Abstract
Our understanding of arthropod responses to environmental pressures is limited, especially for the poorly studied Mediterranean region. In the light of likely further environmental change and the need for protocols for rapid biodiversity assessment, we measured how the abundance and species richness of two taxa, ground spiders and Orthoptera, belonging to different functional groups, fluctuates intra- seasonally (early-mid-late summer) and across habitat types (grasslands, maquis, forests). We also tested their surrogate value. Spiders were found to have higher species richness and abundance almost throughout the investigation. Orthoptera had lower species richness and abundance in forests compared to grasslands and maquis, while no significant difference between habitats was revealed for spiders. Early-summer was the richest period for spiders while mid-summer was the richest for Orthoptera. Canopy cover was found to significantly influence community composition of both groups, while herb height and cover of stones was a determinant factor for Orthoptera only. There was a significant congruence between the two groups and Orthoptera provided the best complementary network. Our results show that diversity patterns of both spiders and Orthoptera are sensitive to environmental changes even over short time-scales (e.g. within the summer period) and space (e.g. across different habitat types), suggesting that small inexpensive experimental designs may still reveal community dynamics. For conservation purposes, we advise a focus on variables regulating habitat heterogeneity and microhabitat characteristics. We provide a list of the most influential species and propose the most effective network for obtaining information on the local fauna.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the fact that 85% of all animal species are arthropods, studies examining diversity shifts in ground-dwelling arthropods are less common than those examining shifts in vertebrates or charismatic arthropod taxa such as butterflies and moths (Chen et al. 2011). In the Mediterranean, and especially in Greece, arthropod surveys are scarce and our taxonomic knowledge is still limited, mainly due to the extreme diversity of the fauna and the relatively few taxonomists that specialize on this group (Behan-Pelletier and Newton 1999). Additionally, practical difficulties for their collection (e.g. cryptic lives, inaccessible microhabitats, short biological cycles) make primary data difficult to obtain. The lack of basic knowledge on their diversity in an area hinders ecological research or conservation (Agnarsson and Kuntner 2007). This explains the almost complete absence of arthropods from conservation planning at a national level (Sfenthourakis and Legakis 2001).
Functional groups of species (i.e. sets of species encompassing a variety of similar functional attributes) (Díaz and Cabido 1997) contribute in a similar way to ecosystem services and properties and thus permit generalizations on species responses to environmental change (Wilson 1999). The use of systems of interacting functional groups that reflect environmental change differently (rather than individual species) is an effective way of organizing empirical research in ecology, thus increasing the value of monitoring (Barton 2011). Investigating changes in arthropod functional groups is a very efficient biodiversity shortcut that could improve understanding of their ability to respond to environmental change and provide a useful gateway to more efficient conservation planning.
This has led us to focus on understanding the environmental factors influencing diversity patterns of arthropods in the Mediterranean region by combining data on two different functional groups in different habitats. We chose ground dwelling spiders (predators) and Orthoptera (herbivores) in one of the most diverse areas in N.E. Greece, the Dadia-Leukimi-Soufli National Park (Dadia NP hereafter). Our aim was also to provide conservation biologists with a tool to help develop effective strategies for conservation planning in Dadia NP, focusing specifically on arthropods.
Spiders are among the most abundant predators of invertebrates in terrestrial ecosystems (Symondson et al. 2002). They are significant predators of many herbivore species and also provide food for other canopy foragers, such as ants and birds (Halaj et al. 1997). They also contribute to many ecosystem processes (e.g. nutrient cycling (Lawrence and Wise 2000)). The high taxonomic and functional diversity of spiders justifies their use as a model group for studying large-scale ecological patterns (Birkhofer and Wolters 2012; Cardoso et al. 2011). Orthopterans are apparently under threat as their diversity declines in many temperate regions (Marini et al. 2010), and in Europe 50% are considered endangered (Hochkirch et al. 2016). In addition, they are important primary and secondary consumers in grassland ecosystems, and they provide an abundant source of prey for many predators (e.g. insectivorous birds, spiders). For several regions and biomes Orthoptera have been suggested as appropriate bioindicators (Báldi and Kisbenedek 1997) due to their sensitivity to microclimatic conditions (Zografou et al. 2009).
Thermal conditions are known to greatly affect all arthropods because increased ambient temperatures have a direct effect on their metabolic rates, activity patterns and developmental rates. For Orthoptera, thermal conditions might even determine their ability to avoid predators (Pitt 1999). For spiders, different climatic conditions between early and late spring were found to affect their activity and density patterns (Öberg et al. 2008). Given that climatic variability operates over all time scales, even very short ones (Ghil 2002), different thermal conditions within seasons may control species activity patterns (e.g. within season abundance shifts), leading to sizeable effects at the community level.
Another factor that is known to drive diversity patterns of both ground spiders and Orthoptera is vegetation composition and structural characteristics including herb cover and height, bare ground, and cover of stones (Standish 2004; Stromberg and Tellman 2012). Structural differences can modify temperature, light intensity, and soil moisture, which in turn can determine the distribution and diversity patterns of arthropods (Guido and Gianelle 2001; Nufio et al. 2010). An example of changes in vegetation structure is forest re-establishment at the expense of open areas (Barbero et al. 1990; Debussche et al. 1999) due to the reduction of human densities in many rural areas in the Mediterranean. This, in turn, has led to reduced species richness and diversity of ground spiders (Zakkak et al. 2014). Likewise, vegetation coverage and habitat desiccation were found to constrain the distribution of spiders (Lambeets et al. 2008; Perner and Malt 2003). Changes in other habitat-specific variables such as the cover of flower-heads, presence of shrubs or altitudinal changes were shown to greatly influence the Orthoptera community (Zografou et al. 2009).
The northern part of Greece was recently found to have the most prominent seasonal trend in terms of temperature alterations during the summer as opposed to the winter or autumn period (Mamara et al. 2016). Given that weather conditions from June to August are clearly distinct, Dadia NP provides an excellent opportunity to study the intra-seasonal activity of arthropods over a short time span i.e. within summer.
