Abstract
The organization of ecological communities along local environmental gradients provides important information about how such communities may respond to environmental change. In the Arctic, the importance of gradients in shrub cover and soil moisture for non-marine arthropod communities has been clearly demonstrated. By replicating studies along shrub and moisture gradients at multiple elevations and using space-for-time substitution, it is possible to examine how arthropod communities may respond to future environmental change. We collected and identified 4640 adult specimens of spiders and beetles near Narsarsuaq, South Greenland between 8 July and 25 August, 2014 from 112 pitfall traps. The traps were arranged in eight plots covering local gradients in either soil moisture or tall shrub dominance at both low and high elevation. Multivariate generalized linear models revealed that community composition was significantly related to shrub height and soil moisture, and that this relationship varied between low and high elevation. Among the 46 species we found, more species were unique to the high elevation plots than to the low elevation plots, a finding that was most pronounced for spiders in plots along soil moisture gradients. Indicator species analysis corroborated earlier findings of the indicator value of specific species in Greenland and suggested that beetles may serve as better indicators of specific habitats than spiders. The location of plots along local environmental gradients allowed us to detect fine-scale variation in arthropod communities. Together, our results suggest that Arctic arthropod community responses to environmental change may differ among low and high elevation sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how and why ecological communities vary in space and time is a central challenge in ecology (Morin 2011). Paleoclimate and past glaciation events are clearly important for the broad–scale distribution of high latitude species (Normand et al. 2013; Svenning et al. 2015). Yet, the organization of ecological communities across local environmental gradients provides important additional information about how such communities may respond to future environmental change. In Arctic tundra, variation in shrub cover, soil moisture and temperature are important environmental gradients for non-marine arthropods, which make up the bulk of species richness (Hodkinson et al. 2013).
In the Arctic, increasing temperatures are changing soil moisture levels and shrub growth and dominance. Satellite imagery has revealed a widespread decrease in the number of lakes in Arctic Siberia linked to thawing permafrost (Smith et al. 2005; Wrona et al. 2016). At higher latitudes, warming may increase wetland areas due to shallow active layers and limited soil drainage, while in areas with deeper active layers, warming may lead to drying of top soils (Avis et al. 2011). Such changes may have profound effects on Arctic biota including plants (Elmendorf et al. 2012) and animals (Høye et al. 2013; Park 2017). Similarly, increased shrub growth (Myers-Smith et al. 2011) may change habitats for both vertebrates (Ehrich et al. 2012; Ims and Henden 2012; Boelman et al. 2015; Tape et al. 2016; Wheeler et al. 2017) and invertebrates (Rich et al. 2013; Sweet et al. 2015; Hansen et al. 2016a; b).
Soil moisture and cover of tall shrubs vary across Arctic climate zones and in relation to local topographic variation (Myers-Smith et al. 2011; Wrona et al. 2016). Following the premise of space-for-time substitution, local gradients in shrub dominance can be used to mimic the likely encroachment of dwarf shrub heath by taller woody species due to climate change. Indeed, such variation in shrub cover has been exploited to assess the effects of varying shrub dominance on arthropod abundance and family level diversity (Rich et al. 2013) and biomass (Sweet et al. 2015). Variation in soil moisture from fens toward drier soils with dwarf shrub heath could also provide a basis for predicting how e.g. arthropod communities may respond to altered hydrology as a consequence of climate change. In a first attempt to examine how local-scale variation in shrub cover and soil moisture determines arthropod communities in the Arctic, Hansen et al. (2016b) sampled spiders and beetles inside and outside shrub and fen patches near Nuuk in West Greenland. Communities varied across local habitat transitions although the magnitude of the variation differed among sampled sites (Hansen et al. 2016b). In the Low-Arctic and the Sub-Arctic, soil drainage is better, temperatures are higher, and in sheltered areas shrubs grow taller than in the High-Arctic (Avis et al. 2011; Damgaard et al. 2016). The transition zone between the Sub-Arctic and the Low-Arctic could serve as a particularly relevant place for space-for-time studies of the effects of soil moisture and shrub dominance on arthropod communities.
Temperature variation along elevational gradients can be used as a surrogate for climate-related environmental filtering of arthropod communities (Hodkinson 2005; Hodkinson and Jackson 2005; Pizzolotto et al. 2016). While declining species richness with elevation is commonly observed (e.g. Bowden and Buddle 2010b), mid-elevational peaks in species richness have also been observed and the available regional species pool as well as habitat and microclimatic diversity may shape the exact configuration of communities at a given elevation (Hodkinson 2005; Peters et al. 2016). Hence, the species pool available to inhabit local gradients in shrub dominance and soil moisture will likely differ between low elevation and high elevation sites. The strength of local environmental gradients in e.g. soil moisture and vegetation structure may also vary between benign conditions at low elevation and the harsher conditions at high elevation (Hodkinson 2005).
