period (Table 1, Fig. 1). Most imaging studies were performed without intravenous contrast initially with a total imaging time of 45 min. One patient was called back for intravenous gadolinium examination following detection of a mass within the IAN and one had contrast examination during the same study. Patient demographics in terms of age (mean and SD) and sex and clinical findings including the inciting event, prior nerve surgery, duration of symptoms before MRN, pain, other sensory symptoms, motor symptoms and palpable mass were recorded. There were 17 patients including 10 females with age 43.4 ± 18.7 years (mean ± SD) and 7 males with age 47.5 ± 14.2 years (mean ± SD) and symptoms ranged from 2 weeks to 17 years (2.2 ± 4.0 years, mean ± SD) (Table 2). Prior dental work artefact caused suboptimal visualisation of both IAN and LN in 1/17 and isolated lingual nerve in another 1/17 patients. Prior nerve surgery history was present in 2/17 patients; 1 had repair of the distal mental branch of the IAN and the other had prior lingual nerve repair for class 5 injury. Clinical findings included pain (14/17), other sensory changes (15/17), motor changes (2/17) and palpable mass (3/17). Inciting events included prior dental surgery (12/17), trauma (1/17) and idiopathic (4/17). During the image evaluation, from 3D isotropic MRN sequences, thick slab maximum intensity projections (MIPs) were obtained in nerve longitudinal planes (coronal and oblique sagittal) on an independent workstation (Aquarius, Tera Recon, Foster City, CA, USA). The MRN findings of nerve signal and calibre alteration were read as part of clinical care by two readers (attending: 19 years and multiple fellows: 5 years of radiology experience) in consensus in light of the clinical findings, but blinded to the side of abnormality, and no separate independent reads were performed. The final impression was generated in terms of normal, entrapment, injury or a mass lesion. Perineural fat planes were assessed for the presence of scarring or mass lesions. The CISS 3D sequence was employed in the protocol to exclude any intracranial neural pathology and the axial T1W and T2 SPAIR sequences were extended to the corpus callosum to the chin to exclude any intra-nuclear or adjacent intra-cranial pathology. The impact on the diagnosis and clinical management was assessed as none (no change in diagnosis, nerve involved or pre-MRN treatment strategy), mild (change in diagnosis, nerve or alternative diagnosis but no change in treatment) and substantial (change in diagnosis or change in involved nerve with change in proposed treatment, such as change from proposed follow-up to immediate surgery or change from surgery to non-surgical management). Based on the MRN results, the surgeons offered non-operative treatment, operative treatment (for entrapment, mass and high-grade nerve injuries—neuroma in continuity and transection cases) and rhizotomy (long-standing low-grade injury cases). Surgical correlation of MRN findings was obtained in the operated cases and was classified as poor (no substantiation of MRN findings), moderate (partial correlation) or excellent (full substantiation of MRN findings) correlation. Follow-up clinical status after imaging and surgery was also recorded.
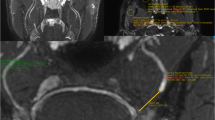
a−g Peripheral trigeminal nerve MRN. a Axial T2 SPAIR, b axial T1W, c axial CISS-3D and d axial DTI images in the top row show the normal left inferior alveolar nerve and intracranial trigeminal nerve (arrows). e Coronal 3D STIR SPACE (non-vessel-suppressed and f coronal 3D DW PSIF (vessel-suppressed nerve selective) imaging shows the left inferior alveolar nerve in the mandibular canal (arrows). g Sagittal oblique MIP reconstruction from 3D DW PSIF shows the entire course of the left inferior alveolar nerve (arrow)
Results
On MRN, the nerve abnormalities were seen in the IAN (13/17), LN (2/17), and both IAN and LN (2/17) (Table 3). Ipsilateral signal changes were seen in 13/17, diffuse nerve thickenings in 12/17, focal mass-like nerve thickenings in 4/17 and perineural fibrosis in 7/17. Enhancement on contrast administration was seen in 1/17 patient with focal mass-like thickening in the proximal mandibular canal, which decreased slightly on follow-up MRN examination after 2 months. The contralateral nerves and trigeminal nerve in the intracranial and skull base course were normal in all cases. Associated submandibular gland and sublingual gland oedema-like changes were seen in 3/17 cases because of parasympathetic effects. Final diagnoses were nerve inflammation (4/17) (Fig. 2), scar entrapment (3/17) (Fig. 3), neuroma in continuity (2/17) and LN transection (1/17) (Figs. 4 and 5), infectious granuloma (1/17), low-grade injuries (3/17) and no abnormality (3/17) (Figs. 3 and 4). Six of 17 studies resulted in a substantial impact on management, 6/17 in a mild impact and 5/17 did not change management. Moderate-to-excellent MRN-surgical correlation was seen in operated (8/17) patients. Neuroma, nerve entrapment and nerve transection were prospectively identified in all cases on MRN with excellent correlation (Fig. 5). One patient had class 4 injury on surgery, which was overcalled on MRN as class 4/5 injury because of suboptimal identification of a small segment of the LN on MRN related to encasement in scarring. It was classified as moderate correlation. Other cases of low-grade injuries and entrapments were classified as moderate correlation, where the surgeon found more scarring than was seen on MRN and in one case, there was foreign material in the inferior alveolar nerve from a prior injury, which was not prospectively seen on MRN. Follow-up ranged from 1 week to 19 months and most cases improved following surgery or intervention (balloon rhizotomy), i.e. 8/9 cases with intervention.