Recent studies in Dadia NP showed that changes in temperature have already impacted diversity patterns, community composition and phenological patterns of butterflies and orthopterans (Zografou et al. 2014, 2015). Further climate warming (IPCC 2013) is likely to affect spiders and orthopterans differently (Barton 2010, 2011), and also cause changes in vegetation structure (Debussche et al. 1999). We adopted this combined approach of time- and habitat-dependent structure and we documented the differences on diversity patterns of these two functional groups.
Specifically, we addressed the following questions: (a) is there a significant difference in abundance and richness of spiders and orthopterans intra-seasonally? (b) is there a consistent effect of habitat type on diversity of spiders and orthopterans? (c) are there any common habitat-specific variables shaping spider and orthopteran community composition? and (d) which species are responsible for possible differences in the community structure intra-seasonally and between habitats? Since each taxon displays an individualistic response to current pressures, dictated by its specific ecological demands (Peñuelas et al. 2013; Rapacciuolo et al. 2014) we expected to find significant differences in activity patterns of the study organisms. In particular, we expected that microhabitat characteristics (e.g. flower-heads, shrubs, stones) will have a significant impact on Orthoptera (Kati et al. 2004; Zografou et al. 2009), while habitat characteristics such as openness (e.g. grasslands) or closeness (e.g. forests) would affect spiders (Buchholz 2010; Zakkak et al. 2014). For spiders, we expected to find increased activity in grasslands or maquis in the early-summer period, as spiders are known to populate open areas during that time, after leaving their overwintering sites (Bertrand et al. 2016). Previous studies on the phenology of ground spiders in other Mediterranean areas (i.e. Majadas and Urones 2002; Jiménez-Valverde and Lobo 2006; Cardoso et al. 2007) or in Greece (i.e. Chatzaki et al. 1998, 2005) confirm that late spring to early autumn is the period of maximum activity, with variations depending on local climate fluctuations. Our previous studies of Orthoptera in the study area (Kati et al. 2004) and elsewhere (Kati et al. 2012; Zografou et al. 2009) showed that peak abundance occurs in mid-summer, i.e. the hottest period of the season. Finally, we explored the value of ground spiders and Orthoptera as biodiversity surrogates as well as their complementarity. When data availability and funds are limited but diversity is high, it is suggested to rely on few biodiversity surrogates for data acquisition, suitable for proper prioritization of conservation efforts.
Materials and methods
Study area and sites
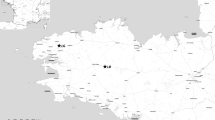
The study area was Dadia NP in N.E. Greece (41°07′–41°15′N, 26°19′–26°36′E) (Fig. 1). It is a hilly area extending over 43,000 ha, with altitudes ranging from 20 to 650 m. It includes two strictly protected core areas (7290 ha), where only low-intensity activities such as extensive grazing and selective wood cutting are allowed on a periodical basis. The climate is sub-Mediterranean, with a mean annual rainfall of 653 mm and a mean annual temperature of 14.3 °C, (min in January and max in July–August), while the hot and arid summer season extends from July to September (mean temperature 25 °C, mean rainfall 210 mm) (Maris and Vasileiou 2010). Along with the dry and hot summers that characterise Dadia NP, a significant increase in average temperature of 0.95 °C has been documented in a 22 year-period (1990–2012) (Zografou et al. 2014). Seventy-five percent of the reserve is covered by pinewoods (Pinus nigra, Pinus brutia), broad-leaved woods (Quercus frainetto, Quercus cerris, Quercus pubescens), mixed pine-oak woods and maquis, whilst the semi-open forested zone covers 5%, agricultural fields cover 16% and grasslands and open habitats cover only 4% (Poirazidis et al. 2002). Both pine and oak habitats reflect the original Mediterranean vegetation at low altitudes. We recognised three dominant habitat types: forests, grasslands and maquis and we located four sampling sites in each, resulting to a total of 12 sites (Supplementary Online Material, Table S1). The mean distance between sites was 1.2 km ± 0.5 (SE) so that each site represented an independent sample in which diversity measures depend much more on local activity of organisms than on immigration from neighbouring sites.
Map of Greece showing the location of Evros prefecture (left figures), the area of Dadia-Lefkimi-Soufli National Park (right figure) with the core areas (polygons in dotted lines) and the 12 sites where sampling took place. Two out of the twelve sites are located outside the borders of the National Park
Arthropod sampling
On the basis of a significant intra-seasonal variation of mean temperature values from early to late summer (June has significantly lower temperature from July and July greater than August) sampling was conducted during the extended summer period from late May to early September 2011. Orthoptera sampling was conducted along two transects of 30 m length and 2 m width in each sampling site, with a minimum distance of 100 m between the 24 transects. Adult specimens of Orthoptera were caught in a sweep net, counted, and identified ex situ using the Greek Orthoptera guide (Willemse 1985). Ground dwelling spiders were sampled using pitfall traps. Five traps were located at 10 m intervals along every orthopteran transect, (5 traps/transect and 10 traps/site) resulting in a total of 120 traps. The traps remained active for 94 (± 6) days on average from late May to early September and were emptied three times. The three sampling periods were: 20. 5–25. 6. 2011 (early-summer), 25. 6–27. 7. 2011 (mid-summer) and 27. 7–3. 9. 2011 (late-summer). On average only nine (± 1) traps were active per site due to losses from animal interference and heavy rain. Each trap was filled with 200 ml of ethylene glycol (preservative) and was covered with a stone roof for protection against rain. Samples were transferred to 95% isopropyl-alcohol and subsequently sorted. As there are no extensive keys available for Greek spiders, identification was from existing literature related to the region and the online database for European spiders (http://www.araneae.unibe.ch/). Our taxonomic results were presented in a separate paper (Komnenov et al. 2016). Species names follow the nomenclature of the World Spider Catalog (Catalog 2016). The material is deposited in the Araneae collection of the Natural History Museum of the University of Crete.