Spiders and beetles are known to respond to a wide variety of environmental parameters (Wheater et al. 2000) and have been widely recommended as bioindicators (Pearce and Venier 2006). Better spatial replication of arthropod sampling and local measurements of critical environmental factors, e.g. soil moisture and vegetation height, would therefore add substantial new information to the study of Arctic arthropod community responses to future climate change. This paper investigates the importance of local gradients in soil moisture and vegetation height for the structure of spider and beetle communities in South Greenland and whether the importance may vary with elevation in plots representing Sub-Arctic and Low-Arctic conditions, respectively. Due to the specific geography in Greenland with a limited area with Sub-Arctic conditions surrounded by the Atlantic Ocean, we predict that high elevation communities are subsets of low elevation communities. We also identify potential indicator species, which can serve as sensitive biological indicators of future environmental changes in Arctic ecosystems. As such, the study can be seen as a baseline for future Arctic biodiversity assessments (Hodkinson et al. 2013; Høye and Sikes 2013; Post and Høye 2013).
Materials and methods
Study area and data
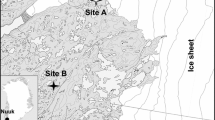
Spiders and beetles were collected with uncovered pitfall traps during the period 8 July to 25 August 2014 arranged in eight plots near Narsarsuaq, South Greenland (61.16°N, 45.40°W, Fig. 1). We used yellow pitfall traps, because they combine the benefits of pitfall and pan traps in low stature vegetation (Ernst et al. 2016). The traps were 10 cm in diameter and contained a 50% propylene glycol:water mixture as preservative and a few drops of detergent to break the surface tension of the trapping liquid. Four plots (fen plots) were established to cover local gradients in soil moisture and four plots (shrub plots) were established to cover local gradients in cover of Salix glauca, which is the dominant tall shrub species of the area (Fig. 1). Two fen plots (LF1 and LF2) and two shrub plots (LS1 and LS2) were located at 50 m above sea level and two fen plots (HF1 and HF2) and two shrub plots (HS1 and HS2) were located at about 450 m above sea level. Besides mosses, the fens were dominated by Betula glandulosa and different species of Carex with higher dominance of lichens at high elevation. The heaths had a higher cover of dwarf shrubs like Vaccinium uliginosum and Empetrum hermaphroditum and lichens and the shrub patches had an almost complete cover of Salix glauca with occasional herb species like Campanula rotundifolia.
Map of the study area. The light and dark grey dots indicate the location of fen (HF) and shrub (HS) plots at high elevation, respectively. The white and black dots indicate the location of the fen (LF) and shrub (LS) plots at low elevation, respectively. The airport at Narsarsuaq is marked with a white square. The interval between contour lines is 100 m. The inset indicates where the study area is located in Greenland
Fen plots were 5 m × 40 m and consisted of 18 pitfall traps arranged in two rows five meters apart with nine pitfall traps five meters apart in each row (Fig. 2). The two traps in the middle of each row were placed in the transition between fen and dwarf shrub heath with eight traps on one side placed in the fen and eight traps on the other side placed in the heath (Fig. 2). Shrub plots were 5 m × 20 m and consisted of 10 pitfall traps arranged in two rows five meters apart with five pitfall traps five meters apart in each row. Similar to the fen plots, the two traps in the middle of each row in shrub plots were placed in the transition between Salix glauca shrub patches and dwarf shrub heath with four traps on one side placed in shrub habitat and four traps on the other side placed in heath (Fig. 2). The limited size of shrub patches at our site did not allow us to make shrub plots as large as fen plots. Next to each pitfall trap, a 1 × 1 m vegetation subplot was established (Fig. 2).
Plot design for fen and shrub plots. Each dot is a pitfall trap and fen plots consisted of 18 pitfall traps while shrub plots consisted of 10 pitfall traps. Fen plots are extended 10 m (four pitfall traps) at both ends relative to shrub plots. Each plot consists of two lines of pitfall traps and associated vegetation subplots perpendicular to the gradient of soil moisture and cover of Salix glauca in fen and shrub plots, respectively. The two traps in the middle of each plot are regarded as the transition section
The height of the vegetation was recorded to the nearest cm as the mean of nine measurements of vegetation height arranged in a 1 × 1 m cross in each vegetation subplot. Measurements of vegetation height were carried out at peak growing season in July 2016. Soil moisture was measured as volumetric water content at 12 cm depth on a weekly basis between 8 July and 25 August during the 2015 and 2016 growing seasons by taking three measurements in each subplot using a FieldScout TDR 300 Soil Moisture Meter from Spectrum Technologies, Inc. Unfortunately, measurements of vegetation height and soil moisture were missing for one high elevation fen plot (HF2). We compared vegetation height and soil moisture using a general linear model with each subplot as the observational unit. For soil moisture, we used the mean soil moisture across weekly measurements in both years for each subplot as the observational unit. Within each plot, we used a group variable to classify subplots on either side of the transition and omitted subplots in the transition. As predictor variables, we used group, plot and elevation and ran one model for vegetation height using data from shrub plots and one model for soil moisture using data from fen plots.
