Abstract
Background
Idiopathic trigeminal neuralgia (TN) is caused by neurovascular compression and is often related to morphological changes in the trigeminal nerve. The aim of this study was to quantitatively measure atrophic changes of trigeminal nerves in patients with TN, and to further investigate whether nerve atrophy affected the efficacy of microvascular decompression (MVD).
Methods
We conducted a prospective case-control study of 60 consecutive patients with TN and 30 sex- and age-matched healthy controls. All subjects underwent high-resolution three-dimensional MRI. The volume of the cisternal segment of trigeminal nerves was measured and compared using 3D Slicer software. Patients with TN underwent primary MVD and regular follow-up for at least 2 years. Associations of nerve atrophy with patient characteristics and operative outcomes were analyzed.
Results
The mean volume of the affected trigeminal nerve was significantly reduced in comparison to that of the nonaffected side (65.8 ± 21.1 versus 77.9 ± 19.3 mm3, P = 0.001) and controls (65.8 ± 21.1 versus 74.7 ± 16.5 mm3, P = 0.003). Fifty-two patients (86.7%) achieved complete pain relief without medication immediately after surgery, and 77.6% of patients were complete pain relief at the 2-year follow-up. The Spearman correlation test showed that there was a positive correlation (r = 0.46, P = 0.018) between the degree of trigeminal nerve indentation and nerve atrophy. In multivariate logistic regression analysis, two factors, indentation on nerve root (OR = 2.968, P = 0.022) and degree of nerve atrophy (OR = 1.18, P = 0.035), were associated with the long-term outcome.
Conclusions
TN is associated with atrophy on the affected nerve. Furthermore, greater nerve atrophy is associated with more severe trigeminal nerve indentation and better long-term outcome following MVD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The specific pathophysiological cause of trigeminal neuralgia (TN) is still unclear. It has so far been widely accepted that neurovascular conflict (NVC) at the nerve’s root entry zone (REZ) plays a major role in the etiology of TN [4, 19, 18, 15, 17]. Microvascular decompression (MVD) leads to NVC elimination and successful pain relief, strongly supporting this theory, which is widely regarded as a logical and valuable method for curing the disease [24, 26]. However, NVC has been demonstrated in healthy subjects as well [1]. Furthermore, TN can occur in patients with no clear NVC [13]. These observations suggest that NVC alone is insufficient to cause TN.
It is suggested that structural and morphologic changes that occurred in the affected trigeminal nerve may be involved in the pathophysiology of TN [7]. Morphologic changes in the nerve include nerve distortion, deviation, groove formation, and nerve atrophy. Atrophy of the trigeminal nerve is seen in most patients with TN and is possibly due to structural abnormalities, such as axonal loss and demyelination [10, 7]. Neurovascular compression may represent a continued source of irritation to the trigeminal nerve that is believed to be the main cause of nerve atrophy [14]. Erbay et al. measured the diameter and cross-sectional area of the trigeminal nerve in patients with TN and concluded that trigeminal nerve atrophy can be depicted noninvasively [10]. However, the precise nerve atrophy on MRI was difficult to delineate.
High-resolution three-dimensional (3D) MRI proved to be reliable in detecting NVC and in predicting the degree of nerve root compression [15], and also made it possible to identify fine anatomic structures of trigeminal nerve in the cerebrospinal fluid. In addition, previous studies demonstrated that patients with severe trigeminal nerve indentation tended to exhibit greater clinical improvement following MVD [24]. However, it is unclear whether nerve atrophy correlates with degree of trigeminal nerve indentation, and whether it affects the surgical outcome of MVD. To address these problems, we conducted a prospective case-control study to quantitatively measure the volume of the cisternal segment of trigeminal nerves in patients with TN in comparison with age- and sex-matched control subjects. Furthermore, we further analyzed the associations of nerve atrophy with patient characteristics and operative outcomes.
Materials and methods
Patients and control subjects
Between September 2014 and March 2015, 60 consecutive patients with idiopathic TN who were treated at the neurosurgery department of West China Hospital were included in the study. Idiopathic TN was diagnosed according to the criteria of the second edition of the International Classification of Headache Disorders [25]. Patients diagnosed with secondary TN, presenting with bilateral symptoms, or having been treated previously with other procedures were excluded. All patients underwent primary MVD and received regular follow-up after operation. The following subject characteristics were recorded: age, sex, symptom duration, distribution of pain, operation date, surgical findings, follow-up duration, and operative efficacy. Controls were healthy age- and sex-matched volunteers. All patients and control subjects were informed about the imaging procedure and gave written informed consent. This study was approved by the West China Hospital Ethics Committee.