Habitat-specific variables
We recorded ten habitat-specific variables for orthopterans and spiders in standard plots (5 × 2 m2) by systematically locating four orthopteran plots along each transect (every 10 m: 96 plots) and one spider plot around every pitfall trap (120 plots). Using a hand GPS we took the coordinates and recorded the altitude (1). To estimate the temperature (2) and soil humidity (3) at each site, we extracted data from a Hobo data logger (U12) that was placed at the beginning of each orthopteran transect and remained there for as long as the sampling was conducted (15 min with one record per minute). In addition, we estimated the average percentage canopy cover (4) per plot using a spherical densiometer (measures at four cardinal directions in July 2011), as well as the cover of herbs (5), shrubs (6), bare ground (7) and stones (8) using the Braun–Blanquet scale (5: 75–100%, 4: 50–75%, 3: 25–50,% 2: 5–25%, and 1: 0–5%). Finally, we estimated mean herb height (9) as the average of four measures corresponding to four dominant herb species as well as the number of flower-heads (10) in May (2011) using a five grade scale (1 ≤ 10, 2 > 10, 3 > 50, 4 > 100, 5 > 200).
Data analysis
Our dataset consisted of 12 sites and three samples (early-mid-late summer period). In order to standardize sampling effort in the whole experiment, for each sample in each site, we estimated spider abundance as the total number of individuals caught in all pitfall traps, divided by the number of active traps and the number of active sampling days, multiplied by 1000 thus rendering it to a standardised measure of number of individuals per 1000 trap-days. For Orthoptera, we considered both transects in each site as one sample and we estimated a mean value of Orthoptera abundance in terms of the number of individuals counted per site. Beta-diversity was also used to quantify species turnover within the summer period and across the habitat types using Whittaker’s formula for beta diversity (bw = S/ā − 1), where S is the total number of species in that period or habitat and ā the average number of species in these periods or habitats (Whittaker 1960). We calculated the proportion of species exclusively found in only one repetition for each target group separately.
The time and habitat effect in diversity patterns
We used general linear models to investigate differences in ground spider and Orthoptera species (a) richness and (b) abundance in time and habitat types. In particular, each model described the response variable (species richness or species abundance) as a function of the factors “taxon”, “intra-season”, “habitat type”, “site” and the interactions of “taxon × intra-season” and “taxon × habitat type”. The response variables were normalized using log-transformation allowing the specification of a normal error distribution. Models were validated by checking for homoscedasticity and normality of the residuals (Zuur et al. 2009), and in all cases, diagnostic graphs showed that model assumptions were met. Post-hoc tests (Tukey) were conducted to further explore the interaction terms. Statistical analyses were performed in R (R Core Team 2014) using library faraway for lm () function, and library estimability for lsmeans () function for post hoc tests (Lenth 2016).
The effect of habitat-specific variables in community composition
In order to explore the effect of habitat-specific variables (ten in total) on species composition, we conducted Redundancy Analysis (RDA) using CANOCO software (ter Braak and Smilauer 2002). Preliminary analyses were made using Detrended Correspondence Analysis [DCA, Hill and Gauch (1980)] to check the magnitude of change in species composition along the first ordination axis. Since the gradient length was found to be smaller than 4 SD-units (gradient length in standard deviation (SD) units, RDA was the appropriate ordination method to perform analysis and determine how much of the variation in species data is accounted for by the environmental data (Ter Braak and Prentice 1988). The RDA method extracts the major gradients in the data that are accounted for by the measured environmental parameters. The position of a species in the resulting diagram indicates the degree of dependence on the closest environmental parameters (arrows). The diagram shows only the species sufficiently influenced by the parameters (fit > 10%), and only the significant (P < 0.05) environmental variables that did not show collinearity (1,000 iterations of the Monte–Carlo test).
The most influential species
To pinpoint the most influential spider and orthopteran species that contributed most to the observed differences during the three summer periods and between open and closed canopy cover areas, we used the SIMPER (Similarity Percentage) method and the Bray-Curtis similarity measure (Clarke 1993). The classification between open and closed areas was done on the basis of canopy cover percentage (%): sites with >50% of canopy cover were classified as closed and sites with <50% as open. In addition, each species was given a vulnerability index from one (the least vulnerable) to three (the most vulnerable). An index of “one” was attributed to species that were found to be the most influential during the mid-summer period. This is because they are expected to be better synchronized to their food resources compared to species represented earlier or later (Donoso et al. 2016). An index of “two” was given to species that increased their influential role in late-summer, but for which the open/closed areas did not seem to be effective. An index of “three” was given to species that were found to be most influential both in the early- or late-summer period and in open habitats. One of the best studied species responses to climate change is the advanced appearance. The response is more pronounced for species that appear early or late in the season (McKinney et al. 2012). Therefore, species that will be found to be the most influential in terms of the early- or late-summer period are expected to have a higher vulnerability in the light of climate warming. Also, as the open areas are known to positively affect arthropod communities, if these areas are lost or decreased at the expense of forests, this might present a possible threat to these arthropods. We used PAST version 2.17c for SIMPER analysis (Hammer et al. 2001).
Surrogate value
We tested the surrogate value of (a) ground spiders and (b) Orthoptera by comparing the congruence of their species richness patterns using the Spearman rank correlation coefficient. Using one group as the surrogate group and the other as the target group, we formed the complementary network after the surrogate group, starting with the most species-rich site and adding those sites progressively contributing more new species, until all species were included in the network (Cardoso et al. 2004; Zografou et al. 2009). By calculating the proportion of species in every group included in each step of the networking procedure, we were able to assess the most efficient complementary network, thus eliminating the bias of the different number of sites forming the network.