For this study, a total of 112 pitfall traps were in operation for a total of 5040 trap days. Adult spiders and beetles were identified using a stereo microscope and the recent key to spiders and insects from Greenland (Böcher et al. 2015), cross-referencing Marusik et al. (2006) and Spiders of Europe at: http://www.araneae.unibe.ch. Specimens are preserved in 75% ethanol and stored at the Natural History Museum Aarhus, Denmark. The dataset is available through the Global Biodiversity Information Facility (http://doi.org/10.15468/kjwngr).
Species diversity
We used Venn diagrams to summarize how many species of spiders and beetles were found at high and low elevation for fen and shrub plots separately. Venn diagrams offer an intuitive solution to visualize the number of unique and shared species among groups of habitats or sites (Micallef and Rodgers 2014). Species diversity was calculated using abundance-based as well as incidence-based data. For some groups of arthropods, e.g. ants, incidence-based diversity estimates are recommended due to the clumped distribution of individual species, while for other groups, e.g. spiders, abundance-based diversity estimates may offer the best solution (Hsieh et al. 2016). For this reason, we opted to repeat our diversity analyses using both incidence and abundance-based estimation. We calculated diversity using Hill-numbers: 0 = species richness, 1 = Shannon diversity, and 2 = Simpson diversity with the R package iNEXT (Chao et al. 2014; Hsieh et al. 2016) by each plot type at each elevation (i.e. by pooling the data in four groups, HF, HS, LF and LS). We compared samples at the same level of sample completeness i.e. the proportion of species in the community, which is represented in the sample. Since sample completeness was very high for both abundance-based and incidence-based estimation (>0.94 in all cases), we used the level of sample completeness equal to the least well-sampled group (Chao et al. 2014).
Indicator species
We performed indicator species analysis (Dufrene and Legendre 1997) separately for high and low elevation plots based on the abundance of each species in each of the four habitat types: shrub, heath, fen as well as the transition sections (Fig. 2). We used the multipatt function in the R package indicspecies and included site group combinations to allow species to indicate more than one habitat (De Caceres et al. 2010, 2012). The multipatt function calculates a specificity value ‘A’ (range 0–1), which indicates the conditional probability that a trap belongs to a certain habitat given that the species has been found in the trap, as well as a sensitivity value ‘B’ (0–1), which indicates how many of the traps in a certain habitat the target species has been found in. Significance of the relationship between species and habitat was based on permutation tests using 9999 random permutations to estimate p-values.
Community composition
To analyse variation in community composition, we employed latent variable modelling, a Bayesian model-based approach which explains community composition through a set of underlying latent variables to account for residual correlation among species, for example due to biotic interaction (Warton et al. 2012). This method offers the possibility to adjust the distribution family to e.g. “poisson” or “negative binomial” to account for over-dispersion in data without confounding location with dispersion (Hui et al. 2015). We used the R package boral (Hui 2016) to perform model-based unconstrained ordination (Hui et al. 2015). We modelled combined spider and beetle communities with two latent variables and poisson distribution to visualize the clustering of arthropod communities across elevation and plot types. We scaled symbol size according to soil moisture level and vegetation height. To examine if a potential role of soil moisture and vegetation height interacted with each other and with elevation we constructed a multivariate generalized linear model using the manyglm function in the R package mvabund (Wang et al. 2012). We included all two-way interactions in the model structure and used a negative binomial distribution based on inspection of residual plots. All analyses were done in R version 3.2.5.
Results
Across the study period, our sampling resulted in the capture of 8177 spiders and 549 beetles. Among these, 4091 adult spiders and all beetles were identified to species level and all species were previously known from Greenland (Böcher et al. 2015; Hansen et al. 2016a, b). Juvenile spiders were not used in any analysis. The spiders were distributed across 35 species belonging to 11 families. The three most abundant species in the traps, the wolf spiders Pardosa furcifera and Pardosa hyperborea (Lycosidae) and the crab spider Xysticus durus (Thomisidae), were abundant at both low and high elevation. A total of 12 of the 35 species collected were only represented by one or two specimens (Table 1). Among the beetles, 11 species were found across six families. The three most abundant species in the traps were the weevil Otiorhynchus nodosus (Curculionidae), the ground beetle Patrobus septentrionis (Carabidae) and the ladybird beetle Coccinella transversoguttata (Coccinellidae). The first two species were most abundant at low elevation, whereas the latter was most abundant at high elevation. Three beetle species were only represented by a single specimen (Table 1).