Imaging examinations
All patients and control subjects were examined with the same MRI scanner (Philips Achieva, 3.0 T). A standard protocol of conventional axial T1WI, axial T2WI, and a special three-dimensional sequence focused over the posterior fossa: 3D–T2-DRIVE (TR, 2000 ms; TE, 200 ms; matrix, 256 × 168; ACQ voxel MPS, 0.59/0.89/1.60 mm; REC voxel MPS, 0.29/0.29/0.80 mm; thickness, 0.5 mm) was performed. The image was jointly interpreted by neurosurgeons and neuroradiologists. Subjects with structural abnormalities were excluded.
Measurements
Three-dimensional T2-DRIVE images were transferred to a personal workstation for postprocessing and analysis. Segmentation and measurements of trigeminal nerve volume were performed using the imaging software 3D Slicer (version 3.6.3, U.S.), a free download available on the website https://www.slicer.org/.
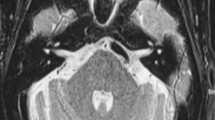
The volume of the cisternal segment of trigeminal nerve was selected to evaluate the general alteration of the trigeminal nerve. The trigeminal nerve was manually delineated on the axial images from the pons to the narrow aperture of Meckel’s cave with section thickness of 0.5 mm, and the volume of the cisternal segment of trigeminal nerve was automatically calculated with the 3D reconstruction software (Fig. 1). Nerve atrophy was defined when the volume of the nerve was smaller than that of the contralateral nerve. The degree of trigeminal nerve atrophy was calculated as the volume loss of the affected side divided by the volume of the contralateral side, expressed as a percentage. All work were performed by one radiologist blinded to the clinical information and experienced with the software.
Surgical techniques
All patients were operated on by microvascular decompression via a standard suboccipital retrosigmoid approach, which has been described in detail in our previous study [26]. When the NVC was identified, Teflon felt was inserted between the trigeminal nerve and the offending vessel for decompression. Meanwhile, patterns of offending vessels and degree of trigeminal nerve indentation were documented. Due to the lack of a universal approved grade to assess the degree of trigeminal nerve indentation during operation, we used three grades: grade 1 means no or mild indentation, grade 2 means moderate indentation, and grade 3 indicates severe indentation with nerve discoloration and displacement. The grade of trigeminal nerve indentation reflects the severity of neurovascular compression.
Follow-up and outcome assessment
All patients were followed-up at the outpatient department or by telephone for at least 2 years. Surgical outcomes were evaluated based on the Barrow Neurological Institute (BNI) Pain Intensity Scale [21] by an independent neurosurgeon who was blinded to the measurements and intraoperative findings. Clinical outcomes were categorized as complete pain relief (BNI pain score I), partial pain relief (BNI pain score II–III), and failure (BNI pain score IV–V). Short-term outcome was evaluated immediately after the surgery, and long-term outcome was evaluated at follow-up for more than 2 years.
Statistical analyses
Descriptive statistics were used to summarize patient characteristics and clinical features. The volume of the cisternal segment of trigeminal nerve on the affected and nonaffected sides, or patient and control groups were compared using Student’s t test. Correlations between patient characteristics, such as symptom duration, distribution of pain, offending vessel and nerve indentation, and degree of nerve atrophy were calculated using the Spearman correlation test. Uni- and multivariate logistic regression analysis were designed to determine possible prognostic factors of MVD surgery outcomes. Independent variables included sex, age, symptom duration, distribution of pain, offending vessel type, trigeminal nerve indentation, and degree of nerve atrophy. The dependent variable was long-term operative outcomes (1 = complete pain relief without medication [BNI pain score I], 0 = partial pain relief or failure [BNI pain score II–V]). A Kaplan–Meier survival analysis was performed to evaluate the long-term outcome of MVD. Associations were considered statistically significant when P < 0.05. SPSS software was used for statistical analyses (version 19.0).