Results
Single taxon diversity
Our dataset consisted of 136 spider species (22 families, 2,403 individuals) out of which 12 species are new records for Greece and seven species are new for science (Komnenov et al. 2016) (Supplementary Online Material, Table S2). The most abundant families were Gnaphosidae (36.5%; 878 ind.), Dysderidae (20.6%; 496 ind.) and Lycosidae (16%; 392 ind.). More spider species (26%) were encountered exclusively in early summer and fewer in mid (13%) and late summer (12.5%). Species turnover was lower from the first to the second period (bw = 0.36) and second to third (bw = 0.48) than first to third (bw = 0.54). Accordingly, species turnover was greater between forests and maquis (b w = 0.67), and between forests and grasslands (b w = 0.6) than grasslands and maquis (bw = 0.34). We recorded 39 species of orthopterans (five families, 2,628 individuals), with Acrididae (57%; 1,497 ind.) and Tettigoniidae (34%; 893 ind.) being the most abundant families (Table S2). The most important species in terms of conservation, listed as endangered in the IUCN 2016 list (Hochkirch et al. 2016) is Paranocarodes chopardi, an apterous pamphagid, with low dispersal ability that renders it prone to extinction if its habitat changes (Kati and Willemse 2001). Most orthopteran species were encountered exclusively in mid-summer (20.5%), fewer in early summer (15.4%) and the least in late summer (8%). Species turnover was higher from the first to the second period (bw= 0.7) and first to third (bw= 0.6) than second to third (bw= 0.37). Species turnover across habitat types was higher between forests and grasslands or maquis (bw = 0.5) than between grasslands and maquis (bw = 0.38).
The effect of time and habitat type on diversity patterns
The main effects of “taxon”, “habitat type” and “site” on abundance were significant, while no significant main effect of “intra-season” was revealed (Table 1). The post-hoc Tukey test for the factor “taxon” showed a significantly higher abundance for spiders than Orthoptera (P < 0.001). Forests had significantly lower abundance than both grasslands and maquis habitats (Tukey: P = 0.003 and P = 0.038 respectively), while grasslands and maquis had similar abundance (Tukey: P = 0.61). The two interactions included in the model were found to be significant at the 0.05 level (Table 1) indicating that differences between both habitat types and intra-seasonally affected the response of abundance to taxon. The post-hoc Tukey tests revealed that spiders always had higher abundance than Orthoptera (Fig. 2a, b) except in the maquis habitats (P = 0.07; Fig. 2a) and in the mid-summer period which were marginally significantly different in abundance (P = 0.056; Fig. 2b). Orthoptera were significantly less abundant in forests than in grasslands (Tukey: P = 0.005; Fig. 2a) and maquis habitats (Tukey: P = 0.007; Fig. 2a). However, the abundance of orthopterans was similar in grasslands and maquis (Tukey: P = 0.062; Fig. 2a). Interestingly, spider abundance did not significantly differ in the different habitat types (Tukey: P > 0.05 in all cases; Fig. 2a). Finally, there was no significant difference in abundance of either taxa intra-seasonally (Tukey: P > 0.05 in all cases; Fig. 2b).
The second model, with species richness as the response variable, showed that the main effects of the factors “taxon” and “habitat type” were significant, but not significant was the effect of “intra-season” and “site” (Table 1). Species richness was significantly higher for spiders than for Orthoptera (Tukey: P < 0.001). In addition, grasslands and maquis had higher species richness than forests (Tukey: P < 0.001 in both cases; Fig. 3a), while grasslands and maquis had similar species richness (Tukey: P = 0.82; Fig. 3a). The only significant interaction “taxon × habitat type” indicates that differences between habitat types did not affect the response of species richness to taxon. Spiders had significantly higher species richness than Orthoptera, across all different habitat types (Fig. 3a) and intra-seasonally (Fig. 3b). Orthoptera had significantly lower species richness in forests than in grasslands (Tukey: P = 0.005; Fig. 3a) and maquis habitats (Tukey: P = 0.006; Fig. 3a). However, orthopterans had similar species richness in grasslands and maquis (Tukey: P > 0.05; Fig. 3a). The species richness of spiders was not significantly different across the different habitat types (Tukey: P > 0.05 in all cases; Fig. 3a). Orthoptera presented significantly lower species richness values in the early-summer than in the two following periods (Tukey: P = 0.002 and P = 0.001 respectively; Fig. 3b), while the mid and late-summer had similar species richness (Tukey: P > 0.05 in all cases; Fig. 3b). On the contrary, spiders had significantly higher species richness in early-summer than in the late-summer (Tukey: P = 0.04; Fig. 3b), while the mid-summer period had similar species richness to all other periods (Tukey: P > 0.05 in all cases, Fig. 3b).
Environmental factors shaping community composition
Among habitat-specific variables, increased canopy cover was found to be the most influential factor for spider communities, negatively affecting 72% of the species (61% of the total variance explained, Fig. 4). Among newly described spider species, some (Dysdera kati, Dysdera krisis and Harpactea wolfgangi) were positively affected by increased canopy cover, while others (Harpactea ice, Phrurolithus thracia and Zodarion beroni) were negatively affected (Fig. 4, Table S2). In addition to canopy cover, herb height and cover of stones were found to significantly influence the Orthoptera community. Increased canopy cover negatively affected 74% of species, while P. chopardi, a typical species for semi-shaded oak woods with Mediterranean scrub undergrowth and leaf litter (Kati et al. 2010), was positively affected by this factor. Increased herb height and cover of stones positively affected 59 and 64% of species respectively (46.5% of the total variance explained, Fig. 4, Table S2).
Redundancy analysis diagram (RDA) presenting the significant (P = 0.05) environmental factors affecting the community of spiders and Orthoptera. Only species with a fit of more than 10% are shown. Full names of the newly described spider species (>10% fit) are given on the plots while all others are symbolized with a number (for corresponding species numbers and names see Table S2). For Orthoptera, only the name of the most important species in terms of conservation concern is provided
Most influential species
Nine spider species belonging to six families were responsible for more than 50% of the intra-seasonal differences, while six species were responsible for the differences between open and closed canopy areas; six species were common in both cases (Table 2). For Orthoptera, SIMPER analysis indicated seven species that explained more than 50% of the differences intra-seasonally and between closed and open canopy areas (Table 2).