Soil moisture was higher in the fen section compared to the heath section of fen plots, but we found no differences among plots or between high and low elevation (ANOVA, βfen = 23.4 ± 1.54, F1,46 = 230.8, p < 0.0001, Fig. 3a). Similarly, the vegetation was higher in the shrub section compared to the heath section of shrub plots with no effect of elevation or plot (ANOVA, βshrub = 36.7 ± 4.33, F1,30 = 71.71, p < 0.0001, Fig. 3b).
The measured variation in soil moisture (volumetric water content in %) and vegetation height (in cm) in fen (a) and shrub (b) plots presented as box plots for each position along the local environmental gradient, respectively (see Fig. 2). Soil moisture measurements were taken weekly between 8 July and 25 August in 2015 and 2016. We used the mean soil moisture across weekly measurements in both years in each subplot as observational unit. Vegetation height measurements were taken at peak growing season in 2016. Dots indicate observations falling outside 1.5 times the interquartile range
Among the 26 spider species found in shrub plots, seven species were only found at high elevation. Similarly, among the 29 spider species found in fen plots, 11 species were only found at high elevation. In contrast, all beetle species found at high elevation, except the water beetle Hydroporus morio (Dytiscidae) represented by a single specimen, were also found at low elevation (Fig. 4). Total species richness was higher for fen plots at high elevation than for fen plots at low elevation and shrub plots at both high and low elevation using both abundance-based and incidence-based data (Fig. 5a, b). However, for the incidence-based data, low elevation shrub plots showed intermediate levels of species richness (Fig. 5a). There were no differences among plot types for Simpson diversity nor for Shannon diversity using incidence-based data (Fig. 5c, d, e). For abundance-based data, Shannon diversity was significantly higher for high elevation plots of each type (shrub or fen) relative to low elevation plots (Fig. 5f).
The species richness (a, b), Shannon diversity (c, d) and Simpson diversity (e, f) for each of the four plot types high elevation fen (HF) and shrub plots (HS) and low elevation fen (LF) and shrub (LS) plots for both incidence-based (a, c, e) and abundance-based (b, d, f) data. The effective species number is given ± 95% confidence intervals by comparing samples at the level of sample completeness equal to the least well-sampled plot type. Plot types sharing the same letter at the bottom of each panel were not significantly different. Plot types were not significantly different in panels without letters
Among spiders, we found six significant indicator species, which indicated shrub habitats or shrub habitats in combination with other habitats. Four species were significant indicators at either high or low elevation and two species, Hybauchenidium gibbosum and Improphantes complicatus, were significant indicator species at both low and high elevation. Arctosa insignita indicated fens at low elevation and Wabasso quaestio indicated fens at high elevation, while Hahnia glacialis and Agyneta nigripes indicated transition sections although both species were found in low numbers (Table 1). Four of the twelve species of beetles were significant indicator species. At low elevation, these four species were indicators for transition sections in combination with either heath sections (Byrrhus fasciatus and C. transversoguttata), or shrub and fen sections (P. septentrionis), or shrub and heath sections (O. nodosus). At high elevation, P. septentrionis was also an indicator of shrub sections (Table 1).
The model-based unconstrained ordination showed clear separation of communities of spiders and beetles at both low and high elevation, with some separation within each elevation of plots varying in soil moisture and vegetation height (Fig. 6). The multivariate generalized linear models revealed significant effects of soil moisture and vegetation height, as well as elevation. In addition, the interaction between soil moisture and vegetation height, between soil moisture and elevation and between vegetation height and elevation were significant (Table 2). Thus, the arthropod community response to the local gradients in shrub dominance and soil moisture differed between low elevation and high elevation plots.
Latent variable modelling of spider and beetle communities from all 112 traps. Data from fen (HF) and shrub (HS) plots at high elevation are indicated by light and dark grey dots, respectively. Data from fen (LF) and shrub (LS) plots at low elevation are indicated by white and black dots, respectively. The circle sizes represent soil moisture levels (a) and vegetation height (b). Traps with missing data are indicated by crosses (×)
Discussion
In this study, we assessed how elevation can modulate the structure of non-marine Arctic arthropod communities at sites along local environmental gradients. Our results confirm that local gradients in soil moisture and tall shrub dominance are important factors determining the structure of beetle and spider communities. By using the same number of pitfall traps in the same number of plots covering local environmental gradients, our results offer an equal effort comparison, which can be repeated to track the consequences of climate change as has recently been recommended by Ernst and Buddle (2015). Our results highlight that elevational variation is an important additional factor to consider along with soil moisture and vegetation height which were identified in a study from Godthåbsfjorden about 1000 km further north in West Greenland (Hansen et al. 2016b). We measured two central variables (soil moisture and vegetation height), but other environmental factors, such as temperature and precipitation, typically vary with elevation and could also be important for arthropods (Hodkinson 2005). Since the community response to the local environmental gradients varied among elevations, communities at different elevations are also likely to change at different rates in response to ongoing global warming. Therefore, we recommend that such local gradients in soil moisture and vegetation height should be replicated along an elevational gradient in long-term monitoring of Arctic arthropod responses to climate change.