Results
Demographics and clinical characteristics
A total of 90 study subjects, comprising 60 TN patients and 30 healthy controls, were included in this study. The mean ages of patients with TN and controls were 48.3 ± 9.1 (range, 23–70) and 48.6 ± 9.4 (range 23–69) years; there was no statistical difference in mean age between the two groups (P = 0.43). The female/male ratio was 1.5:1 in both groups. The basic patient characteristics and clinical features are summarized in Table 1.
Surgical findings
In three cases (5%) of the 60 patients with TN, no offending vessel was found during the operation despite a careful and complete exploration of the trigeminal root from the pons to the porus of Meckel’s cave. The superior cerebellar artery (SCA) was identified as the most frequent offending vessel in 38 cases (63.3%), the anterior inferior cerebellar artery (AICA) in ten cases (16.7%), the vertebral artery (VA) in three cases (5%), and the basilar artery (BA) in one case (1.7%). In addition, a vein was found to be the main offending vessel in five cases (8.3%) (Table 1).
According to our established classification, the degree of trigeminal nerve indentation was grade 1 in 13 patients (21.7%), grade 2 in 21 patients (35%), and grade 3 in 26 patients (43.3%) (Table 1).
Trigeminal nerve atrophy
The mean volume of the cisternal segment of trigeminal nerve was 65.8 ± 21.1 mm3 on the affected side, 77.9 ± 19.3 mm3 on the nonaffected side, and 74.7 ± 16.5 mm3 in the control group. The volume of the affected trigeminal nerve was significantly reduced in comparison to that of the nonaffected side (P = 0.001) and controls (P = 0.003). However, there was no statistical difference in mean volume between the nonaffected side nerve of patients with TN and controls (P = 0.62), nor between the right and left sides of the trigeminal nerve in the control subjects (P = 0.34). The loss of volume on the affected side compared with that on the nonaffected side (15.5%) in patients with TN was greater than the difference between the right and left sides in the control group (2.3%).
Correlations between patient characteristics and nerve atrophy
The Spearman correlation test showed that there was a positive correlation (r = 0.46, P = 0.018) between the degree of trigeminal nerve indentation and nerve atrophy. However, other factors, such as age, sex, symptom duration, distribution of pain and type of offending vessel, were not significantly correlated with the trigeminal nerve atrophy.
Correlations between nerve atrophy and surgical outcome
Fifty-two patients (86.7%) achieved complete pain relief without medication (BNI pain score I) immediately after the surgery, six patients (10%) achieved partial pain relief (BNI pain score II–III) postoperatively, and only two patients (3.3%) failed to obtain pain relief (BNI pain score IV–V) immediately after the operation. These patients were followed up from 24 to 30 months (median, 26 months). Two patients were lost to follow-up and were not included in any statistical analyses. At the 2-year follow-up, 45 patients (77.6%) were pain free without medication (BNI pain score I), nine patients (15.5%) suffered from partial pain relief in different degrees (BNI pain score II–III) and four patients (6.9%) failed to obtain pain relief (BNI pain score IV–V). A Kaplan–Meier survival analysis was performed to evaluate the long-term outcome of MVD (Fig. 2).
Univariate logistic regression analysis showed a significant association between the long-term outcome of MVD surgery and type of offending vessels (OR = 2.161, P = 0.017), trigeminal nerve indentation (OR = 3.182, P = 0.023), and degree of nerve atrophy (OR = 2.424, P = 0.031) (Table 2). However, in multivariate logistic regression analysis, only two factors, trigeminal nerve indentation (OR = 2.968, P = 0.022), and degree of nerve atrophy (OR = 1.18, P = 0.035), were associated with the long-term outcome (Table 3).
Discussion
Since Dandy [6] first described the compression of blood vessels on the REZ of trigeminal nerve as the reason for TN, the theory of NVC was widely accepted. However, it was unclear how vascular compression leads to TN, and it was also puzzling why a substantial number of patients with TN did not seem to have any evidence of NVC. This demonstrated that NVC might not necessarily be required for the development of TN in some patients [5]. Jannetta [12] proposed that vascular compression exerted constant pressure on the nerve and thus caused focal demyelination of trigeminal sensory root axons and, thereby, ephaptic “short circuits” that he believed were responsible for the neuralgia. Previous studies [7, 18] examined the ultrastructure of the trigeminal sensory root in patients with TN, and examination revealed a zone of chronic demyelination in the proximal part of the nerve root near its junction with peripheral nerve [18]. These observations suggest that focal demyelination of the trigeminal nerve root is probably involved in the pathogenesis of TN. Trigeminal nerve atrophy ipsilateral to the symptomatic side can be visible both on preoperative MRI and during the operation [10, 23], which is found in most cases of TN and is thought to be a late consequence of the chronic physical stress by vascular compression following demyelination [7]. Atrophic change is one of the signs of nerve damage.