Surrogate value
We found significant congruence between patterns of species richness of spiders and Orthoptera (rho = 0.73, P = 0.007). Orthoptera served as the best surrogate group where complementarity is concerned. Their network included nine sites as opposed to twelve sites of the spider network, which, basically, corresponded to the whole study system (12 sites). The Orthoptera network conserved 88.9% of the ground spider species and when comparing the average species richness included in each step of the complementary network selection, it succeeded in conserving even more orthopterans (13%) and lost only 6.6% of spider species.
Discussion
Time and habitat effect
In this study, we found both season and habitat type significantly influenced the arthropod assemblages in the Dadia NP. As expected, responses were not consistent between Orthoptera and spiders and their diversity measures. Regarding the within-season results, patterns of variation in both species richness and abundance between the two groups were different. Also, ground spiders were always more abundant and rich in species than Orthoptera, except during the mid-summer period where differences in abundance of the two groups were not significant. The species turnover analysis showed that many of the spider species that are highly active in the early-summer period, also persist into mid-summer, but then they are replaced by a few other species that are only highly active in late summer. This phenological pattern has been reported for Mediterranean Gnaphosidae before (Chatzaki et al. 2005). The analysis of the most influential species showed that there are two highly influential spider species with maximum activity in the early summer (Harpactea babori and Pardosa alacris) and two in the late summer (Scytodes thoracica and Zelotes erebeus), but only one in mid-summer (Civizelotes caucasius). These observations, in conjunction with the short life cycle of Orthoptera with peak activity in mid-summer (Kati et al. 2004) could explain the reduced differentiation between the two groups during this period. The higher species richness of spiders in the early-summer period indicates the importance of this season for local biodiversity suggesting it is the optimal time for sampling spiders in case of low duration/budget inventories.
The results showed that grasslands and maquis hosted more species than forests in both taxa examined. Habitat openness, therefore, is the main driver of spider and Orthoptera species richness in Dadia NP (Kati et al. 2004). In fact, the positive effect of the open habitat structure explains the lack of any significant interaction between the factors “taxon” and “habitat type” in our models. However, for spider abundance, the pattern is not repeated since both forests and grasslands host an equally high number of individuals, resulting in non-significant variation among habitat types (Fig. 2a). Five out of the six most important spider species that affect differences between open and closed canopy cover areas had maximum activity in closed forest habitats. Species turnover analysis, however, showed that species inhabiting forests differed from those of grasslands or maquis, clearly demonstrating niche separation of ground spiders. Given the lower species richness in forests, a lessened inter-species competition may be expected and hence an increase in the numbers of individuals of the species that persist there. The preference of some of the species for closed habitats has been previously documented, as in the case of P. alacris which is known to prefer deciduous oak and beech woods (Töpfer-Hofmann et al. 2000). However in most cases this study is the first to demonstrate clear ecological preferences of the spider species recorded.
Orthoptera showed a consistent pattern of preference for open canopy cover areas. As found in previous studies in Dadia NP (Kati et al. 2004) and elsewhere (Kati et al. 2012; Zografou et al. 2009), our results for Orthoptera demonstrated the importance of open habitats for the conservation of this group. The conservation value of open habitats, and the importance of habitat-specific variables (e.g. shade, herb height, stones) at a community level are discussed below (Guido and Gianelle 2001; Zografou et al. 2009).
Habitat-specific variables shaping community composition
Both spider and orthopteran communities were negatively affected by canopy cover (72 and 74% respectively). In a recent review of biodiversity indicators for forest ecosystems in Europe, the authors found that there is strong evidence for a negative correlation between tree canopy cover and spider species richness (Gao et al. 2014). Similarly, a study conducted in the Greek mountains of Pindos (Zakkak et al. 2014) showed that community composition of ground spiders of the family Gnaphosidae is significantly affected by land-abandonment with their diversity and species richness being negatively correlated with forest cover. Orthoptera are well known to prefer open, dry and warm habitats with short vegetation (Uvarov 1966), although there are a few exceptions such as P. chopardi, a species typical to oak woodlands with a very narrow distribution restricted to Dadia NP and few localities in Bulgaria (Kati et al. 2004).
The Orthoptera community was also found to be greatly affected by herb height and concomitant changes in rock cover. As herbs are the main food source for all orthopterans, although bush-crickets have a mixed diet consisting of other insects and flower parts, it seems reasonable that increased herb cover positively affected 59% of the species. With a range of more than half a meter, herb height creates multiple microhabitats that can support a great variety of species with different ecological requirements, providing also shelter from predators and extreme climatic conditions (Kemp et al. 1990). However, tall herbs might be an indication of increased shading or lack of grazing that are well-known to negatively affect egg and nymph development of some orthopteran species (van Wingerden et al. 1991). The second element, rock cover, seems to have an even greater positive influence on Orthoptera (64%). It is known that rocks are important structures, aiding in orthopteran thermoregulation as well as providing shelter (Chappell 1983) and their positive correlation to species richness has been previously demonstrated (Crous et al. 2013).
The most influential species
Another interesting finding of this study is the list with the most influential species (Table 2). As climate warming is linked to a changing onset of phenological events for a variety of taxonomic groups (Menzel et al. 2006; Parmesan 2007; Primack et al. 2009) we expect that species most abundant in early or late summer (e.g. Chorthippus bornhalmi, Poecilimon brunneri, H. babori) will be strongly influenced by climate warming. It is also plausible to assume that species that are most abundant in open areas (e.g. Tylopsis lilifolia, Civizelotes caucasius) might be seriously threatened by shifts in vegetation structure such as, for example, forest encroachment on open habitats (Debussche et al. 1999; IPCC 2013). On the basis of anticipated further changes in climate and landscape, we proposed a “vulnerability index” for each of the most influential species so that conservation efforts could be prioritized accordingly.