We had expected to find higher species diversity at low elevation than at high elevation. In contrast to previous studies from Canada (Bowden and Buddle 2010b) and in an alpine region of Norway (Hein et al. 2014), we found limited evidence of nestedness in spider communities i.e. that high elevation communities consisted of a subset of the species found in lower elevation communities. In fact, there were as many or more spider species unique to our high elevation shrub and fen plots, respectively. We cannot rule out that phenological differences between low and high elevation could have affected our results. For instance, if early season species had ceased to be active at our low elevation plots before we started sampling, we could have found a different pattern by starting our sampling even earlier. Further studies could explore this possibility, but we consider it an unlikely explanation, since we already started our sampling in early July and thus covered most of the growing season. Among beetles, we found a clearer pattern of nestedness, except that three of the ten beetle species found were represented by a single specimen. There is little published literature on elevational variation in high latitude beetle communities, but a gradual decrease in species richness with latitude has been documented across the Canadian Arctic (Ernst and Buddle 2015) and the Arctic in general (Chernov et al. 2014). Sub-Arctic conditions found at low elevation at our site are confined to a small area of South Greenland. Further studies at a broader range of sites in Greenland or at the same sites across multiple years are needed to identify species that may benefit from climate change related expansion of Sub-Arctic conditions to areas at higher elevation.
Higher vegetation structure could offer additional niches and therefore host higher diversity of spider species (Avila et al. 2017; Bowden and Buddle 2010a) or arthropods in general (Rich et al. 2013). While spider and beetle communities varied along the local gradients in soil moisture and tall shrub dominance, we found no evidence of differences in diversity among plot types. Generally, our diversity estimates based on abundance-based and incidence-based data were in agreement. Only high elevation fen plots had higher species richness than other plot types. High elevation plots also had higher Simpson diversity than low elevation plot, when fen plots and shrub plots were compared, although this was only the case when using abundance-based data. In contrast, Bowden and Buddle (2010a) found higher spider species richness in sites with higher vegetation structure across a latitudinal gradient in Arctic Canada. Arthropod species richness and diversity were significantly greater in shrub than open tundra in Alaska although with some differences among sites (Rich et al. 2013). In West Greenland, arthropod diversity in heath, fen and shrub habitats differed significantly, with fen habitats being the least diverse (Hansen et al. 2016b). We note that about one-third of the species we found were represented by one or two specimens, which could suggest that, despite a total of almost 5000 individuals across more than 5000 trap days and 112 different traps as well as our efforts to account for variation in sample completeness, our estimates of habitat-specific variation in richness should be regarded as preliminary.
Our study provides new information on the indicator value of arthropods for tundra habitats. The biology of the spider species H. gibbosum is not well known for Greenland (Böcher et al. 2015), yet our results identify this species as indicator of shrub habitats. Given its high abundance at our site, it will be straightforward to gain further knowledge about its phenology and specific habitat requirements. The wolf spider A. insignita was an indicator species of fens, which is in line with previous descriptions of the species’ biology (Böcher et al. 2015) and what has been found on Belcher Islands, Hudson Bay, Canada (Koponen 1992). Arctosa insignita appeared to be widely distributed across habitats at both sea level and at 600–700 m above sea level at study sites near Godthåbsfjorden further north in Greenland. Another spider species W. quaestio, which in our study was an indicator species for fens at high elevation, was only found in fen habitats at sea level near Godthåbsfjorden (Hansen et al. 2016a b). Since they are likely to be sensitive to future changes in precipitation patterns and associated soil moisture levels. Arctosa insignita and W. quaestio are therefore relevant indicator species in a climate change context.
Among the beetles, C. tranversoguttata and B. fasciatus have been described as eurytopic and highly mobile species with fully developed wings, but also with a preference for dry heath (Böcher et al. 2015). Coccinella transversoguttata was also a significant indicator species for heath habitat near Godthåbsfjorden (Hansen et al. 2016a, b). Otiorhynchus nodosus has been described as highly eurytopic with a wide range of host plants in both adult and larval stages and a preference to moist areas (Böcher et al. 2015). It was not found at high elevation sites near Godthåbsfjorden, and although it was present at sea level near Godthåbsfjorden, it was not a significant indicator species at this more northerly site. In our study, it was only an indicator species at low elevation. This species lacks hind wings which makes it much less mobile than C. transversoguttata (Böcher et al. 2015). The association of the ground beetle P. septentrionis with shrub habitat fits with the previous analysis of Hansen et al. (2016b) who identified this species as indicator of shrub and fen habitats at sea level in Godthåbsfjorden. According to Böcher et al. (2015), this species has been found in moist areas, ranging from beaches and lake shores to grassland and lush shrub.