a, b Coronal 3D DW PSIF MIP images showing: a Patient 1: Normal symmetric bilateral IANs (small arrows). b Patient 7: There is mild asymmetric thickening and hyperintensity of the left IAN (long arrows) distally along its course as compared to the right normal IAN (small arrow) suggesting inflammation
a−c Left IAN entrapment: Patient 11. a Axial T1W image shows perineural fibrosis (arrow) along the course of the left IAN with obscuration of the nerve. b Coronal 3D DW PSIF MIP image shows the thickened and hyperintense left IAN (small arrow) with mild contour irregularity due to entrapment. c The respective abnormalities are nicely seen on the MIP image from DTI
a−f Patient 9. a Axial T2 SPAIR image shows thickened and hyperintense right IAN (long arrow) and LN with a small neuroma in continuity (small arrow). b Axial T1W image shows perineural fibrosis around the thickened IAN (long arrow). c Axial DTI shows good vascular signal suppression and confirms the above neural abnormalities (arrows). d 3D DW PSIF MIP image also shows a neuroma in continuity of the LN (small arrow) and abnormally thickened irregular IAN (long arrow). e, f Intraoperative pictures confirm the neuroma in continuity of the LN (small arrow in e) and entrapped thickened IAN with perineural fibrosis (long arrow in f)
a−c. a Patient 2: Coronal 3D DW PSIF image showing enlarged thickened and the discontinuous left lingual nerve (arrow) with the nerve gap measuring 8.5 mm. b Coronal 3D STIR SPACE image showing hyperintense left submandibular gland (arrow) due to parasympathetic effects. c Intraoperative picture showing the discontinuous nerve gap filled with scarring. Following neurolysis to refashion the nerve endings, the nerve gap measured 12 mm and was repaired with allograft
Discussion
This study confirms that high-resolution MRN is very useful in the evaluation of peripheral trigeminal neuropathies. Pain and other sensory changes correctly point to the side of the abnormality; however such symptoms are not specific to the aetiology or location of the lesion and are noted in most cases. MRN correctly showed normal nerves on the asymptomatic side as intermediate signal without hyperintensity or caliber alterations, corroborating the excellent negative predictive value of the examination. The intracranial segment and proximal trigeminal nerve at the base of the brain and in the infratemporal fossa were also normal in all cases, leading to good localisation of the lesion in the periphery, which has not been shown before. On the abnormal side, not only perineural scarring was identified on T1W images, as previously shown by Terumitsu et al. [13], but also nerve signal changes and calibre alterations were seen in most cases on high-resolution fat-suppressed fluid-sensitive 2D and 3D T2W images. For the three cases (one degraded by metal artefacts) where nerves were seen as normal, it may be hypothesised that the sensory nerve abnormality was below MRN resolution. However, a contribution of psychological and other multifactorial causes is not excluded. MRN did not find a surgical cause to address in those cases, which is also important for management purposes.