Surrogate value
Spiders and Orthoptera share no similar traits in their life history and ecology (Barton 2011). However, our results reveal a strong congruence in their species richness patterns, suggesting the possible use of each taxon as a good surrogate for the other. Although Orthoptera have been previously tested for their surrogate value (Bazelet and Samways 2012; Zografou et al. 2009), fewer studies can be found on the surrogate value of spiders (but see Cardoso et al. 2004), and none, to our knowledge, that investigates the surrogate value of spiders for Orthoptera and vice versa. Given that, only recently, the rich spider fauna of Dadia NP started to be investigated or described (Komnenov et al. 2016), the identification of shortcuts such as Orthoptera species network is expected to provide cost- and time-effective monitoring schemes improving our ability to better conserve the local fauna (Margules and Pressey 2000).
Conclusions
Seasonal variation in species activity is a normal event in temperate ecosystems. However, our results demonstrated that significant differentiation of species richness and abundance can occur even within a season, such as the summer period. Our findings highlight the value of low-cost, short-term inventories for revealing community dynamics and confirming that the choice of sampling period is highly taxon-specific. Our study also suggests the importance of open habitats for the conservation of both herbivores and predators. However, some influential spiders’ species seem to benefit from closed habitats. Therefore, although the presence of grasslands or maquis would greatly favour herbivores, forests too play a beneficial role for spiders. It is suggested that for predators and especially for spiders, preservation of landscape heterogeneity rather than openness is the most crucial factor for their diversity as has been proposed in previous studies (Zakkak et al. 2014). In addition, the different effects of other environmental factors we studied, showed that arthropods do not respond in the same way, emphasizing the need for multi-taxa approaches in ecological studies.
In the light of future land-use changes in the Mediterranean (Barbero et al. 1990; Debussche et al. 1999; Gerard et al. 2010), we identified a pool of nine spiders and seven orthopterans that drives community patterns and can be a useful tool for conservation biologists. Apart from its conservation value, this analysis offers a well-documented observational framework of species activity in the Mediterranean region, contributing to the otherwise limited knowledge about their ecological preferences. Finally, the proposal of the Orthoptera species network as the best surrogate group in terms of complementarity accentuates the important role of biodiversity studies in high biodiversity areas such as Greece. The importance of spider and orthopteran diversity in the Dadia NP, merits further ecological study and monitoring in order to obtain detailed information on their population trends.
To conclude, analysing summer samplings that incorporate both open and closed canopy sites with a high variability of habitat-specific variables seems necessary to better assess how time and habitat structure affect groups with different ecological attributes. Conservation managers should prioritize their conservation efforts in the light of the most influential/vulnerable species but also consider the complementarity value of Orthoptera when planning future conservation actions.
References
Agnarsson I, Kuntner M (2007) Taxonomy in a changing world: seeking solutions for a science in crisis. Syst Biol 56:531–539. doi:10.1080/10635150701424546
Báldi A, Kisbenedek T (1997) Orthopteran assemblages as indicators of grassland naturalness in Hungary. Agric Ecosyst Environ 66:121–129. doi:10.1016/S0167-8809(97)00068-6
Barbero M, Bonin G, Loisel R, Quézel P (1990) Changes and disturbances of forest ecosystems caused by human activities in the western part of the mediterranean basin. Vegetatio 87:151–173. doi:10.1007/bf00042952
Barton BT (2010) Climate warming and predation risk during herbivore ontogeny. Ecology 91:2811–2818. doi:10.1890/09-2278.1
Barton BT (2011) Local adaptation to temperature conserves top-down control in a grassland food web. Proc R Soc B 278:3102–3107. doi:10.1098/rspb.2011.0030
Bazelet CS, Samways MJ (2012) Grasshopper and butterfly local congruency in grassland remnants. J Insect Conserv 16:71–85. doi:10.1007/s10841-011-9394-7
Behan-Pelletier V, Newton G (1999) Computers in biology: linking soil biodiversity and ecosystem function - the taxonomic dilemma. Bioscience 49:149–153
Bertrand C, Baudry J, Burel F (2016) Seasonal variation in the effect of landscape structure on ground-dwelling arthropods and biological control potential. Basic Appl Ecol 17:678–687. doi:10.1016/j.baae.2016.07.007
Birkhofer K, Wolters V (2012) The global relationship between climate, net primary production and the diet of spiders. Global Ecol Biogeogr 21:100–108. doi:10.1111/j.1466-8238.2011.00654.x
Buchholz S (2010) Ground spider assemblages as indicators for habitat structure in inland sand ecosystems. Biodivers Conserv 19:2565–2595. doi:10.1007/s10531-010-9860-7
Cardoso P, Silva I, De Oliveira NG, Serrano ARM (2004) Indicator taxa of spider (Araneae) diversity and their efficiency in conservation. Biol Conserv 120:517–524. doi:10.1016/j.biocon.2004.03.024
Cardoso P, Silva I, Oliveira NG, Serrano ARM (2007) Seasonality of spiders (Araneae) in Mediterranean ecosystems and its implications in the optimum sampling period. Ecol Entomol 32:516–526
Cardoso P, Pekár S, Jocqué R, Coddington JA (2011) Global patterns of guild composition and functional diversity of spiders. PLoS ONE. doi:10.1371/journal.pone.0021710
Catalog WS (2016) World Spider Catalog. Natural History Museum Bern, online at http://wsc.nmbe.ch, version 17.0, (Accessed on 30 Jan 2016)