Species may be more restricted in their habitat association towards the margins of their ranges (Strathdee and Bale 1998). Although the exact distributional ranges of most of the species we found are not well known, a large number of the insect and spider species known from Greenland are confined to the northern parts of Greenland (Böcher et al. 2015). Hence, even widespread species may have colonized North Greenland before South Greenland from Canada, Iceland or Svalbard. South Greenland is surrounded by the Atlantic Ocean and Sub-Arctic conditions like those found at our low elevation site are restricted to small areas in the inner fiords of South Greenland. Three beetle species and two spider species were only habitat indicators at low elevation. Despite that all five species were found at sea level near Godthåbsfjorden, only two of these species were also significant indicator species at this more northerly site. This suggests that insect species may generally exhibit tighter habitat associations at our southerly site compared to more northerly sites in Greenland. The abundance of such indicator species may thus be relevant biological indicators of future environmental change. Our indicator species analysis was limited by the low number of specimens of many of the species. Twelve species of spiders and three species of beetles were represented by only one or two specimens. This emphasizes the importance of plot replication, even in relatively species poor regions like the Arctic. Further studies based on large number of traps across several seasons may be necessary to confirm the indicator status of specific species.
The direction and magnitude of future changes in soil moisture levels and vegetation height in the Arctic are uncertain (Phoenix and Bjerke 2016; Rawlins et al. 2010). Although there is a general expectation of increased shrub growth, long-term observations from paired vegetation plots subject to sheep grazing and fenced off to prevent sheep grazing have so far shown little evidence for shrub encroachment in South Greenland (Damgaard et al. 2016). Dendroecological studies of shrub growth in South Greenland also show no relationship between climate and shrub growth (Myers-Smith et al. 2015). Most studies on shrubification are, however, limited to a small number of sites across the Arctic and the understanding of local variation in shrubification is limited (Young et al. 2016). Long-term data on soil moisture have coarse spatial resolution, but with our direct measurements of soil moisture at each pitfall trap, we have provided a basis for future comparison. This will also allow us to assess possible links between changes in soil moisture and rates of shrub growth (Ackerman et al. 2017).
We consider this study as a thorough baseline for further studies on how Arctic arthropods will respond to changes in climate and habitat characteristics. As hypothesized, habitat type and elevation were significant factors determining species assemblages in this area. We have established a clear link between local environmental factors and arthropod communities and recommend that soil moisture and vegetation height are measured in future studies of arthropod community variation in space and time at other Arctic sites. Further studies will allow us to track the temporal dynamics of specific indicator species in response to climate change. We would also recommend that other taxonomic groups are studied from similar plot designs to identify indicator species among e.g. moths, wasps and flies. Such studies will help develop a more detailed understanding of how Arctic arthropod communities are affected by environmental factors and how functional traits like e.g. mobility and generation time affect their spatio-temporal dynamics and response to environmental change.
References
Ackerman D, Griffin D, Hobbie SE, Finlay JC (2017) Arctic shrub growth trajectories differ across soil moisture levels. Glob Change Biol. doi:10.1111/gcb.13677
Avila AC, Stenert C, Rodrigues ENL, Maltchik L (2017) Habitat structure determines spider diversity in highland ponds. Ecol Res 32:359–367. doi:10.1007/s11284-017-1442-7
Avis CA, Weaver AJ, Meissner KJ (2011) Reduction in areal extent of high-latitude wetlands in response to permafrost thaw. Nat Geosci 4:444–448. doi:10.1038/Ngeo1160
Böcher J, Kristensen NP, Pape T, Vilhelmsen L (2015) The Greenland entomofauna: an Identification manual of insects, spiders and their allies. BRILL, Fauna Entomol Scand, p 898