We could produce excellent image quality on 1.5-T scanners using a head coil. In only two cases, the nerves were sub-optimally visualised because of dental hardware-related susceptibility artefacts. The isotropic 3D images could be easily reconstructed to produce long axis images of the peripheral trigeminal branches, which showed asymmetric signal and calibre alterations on the affected sides. The water-selective fat-suppressed 3D DW PSIF imaging sequence was ideal in demonstrating the IAN and LN selectively with excellent fat and vascular signal suppression [10, 12]. The neuroma in continuity (class 4 injury), scar entrapment and lingual nerve transection (class 5 injury) were correctly identified with moderate-to-excellent surgical correlation [16]. Use of T1W, T2W and DTI images in tandem aids in the evaluation of neural abnormalities, perineural fibrosis and the ancillary finding of salivary gland parasympathetic changes. The abnormality shows up as considerably bright signal on DTI and 3D STIR SPACE and 3D DWPSIF images as compared to 2D images. The reconstructions along the long axis of the nerve showed both calibre alterations and discontinuity for the surgeon’s benefit in contemplating pre-operative planning [17–19].
The impact on clinical management was important. Less than 1/3 of the cases (29%) did not have a change in clinical management and in about 35% of cases it changed substantially. Clinical findings thus need objective substantiation from high-resolution imaging, such as MRN [20, 21]. Additionally, pain can be present before and after nerve repair and does not point to resolution or improvement of the nerve injury [22]. This was also seen in one of our previously operated patients; MRN could find an unsuspected neuroma in continuity, which was resected with nerve repair. Currently, there is controversy regarding how to best treat these nerve injuries, with increasing evidence seeming to point towards better outcomes with nerve grafting as compared to the primary repair [3, 23]. However, no studies have reported the effects of the intervention on the remaining primary outcomes of pain, difficulty eating or speaking, or taste or quality of life. This study also did not assess the long-term outcome, although in the short term, most cases improved following surgery. However, that was not the primary objective of the study. Other limitations of the study include selection bias, as these cases came from surgeons and not primary physicians, and the small sample size.
In future, larger prospective studies can be performed in a single or multicentre trial to evaluate the patient outcomes and impact of MRN on management [24]. Further refinement of the techniques may help to image the sensory nerves in both the absence and presence of dental artefacts. Finally, we did not attempt to correlate the imaging findings with objective neurosensory testing [25], which, of note, is not universally performed in different centres managing peripheral trigeminal neuropathy.
Conclusion
To summarise, MRN is useful for the diagnostic work-up of suspected peripheral trigeminal neuropathy patients with significant impact on the clinical management and moderate-to-excellent correlation with intra-operative findings.
References
Ziccardi V, Zuniga J (2007) Nerve injuries after third molar removal. Oral Maxillofac Surg Clin N Am 19:105–115
Tay AB, Lai JB, Lye KW, Wong WY, Nadkarni NV, Li W, Bautista D (2015) Inferior alveolar nerve injury in trauma-induced mandible fractures. J Oral Maxillofac Surg
Coulthard P, Kushnerev E, Yates JM, Walsh T, Patel N, Bailey E, Renton TF (2014) Interventions for iatrogenic inferior alveolar and lingual nerve injury. Cochrane Database Syst Rev 4, CD005293
Tay AB, Zuniga JR (2007) Clinical characteristics of trigeminal nerve injury referrals to a university centre. Int J Oral Maxillofac Surg 36(10):922–927
Ellies LG (1992) Altered sensation following mandibular implant surgery: a retrospective study. J Prosthet Dent 68:664–671
Fujita H, Kokubun N, Sada T, Nagashima T, Komagamine T, Kawabe K, Hirata K (2015) Demyelinating hypertrophic inferior alveolar nerve mimicking a nerve tumor. Intern Med 54(9):1109–1111
Alhassani A, AlGhamdi A (2010) Inferior alveolar nerve injury in implant dentistry: dianosis, causes, prevention, and management. J Oral Implantol 36(5):401–407