Assessment of nerve atrophy
Many parameters have been developed to estimate the nerve atrophy, including diameter (1D), cross-sectional area (2D), and volume (3D) [20, 14, 10]. With the advances of imaging technology, high-resolution 3D MR sequences, such as 3D time-of-flight (TOF) MR angiography, 3D driven equilibrium (DRIVE) sequence, and 3D T1-weighted gadolinium-enhanced sequences, make it possible to quantitatively analyze the anatomical characteristics of cranial nerves [5]. In the present study, we selected the volume of the cisternal segment of trigeminal nerve to evaluate nerve atrophy, instead of the cross-sectional area at the site of NVC of the nerve. Because the volume may more precisely reflect the general alteration of the trigeminal nerve, the cross-sectional area mainly reflects the extent of indentation of the trigeminal nerve due to the chronic physical compression by offending vessels. Furthermore, NVC is not always visible in some patients though with high-resolution 3D MRI, besides, the site of NVC is often variable throughout the whole length of the nerve. Hence it is difficult to measure the cross-sectional area precisely. All subjects in this study underwent a high-resolution 3-T MRI with 3D DRIVE sequence, so the delineation of fine anatomic structure and quantitative measurement of volume of the trigeminal nerve can be achieved with the use of 3D Slicer software.
Trigeminal nerve atrophy
Our results showed that the volume of the affected trigeminal nerve was significantly reduced in comparison to that of the nonaffected side and controls. However, there was no statistical difference in mean volume between the right and left sides of the trigeminal nerve in the control subjects. The significant volume difference between the affected and nonaffected sides in patients with TN is indicative of trigeminal nerve atrophy. In the majority of cases, the loss of trigeminal nerve tissue is believed to be the result of chronic physical stress by vascular compression. This is supported by the fact that up to 42% of symptomatic nerves have gross atrophy that can be directly visualized during operation [20, 7].
These results are in accordance with previous reports. Leal et al. [16] measured the fraction of anisotropy (FA) and the apparent diffusion coefficient (ADC) of trigeminal nerve in patients with TN and control subjects and found that the trigeminal nerve of the affected side presented extensive loss of FA and an increase in ADC. They also found that the loss of volume and cross-sectional area in affected nerves were associated with loss of FA and increase in ADC [16]. Herweh et al. achieved similar results by studying the trigeminal nerve using the diffusion tensor imaging [11]. These studies suggested that the observed FA reduction might be the late consequence of morphologic changes of the trigeminal nerve caused by chronic mechanical irritation [11, 16]. Furthermore, Park et al. [20] conducted a noncontrolled study in which the mean volume and cross-sectional area of the cisternal segment of the trigeminal nerve were measured in 26 patients with TN. They found that 92.3% of the patients had nerve atrophy on the affected side. Moreover, an ultrastructural analysis of trigeminal root specimens obtained during MVD surgery showed that pathological changes in tissue included axonopathy and axonal loss, demyelination, a range of less severe myelin abnormalities (dysmyelination), residual myelin debris, and the presence of excess collagen. These observations strongly support the contention that vascular compression can damage trigeminal root fibers in patients with TN, and thus causes the atrophic changes of trigeminal nerve [7].
Correlations between patient characteristics and nerve atrophy
Various degrees of severity of vascular compression have been defined and qualified from no contact or mere contact to marked distortion or even severe indentation of the nerve root [24, 2]. In the present study, we defined three grades to assess the degree of trigeminal nerve indentation during the operation. Our results showed that there was a positive correlation between the degree of trigeminal nerve indentation and nerve atrophy. This means that patients with severe indentation of the trigeminal nerve root tend to have a high degree of nerve atrophy. These results are consistent with the observations in previous ultrastructural studies. Devor et al. [7] conducted an ultrastructural analysis of trigeminal root specimens obtained during MVD surgery and suggested that nerve root damage was focal, directly related to vascular compression, and in line with the degree of compression and discoloration seen during operation [7]. Furthermore, Leal et al. [14] measured the volume and cross-sectional area of the trigeminal nerve in patients with TN and also found that the severity of compression was significantly correlated with the mean volume. The ipsilateral TN with NVC grade III had a smaller mean volume than the ipsilateral TN with NVC grade I [14].