Boelman NT et al (2015) Greater shrub dominance alters breeding habitat and food resources for migratory songbirds in Alaskan arctic tundra. Glob Change Biol 21:1508–1520. doi:10.1111/gcb.12761
Bowden JJ, Buddle CM (2010a) Determinants of ground-dwelling spider assemblages at a regional scale in the Yukon Territory, Canada. Ecoscience 17:287–297. doi:10.2980/17-3-3308
Bowden JJ, Buddle CM (2010b) Spider assemblages across elevational and latitudinal gradients in the Yukon Territory, Canada. Arctic 63:261–272
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. doi:10.1890/13-0133.1
Chernov YI, Makarova OL, Penev LD, Khruleva OA (2014) The Beetles (Insecta, Coleoptera) in the Arctic Fauna. Communication 1. Faunal Composition. Zool Zhurnal 93:7–44. doi:10.7868/S004451341401005x
Damgaard C, Raundrup K, Aastrup P, Langen PL, Feilberg J, Nabe-Nielsen J (2016) Arctic resilience: no evidence of vegetation change in response to grazing and climate changes in South Greenland. Arct Antarct Alp Res 48:531–549. doi:10.1657/Aaar0016-005
De Caceres M, Legendre P, Moretti M (2010) Improving indicator species analysis by combining groups of sites. Oikos 119:1674–1684. doi:10.1111/j.1600-0706.2010.18334.x
De Caceres M, Legendre P, Wiser SK, Brotons L (2012) Using species combinations in indicator value analyses. Methods Ecol Evol 3:973–982. doi:10.1111/j.2041-210X.2012.00246.x
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. doi:10.1890/0012-9615(1997)067[0345:Saaist]2.0.Co;2
Ehrich D et al (2012) The importance of willow thickets for ptarmigan and hares in shrub tundra: the more the better? Oecologia 168:141–151. doi:10.1007/s00442-011-2059-0
Elmendorf SC et al (2012) Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nature Clim Change 2:453–457. doi:10.1038/Nclimate1465
Ernst CM, Buddle CM (2015) Drivers and patterns of ground-dwelling beetle biodiversity across Northern Canada. PLos ONE 10:ARTN e0122163. doi:10.1371/journal.pone.0122163
Ernst CM, Loboda S, Buddle CM (2016) Capturing northern biodiversity: diversity of arctic, subarctic and north boreal beetles and spiders are affected by trap type and habitat. Insect Conserv Diver 9:63–73. doi:10.1111/icad.12143
Hansen RR, Hansen OLP, Bowden JJ, Normand S, Bay C, Sørensen JG, Høye TT (2016a) High spatial variation in terrestrial arthropod species diversity and composition near the Greenland ice cap. Polar Biol. doi:10.1007/s00300-016-1893-2
Hansen RR, Hansen OLP, Bowden JJ, Treier UA, Normand S, Høye TT (2016b) Meter scale variation in shrub dominance and soil moisture structure Arctic arthropod communities. Peerj 4:ARTN e2224. doi:10.7717/peerj.2224
Hein N, Feilhauer H, Finch OD, Schmidtlein S, Loffler J (2014) Snow cover determines the ecology and biogeography of spiders (Araneae) in alpine tundra ecosystems. Erdkunde 68:157–172. doi:10.3112/erdkunde.2014.03.01
Hodkinson ID (2005) Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev 80:489–513. doi:10.1017/S1464793105006767
Hodkinson ID et al. (2013) Terrestrial and freshwater invertebrates. In: Meltofte H (ed) Arctic biodiversity assessment. Status and trends in arctic biodiversity. Conservation of Arctic Flora and Fauna, Akureyri, pp 246–276
Hodkinson ID, Jackson JK (2005) Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ Manag 35:649–666
Høye TT, Sikes DS (2013) Arctic entomology in the 21st century. Can Entomol 145:125–130. doi:10.4039/Tce.2013.14
Høye TT, Post E, Schmidt NM, Trøjelsgaard K, Forchhammer MC (2013) Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nat Clim Change 3:759–763. doi:10.1038/Nclimate1909
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. doi:10.1111/2041-210x.12613
Hui FKC (2016) Boral—Bayesian ordination and regression analysis of multivariate abundance data in R. Methods Ecol Evol 7:744–750. doi:10.1111/2041-210x.12514
Hui FKC, Taskinen S, Pledger S, Foster SD, Warton DI (2015) Model-based approaches to unconstrained ordination. Methods Ecol Evol 6:399–411. doi:10.1111/2041-210x.12236
Ims RA, Henden JA (2012) Collapse of an arctic bird community resulting from ungulate-induced loss of erect shrubs. Biol Conserv 149:2–5. doi:10.1016/j.biocon.2012.02.008
Koponen S (1992) Spider Fauna (Araneae) of the Low Arctic Belcher-Islands, Hudson-Bay. Arctic 45:358–362
Marusik YM, Böcher J, Koponen S (2006) The collection of Greenland spiders (Aranei) kept in the Zoological Museum, University of Copenhagen. Arthropoda Selecta 15:59–80
Micallef L, Rodgers P (2014) eulerAPE: drawing area-proportional 3-venn diagrams using ellipses. PLos ONE 9:ARTN e101717. doi:10.1371/journal.pone.0101717
Morin PJ (2011) Community Ecology. 2nd edn. Wiley-Blackwell, West Sussex, p. 407