Liu W, Yin W, Zhang R, Li J, Zheng Y (2015) Diagnostic value of panoramic radiography in predicting inferior alveolar nerve injury after mandibular third molar extraction: a meta-analysis. Aust Dent J
Cassetta M, Pranno N, Pompa V, Barchetti F, Pompa G (2014) High resolution 3-T MR imaging in the evaluation of the trigeminal nerve course. Eur Rev Med Pharmacol Sci 18(2):257–264
Chhabra A, Subhawong TK, Bizzell C, Flammang A, Soldatos T (2011) 3T MR neurography using three-dimensional diffusion-weighted PSIF: technical issues and advantages. Skelet Radiol 40(10):1355–1360
Vargas MI, Viallon M, Nguyen D, Beaulieu JY, Delavelle J, Becker M (2010) New approaches in imaging of the brachial plexus. Eur J Radiol 74(2):403–410
Fujii H, Fujita A, Yang A, Kanazawa H, Buch K, Sakai O, Sugimoto H (2015) Visualization of the peripheral branches of the mandibular division of the trigeminal nerve on 3D double-echo steady-state with water excitation sequence. AJNR Am J Neuroradiol
Terumitsu M, Seo K, Matsuzawa H, Yamazaki M, Kwee IL, Nakada T (2011) Morphologic evaluation of the inferior alveolar nerve in patients with sensory disorders by high-resolution 3D volume rendering magnetic resonance neurography on a 3.0-T system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111(1):95–102
Chhabra A, Soldatos T, Subhawong TK, Machado AJ, Thawait SK, Wang KC, Padua A Jr, Flammang AJ, Williams EH, Carrino JA (2011) The application of three-dimensional diffusion-weighted PSIF technique in peripheral nerve imaging of the distal extremities. J Magn Reson Imaging 34(4):962–967
Chhabra A, Carrino J (2015) Current MR neurography techniques and whole-body MR neurography. Semin Musculoskelet Radiol 19(2):79–85
Seddon HJ, Medawar PB, Smith H (1943) Rate of regeneration of peripheral nerves in man. J Physiol 102:191–215
Kasper JM, Wadhwa V, Scott KM, Rozen S, Xi Y, Chhabra A (2015) SHINKEI—a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging. Eur Radiol 25(6):1672–1677
Breitenseher JB, Kranz G, Hold A, Berzaczy D, Nemec SF, Sycha T, Weber M, Prayer D, Kasparian G (2015) MR neurography of ulnar nerve at the cubital tunnel: a diffusion tensor imaging study. Eur Radiol 25(7):1911–1918
Baumer P, Kele H, Kretschmer T, Koenig R, Pedro M, Bendszus M, Pham M (2014) Thoracic outlet syndrome in 3T MR neurography-fibrous bands causing discernible lesions of the lower brachial plexus. Eur Radiol 24(3):756–761
Jengojan S, Kovar F, Breitenseher J, Weber M, Prayer D, Kasprian G (2015) Acute radial nerve entrapment at the spiral groove: detection by DTI-based neurography. Eur Radiol 25(6):1678–1683
Schwarz D, Weiler M, Pham M, Heiland S, Bendszus M, Bäumer P (2015) Diagnostic signs of motor neuropathy in MR neurography: nerve lesions and muscle denervation. Eur Radiol 25(5):1497–1503
Zuniga JR, Yates DM, Phillips CL (2014) The presence of neuropathic pain predicts postoperative neuropathic pain following trigeminal nerve repair. J Oral Maxillofac Surg 72(12):2422–2427
Miloro M, Ruckman P 3rd, Kolokythas A (2015) Lingual nerve repair: to graft or not to graft? J Oral Maxillofac Surg
Chhabra A, Belzberg AJ, Rosson GD, Thawait GK, Chalian M, Farahani SJ, Shores JT, Deune G, Hashemi S, Thawait SK, Subhawong TK, Carrino JA. Impact of high resolution 3 tesla MR neurography (MRN) on diagnostic thinking and therapeutic patient management. Eur Radiol. 2015 Sep 22. doi:10.1007/s00330-015-3958-y
Zuniga JR (2015) Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft—a case series. J Oral Maxillofac Surg 73(4):734–744
Acknowledgments
The scientific guarantor of this publication is Avneesh Chhabra. The authors of this manuscript declare relationships with the following companies: Avneesh Chhabra serves as a consultant with Siemens for the MSK CAD group. He also receives royalties from the Jaypee, Wolters and Elsevier groups of publishers. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cox, B., Zuniga, J.R., Panchal, N. et al. Magnetic resonance neurography in the management of peripheral trigeminal neuropathy: experience in a tertiary care centre. Eur Radiol 26, 3392–3400 (2016). https://doi.org/10.1007/s00330-015-4182-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4182-5