Chappell MA (1983) Thermal limitations to escape responses in desert grasshoppers. Anim Behav 31:1088–1093. doi:10.1016/s0003-3472(83)80016-5
Chatzaki M, Trichas A, Markakis G, Mylonas M (1998) Seasonal activity of the ground spider fauna in a Mediterranean ecosystem (Mt. Youchtas, Crete, Greece). Proceedings of the 17th European Colloquium of Arachnology, Edinburgh 1997: 235–243
Chatzaki M, Mylonas M, Markakis G (2005) Phenological patterns of ground spiders (Araneae, Gnaphosidae) on Crete, Greece. Ecol Mediterr, 31:33–53
Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026. doi:10.1126/science.1206432
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Crous CJ, Samways MJ, Pryke JS (2013) Exploring the mesofilter as a novel operational scale in conservation planning. J Appl Ecol 50:205–214. doi:10.1111/1365-2664.12012
Debussche M, Lepart J, Dervieux A (1999) Mediterranean landscape changes: Evidence from old postcards. Global Ecol Biogeogr 8:3–15
Díaz S, Cabido M (1997) Plant functional types and ecosystem function in relation to global change. J Veg Sci 8:463–474
Donoso I, Stefanescu C, Martínez-Abraín A, Traveset A (2016) Phenological asynchrony in plant–butterfly interactions associated with climate: a community-wide perspective. Oikos 125:1434–1444. doi:10.1111/oik.03053
Gao T, Hedblom M, Emilsson T, Nielsen AB (2014) The role of forest stand structure as biodiversity indicator. For Ecol Manage 330:82–93. doi:10.1016/j.foreco.2014.07.007
Gerard F et al (2010) Land cover change in Europe between 1950 and 2000 determined employing aerial photography. Prog Phys Geogr 34:183–205. doi:10.1177/0309133309360141
Ghil M (2002) Natural climate variability. In: MacCracken MC, Perry JS (eds). Encyclopedia of global environmental change, vol 1. Wiley, New York, pp 544–549
Guido M, Gianelle D (2001) Distribution patterns of four orthoptera species in relation to microhabitat heterogeneity in an ecotonal area. Acta Oecol 22:175–185. doi:10.1016/s1146-609x(01)01109-2
Halaj J, Ross DW, Moldenke AR (1997) Negative effects of ant foraging on spiders in Douglas-fir canopies. Oecologia 109:313–322. doi:10.1007/s004420050089
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:XIX–XX
Hill MO, Gauch HG (1980) Detrended correspondence analysis: an improved ordination technique. Vegetatio 42:47–58. doi:10.1007/bf00048870
Hochkirch A, Nieto A, Garcνa Criado M, Cαlix M, Braud Y, Buzzetti FM, Chobanov D, Odι B, Presa Asensio JJ, Willemse L, Zuna-Kratky T, Barranco Vega P, Bushell M, Clemente ME, Correas JR, Dusoulier F, Ferreira S, Fontana P, Garcνa MD, Heller K-G, Iorgu I.Ș., Ivković S, Kati V, Kleukers R, Krištνn A, Lemonnier-Darcemont M, Lemos P, Massa B, Monnerat C, Papapavlou KP, Prunier F, Pushkar T, Roesti C, Rutschmann F, Şirin D, Skejo J, Szφvιnyi G, Tzirkalli E, Vedenina V, Barat Domenech J, Barros F, Cordero Tapia PJ, Defaut B, Fartmann T, Gomboc S, Gutiιrrez-Rodrνguez J, Holuša J, Illich I, Karjalainen S, Kočαrek P, Korsunovskaya O, Liana A, Lσpez H, Morin D, Olmo-Vidal JM, Puskαs G, Savitsky V, Stalling T, Tumbrinck J (2016) European red list of grasshoppers, crickets and bush-crickets. Publications Office of the European Union, Luxembourg
IPCC (2013) Summary for Policymakers. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Cambridge University Press, Cambridge
Jiménez-Valverde A, Lobo JM (2006) Establishing reliable spider (Araneae, Araneidae and Thomisidae) assemblage sampling protocols: estimation of species richness, seasonal coverage and contribution of juvenile data to species richness and composition. Acta Oecol 30:21–32
Kati V, Willemse F (2001) The grasshoppers and crickets of the Dadia Forest Reserve (Thraki, Greece) with a new record to the Greek fauna: Paranocarodes chopardi Pechev 1965 (Orthoptera, Pamphagidae). Articulata 16:11–19
Kati V, Dufrêne M, Legakis A, Grill A, Lebrun P (2004) Conservation management for Orthoptera in the Dadia reserve, Greece. Biol Conserv 115:33–44. doi:10.1016/s0006-3207(03)00091-0
Kati V, Zografou K, Tzirkalli E, Chitos T, Willemse L (2012) Butterfly and grasshopper diversity patterns in humid Mediterranean grasslands: The roles of disturbance and environmental factors. J Insect Conserv 16:807–818. doi:10.1007/s10841-012-9467-2
Kemp WP, Harvey SJ, O’Neill KM (1990) Patterns of vegetation and grasshopper community composition. Oecologia 83:299–308. doi:10.1007/bf00317552
Komnenov M, Pitta E, Zografou K, Chatzaki M (2016) Discovering the still unexplored arachnofauna of the National Park of Dadia-Lefkimi-Soufli, NE Greece: a taxonomic review with description of new species. Zootaxa 0:000–000
Lambeets K, Hendrickx F, Vanacker S, Van Looy K, Maelfait JP, Bonte D (2008) Assemblage structure and conservation value of spiders and carabid beetles from restored lowland river banks. Biodivers Conserv 17:3133–3148. doi:10.1007/s10531-007-9313-0
Lawrence KL, Wise DH (2000) Spider predation on forest-floor Collembola and evidence for indirect effects on decomposition. Pedobiologia 44:33–39. doi:10.1078/S0031-4056(04)70026-8
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:33. doi:10.18637/jss.v069.i01
Majadas A, Urones C (2002) Communauté d’araignées des maquis méditerranéens de Cyticus oromediterraneus Rivas Mart. et al. Rev Arachnol 14:31–48
Mamara A, Argiriou AΑ, Anadranistakis M (2016) Recent trend analysis of mean air temperature in Greece based on homogenized data. Theoret Appl Climatol 126:543–573. doi:10.1007/s00704-015-1592-x
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:243–253. doi:10.1038/35012251
Marini L, Bommarco R, Fontana P, Battisti A (2010) Disentangling effects of habitat diversity and area on orthopteran species with contrasting mobility. Biol Conserv 143:2164–2171. doi:10.1016/j.biocon.2010.05.029
Maris F, Vasileiou A (2010) Hydrology and torrential environment. In: Catsadorakis G, KÓ“lander H (eds) The Dadia-Lefkimi-Soufli forest national park, Greece: biodiversity, management and conservation. WWF Greece, Athens, pp 41–45