Myers-Smith IH et al (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6:045509. doi:10.1088/1748-9326/6/4/045509
Myers-Smith IH et al (2015) Climate sensitivity of shrub growth across the tundra biome. Nat Clim Change 5:887–891. doi:10.1038/Nclimate2697
Normand S et al (2013) A greener Greenland? Climatic potential and long-term constraints on future expansions of trees and shrubs. Phil Trans R Soc B 368:20120479. doi:10.1098/Rstb.2012.0479
Park JS (2017) A race against time: habitat alteration by snow geese prunes the seasonal sequence of mosquito emergence in a subarctic brackish landscape. Polar Biol 40:553–561
Pearce JL, Venier LA (2006) The use of ground beetles (Coleoptera : Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: a review. Ecol Ind 6:780–793. doi:10.1016/j.ecolind.2005.03.005
Peters MK et al (2016) Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat Comm 7:ARTN 13736. doi:10.1038/ncomms13736
Phoenix GK, Bjerke JW (2016) Arctic browning: extreme events and trends reversing arctic greening. Glob Change Biol 22:2960–2962. doi:10.1111/gcb.13261
Pizzolotto R, Albertini A, Gobbi M, Brandmayr P (2016) Habitat diversity analysis along an altitudinal sequence of alpine habitats: the Carabid beetle assemblages as a study model. Period Biol 118:241. doi:10.18054/pb.2016.118.3.3924
Post E, Høye TT (2013) Advancing the long view of ecological change in tundra systems. Phil Trans R Soc B 368:20120477. doi:10.1098/Rstb.2012.0477
Rawlins MA et al (2010) Analysis of the Arctic system for freshwater cycle intensification: observations and expectations. J Clim 23:5715–5737. doi:10.1175/2010jcli3421.1
Rich ME, Gough L, Boelman NT (2013) Arctic arthropod assemblages in habitats of differing shrub dominance. Ecography 36:994–1003. doi:10.1111/j.1600-0587.2012.00078.x
Smith LC, Sheng Y, MacDonald GM, Hinzman LD (2005) Disappearing Arctic lakes. Science 308:1429. doi:10.1126/science.1108142
Strathdee AT, Bale JS (1998) Life on the edge: insect ecology in arctic environments. Annu Rev Entomol 43:85–106
Svenning JC, Eiserhardt WL, Normand S, Ordonez A, Sandel B (2015) The influence of paleoclimate on present-day patterns in biodiversity and ecosystems. Annu Rev Ecol Evol Syst 46:551–572. doi:10.1146/annurev-ecolsys-112414-054.314
Sweet SK, Asmus A, Rich ME, Wingfield J, Gough L, Boelman NT (2015) NDVI as a predictor of canopy arthropod biomass in the Alaskan arctic tundra. Ecol Appl 25:779–790
Tape KD, Christie K, Carroll G, O’Donnell JA (2016) Novel wildlife in the Arctic: the influence of changing riparian ecosystems and shrub habitat expansion on snowshoe hares. Glob Change Biol 22:208–219. doi:10.1111/gcb.13058
Wang Y, Naumann U, Wright ST, Warton DI (2012) mvabund- an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474. doi:10.1111/j.2041-210X.2012.00190.x
Warton DI, Wright ST, Wang Y (2012) Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol 3:89–101. doi:10.1111/j.2041-210X.2011.00127.x
Wheater CP, Cullen WR, Bell JR (2000) Spider communities as tools in monitoring reclaimed limestone quarry landforms. Landsc Ecol 15:401–406. doi:10.1023/A:1008171023039
Wheeler HC, Høye TT, Svenning J-C (2017) The effect of shrub expansion on arctic wildlife species responses to shrub cover across trait and niche space. Glob Change Biol. doi:10.1111/gcb.13837
Wrona FJ et al (2016) Transitions in Arctic ecosystems: ecological implications of a changing hydrological regime. J Geophys Res Biogeosci 121:650–674. doi:10.1002/2015jg003133
Young AB, Watts DA, Taylor AH, Post E (2016) Species and site differences influence climate-shrub growth responses in West Greenland. Dendrochronologia 37:69–78. doi:10.1016/j.dendro.2015.12.007
Acknowledgements
We are grateful for logistic support at the field site from Storch Lund and the Icepatrol at the Danish Meteorological Institute. The Natural History Museum Aarhus is thanked for storing arthropod samples. T.T.H. acknowledges funding from the Carlsberg Foundation (Grant Number: CF14-0992), and the Arctic Research Centre at Aarhus University, Denmark.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the special issue on the “Ecology of tundra arthropods”, coordinated by Toke T. Høye and Lauren E. Culler.
Rights and permissions
About this article
Cite this article
Høye, T.T., Bowden, J.J., Hansen, O.L.P. et al. Elevation modulates how Arctic arthropod communities are structured along local environmental gradients. Polar Biol 41, 1555–1565 (2018). https://doi.org/10.1007/s00300-017-2204-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2204-2