Correlations between nerve atrophy and surgical outcome
Our results showed that complete pain relief rate (BNI pain score I) was 86.7% immediately after the surgery, and 77.6% at the 2-year follow-up. This suggests that MVD is an effective and safe method for treating patients with idiopathic TN. However, MVD is not always successful. There are still some patients present with poor surgical outcomes. Failure rates of 15–35% have been reported in the literature [24, 22, 3]. Therefore, the investigation of various factors that may influence the outcome of MVD surgery is of great importance in improving patient selection and treatment strategies.
Univariate logistic regression analysis showed that patients with arterial vascular compression were related to a higher long-term pain relief rate compared with venous or no vascular contact. A previous study also found that a lower success rate was recorded for pure venous compression over arterial compression [24]. However, Dumot et al. concluded that patients with marked venous compression could still achieve the same favorable success rate after MVD [8, 9]. The multivariate logistic regression analysis did not find a relationship between the type of offending vascular and surgical outcome. Furthermore, our results showed that severe trigeminal nerve indentation was associated with good long-term outcomes. Some authors also support the notion that the more severe the degree of compression, the better the results [24, 3]. Sindou et al. [24] quantified the degree of compression at surgery from 1 to 3 and showed that MVD offers a 15-year success rate of 88.1% if the NVC is grade 3, 78.3% if the NVC is grade 2, and only 58.3% if the NVC is grade 1 [24]. The results also demonstrated that greater atrophy of the trigeminal nerve was associated with significantly better long-term outcome. In our opinion, atrophy of the trigeminal nerve seems to be associated with better prognosis, possibly due to its correlation with the degree of vascular compression. Hence, accurate preoperative assessment of the morphologic changes of trigeminal nerve in patients with TN is crucial to predict long-term prognosis and thereby is helpful in choosing the best treatment option.
Conclusions
We conducted a prospective case-control study and the results suggested that morphologic and volumetric changes of the trigeminal nerve in patients with TN can be accurately measured with high-resolution 3D MRI. Our results showed that the volume of the affected trigeminal nerve was significantly reduced in comparison to that of the nonaffected side and controls. In addition, greater nerve atrophy was associated with more severe trigeminal nerve indentation and better long-term outcome following MVD.
Abbreviations
- TN:
-
Trigeminal neuralgia
- MVD:
-
Microvascular decompression
- NVC:
-
Neurovascular conflict
- REZ:
-
Root entry zone
References
Adamczyk M, Bulski T, Sowinska J, Furmanek A, Bekiesinska-Figatowska M (2007) Trigeminal nerve - artery contact in people without trigeminal neuralgia - MR study. Med Sci Monit 13(Suppl 1):38–43
Anderson VC, Berryhill PC, Sandquist MA, Ciaverella DP, Nesbit GM, Burchiel KJ (2006) High-resolution three-dimensional magnetic resonance angiography and three-dimensional spoiled gradient-recalled imaging in the evaluation of neurovascular compression in patients with trigeminal neuralgia: a double-blind pilot study. Neurosurgery 58:666–673
Barker FG 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD (1996) The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 334:1077–1083. doi:10.1056/nejm199604253341701
Bennetto L, Patel NK, Fuller G (2007) Trigeminal neuralgia and its management. BMJ 334:201–205
Burchiel KJ (2016) Trigeminal neuralgia: new evidence for origins and surgical treatment. Neurosurgery 63(Suppl 1):52–55. doi:10.1227/neu.0000000000001276
Dandy W (1934) Concerning the cause of trigeminal neuralgia. Am J Surg 24:447–455
Devor M, Govrin-Lippmann R, Rappaport ZH (2002) Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg 96:532–543. doi:10.3171/jns.2002.96.3.0532
Dumot C, Brinzeu A, Berthiller J, Sindou M (2017) Trigeminal neuralgia due to venous neurovascular conflicts: outcome after microvascular decompression in a series of 55 consecutive patients. Acta Neurochir 159:237–249. doi:10.1007/s00701-016-2994-y
Dumot C, Sindou M (2015) Trigeminal neuralgia due to neurovascular conflicts from venous origin: an anatomical-surgical study (consecutive series of 124 operated cases). Acta Neurochir 157:455–466. doi:10.1007/s00701-014-2330-3
Erbay SH, Bhadelia RA, O’Callaghan M, Gupta P, Riesenburger R, Krackov W, Polak JF (2006) Nerve atrophy in severe trigeminal neuralgia: noninvasive confirmation at MR imaging—initial experience. Radiology 238:689–692. doi:10.1148/radiol.2382042214
Herweh C, Kress B, Rasche D, Tronnier V, Troger J, Sartor K, Stippich C (2007) Loss of anisotropy in trigeminal neuralgia revealed by diffusion tensor imaging. Neurology 68:776–778. doi:10.1212/01.wnl.0000256340.16766.1d
Jannetta PJ (1967) Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg 26(Suppl):159–162. doi:10.3171/jns.1967.26.1part2.0159
Ko AL, Ozpinar A, Lee A, Raslan AM, McCartney S, Burchiel KJ (2015) Long-term efficacy and safety of internal neurolysis for trigeminal neuralgia without neurovascular compression. J Neurosurg 122:1048–1057. doi:10.3171/2014.12.jns14469
Leal PR, Barbier C, Hermier M, Souza MA, Cristino-Filho G, Sindou M (2014) Atrophic changes in the trigeminal nerves of patients with trigeminal neuralgia due to neurovascular compression and their association with the severity of compression and clinical outcomes. J Neurosurg 120:1484–1495. doi:10.3171/2014.2.jns131288
Leal PR, Hermier M, Souza MA, Cristino-Filho G, Froment JC, Sindou M (2011) Visualization of vascular compression of the trigeminal nerve with high-resolution 3T MRI: a prospective study comparing preoperative imaging analysis to surgical findings in 40 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Neurosurgery 69:15–25; discussion 26. doi:10.1227/NEU.0b013e318212bafa
Leal PR, Roch JA, Hermier M, Souza MA, Cristino-Filho G, Sindou M (2011) Structural abnormalities of the trigeminal root revealed by diffusion tensor imaging in patients with trigeminal neuralgia caused by neurovascular compression: a prospective, double-blind, controlled study. Pain 152:2357–2364. doi:10.1016/j.pain.2011.06.029
Love S, Coakham HB (2001) Trigeminal neuralgia: pathology and pathogenesis. Brain 124:2347–2360
Love S, Hilton DA, Coakham HB (1998) Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain pathology (Zurich, Switzerland) 8:1–11 discussion 11-12
Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L (2015) Association between neurovascular contact and clinical characteristics in classical trigeminal neuralgia: a prospective clinical study using 3.0 Tesla MRI. Cephalalgia 35:1077–1084. doi:10.1177/0333102414566819
Park SH, Hwang SK, Lee SH, Park J, Hwang JH, Hamm IS (2009) Nerve atrophy and a small cerebellopontine angle cistern in patients with trigeminal neuralgia. J Neurosurg 110:633–637. doi:10.3171/2008.8.jns08522
Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL (2000) Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the barrow neurological institute. Int J Radiat Oncol Biol Phys 47:1013–1019
Sarsam Z, Garcia-Finana M, Nurmikko TJ, Varma TR, Eldridge P (2010) The long-term outcome of microvascular decompression for trigeminal neuralgia. Br J Neurosurg 24:18–25. doi:10.3109/02688690903370289
Sindou M, Howeidy T, Acevedo G (2002) Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir 144:1–12; discussion 12-13. doi:10.1007/s007010200000
Sindou M, Leston J, Decullier E, Chapuis F (2007) Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg 107:1144–1153. doi:10.3171/jns-07/12/1144
Society HCCotIH (2004) The international classification of headache disorders: 2nd edition. Cephalalgia 24(Suppl 1):9–160
Zhang H, Lei D, You C, Mao BY, Wu B, Fang Y (2013) The long-term outcome predictors of pure microvascular decompression for primary trigeminal neuralgia. World Neurosurg 79:756–762. doi:10.1016/j.wneu.2012.01.040
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Sichuan Science and Technology Support Program (No. 2015SZ0188).
Conflict of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cheng, J., Meng, J., Liu, W. et al. Nerve atrophy in trigeminal neuralgia due to neurovascular compression and its association with surgical outcomes after microvascular decompression. Acta Neurochir 159, 1699–1705 (2017). https://doi.org/10.1007/s00701-017-3250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3250-9