McKinney AM, Caradonna PJ, Inouye DW, Barr B, Bertelsen CD, Waser NM, Irwin RE (2012) Asynchronous changes in phenology of migrating Broad-tailed Hummingbirds and their early-season nectar resources. Ecology 93:1987–1993. doi:10.1890/12-0255.1
Menzel A et al (2006) European phenological response to climate change matches the warming pattern. Global Change Biol 12:1969–1976. doi:10.1111/j.1365-2486.2006.01193.x
Nufio CR, McGuire CR, Bowers MD, Guralnick RP (2010) Grasshopper community response to climatic change: variation along an elevational gradient. PLoS ONE. doi:10.1371/journal.pone.0012977
Öberg S, Mayr S, Dauber J (2008) Landscape effects on recolonisation patterns of spiders in arable fields. Agric Ecosyst Environ123:211–218. doi:10.1016/j.agee.2007.06.005
Parmesan C (2007) Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Global Change Biol 13:1860–1872. doi:10.1111/j.1365-2486.2007.01404.x
Peñuelas J et al (2013) Evidence of current impact of climate change on life: a walk from genes to the biosphere. Global Change Biol 19:2303–2338. doi:10.1111/gcb.12143
Perner J, Malt S (2003) Assessment of changing agricultural land use: Response of vegetation, ground-dwelling spiders and beetles to the conversion of arable land into grassland. Agric Ecosyst Environ 98:169–181. doi:10.1016/s0167-8809(03)00079-3
Pitt WC (1999) Effects of multiple vertebrate predators on grasshopper habitat selection: trade-offs due to predation risk, foraging, and thermoregulation. Evol Ecol 13:499–515. doi:10.1023/a:1006792726166
Poirazidis K, Skartsi T, Katsadorakis G (2002) Monitoring plan for the protected area of Dadia-Lefkimi-Soufli forest. WWF-Greece, Athens
Primack RB, Ibáñez I, Higuchi H, Lee SD, Miller-Rushing AJ, Wilson AM, Silander JA Jr (2009) Spatial and interspecific variability in phenological responses to warming temperatures. Biol Conserv 142:2569–2577. doi:10.1016/j.biocon.2009.06.003
Rapacciuolo G et al (2014) Beyond a warming fingerprint: individualistic biogeographic responses to heterogeneous climate change in California. Global Change Biol 20:2841–2855. doi:10.1111/gcb.12638
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Sfenthourakis S, Legakis A (2001) Hotspots of endemic terrestrial invertebrates in southern Greece. Biodivers Conserv 10:1387–1417. doi:10.1023/a:1016672415953
Standish RJ (2004) Impact of an invasive clonal herb on epigaeic invertebrates in forest remnants in New Zealand. Biol Conserv 116:49–58. doi:10.1016/s0006-3207(03)00172-1
Stromberg JC, Tellman B (2012) Ecology and conservation of the san pedro river. The University of Arizona Press, Tuscon
Symondson WOC, Sunderland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594. doi:10.1146/annurev.ento.47.091201.145240
TerBraak CJF, Prentice IC (1988) A theory of gradient analysis. Adv Ecol Res doi:10.1016/s0065-2504(08)60183-x
TerBraak CJF, Smilauer P (2002) CANOCO reference manual and canoco draw for windows user’s guide: software for canonical community ordination. (version 4.5) Microcomputer power, Ithaca, New York
Töpfer-Hofmann G, Cordes D, von Helversen O (2000) Cryptic species and behavioural isolation in the Pardosa lugubris group (Araneae, Lycosidae), with description of two new species. Bull Br Arachnol Soc 11:257–274
Uvarov B (1966) Grasshoppers and locusts: a handbook of general acridology. Vol. 1. Cambridge University Press, Cambridge
van Wingerden WKRE, Musters JCM, Maaskamp FIM (1991) The influence of temperature on the duration of egg development in West European grasshoppers (Orthoptera: Acrididae). Oecologia 87:417–423. doi:10.1007/bf00634600
Whittaker RH (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr 30:279–338. doi:10.2307/1943563
Willemse F (1985) A key to the orthoptera species of Greece. Hellenic Zoological Society, Athens
Wilson JB (1999) Guilds, functional types and ecological groups. Oikos 86:507–522
Zakkak S, Chatzaki M, Karamalis N, Kati V (2014) Spiders in the context of agricultural land abandonment in Greek Mountains: species responses, community structure and the need to preserve traditional agricultural landscapes. J Insect Conserv 18:599–611. doi:10.1007/s10841-014-9663-3
Zografou K, Sfenthourakis S, Pullin A, Kati V (2009) On the surrogate value of red-listed butterflies for butterflies and grasshoppers: a case study in Grammos site of Natura 2000, Greece. J Insect Conserv 13:505–514. doi:10.1007/s10841-008-9198-6
Zografou K, Kati V, Grill A, Wilson RJ, Tzirkalli E, Pamperis LN, Halley JM (2014) Signals of climate change in butterfly communities in a mediterranean protected area. PLoS ONE. doi:10.1371/journal.pone.0087245
Zografou K, Adamidis GC, Grill A, Kati V, Wilson RJ, Halley JM (2015) Who flies first?—habitat-specific phenological shifts of butterflies and orthopterans in the light of climate change: a case study from the south-east Mediterranean. Ecol Entomol 40:562–574. doi:10.1111/een.12220
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This project was co-funded by the European Union (European Social Fund) and National Resources under the Operational Programme “Education and Lifelong Learning” Action 81324 - SPIDOnetGR, ARISTEIA II Programme, NSRF 2007–2013. In addition, it has been co-financed by the European Union (European Social Fund - ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) - Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund. We are grateful to the Forestry Department of Dadia NP, and WWF for supporting the field research. Finally we wish to thank the two anonymous reviewers for their valuable help in improving the manuscript analyses and structure. We are deeply grateful to Dr A. Russell-Smith who -as a native speaker – performed a thorough linguistic revision of the last version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zografou, K., Adamidis, G.C., Komnenov, M. et al. Diversity of spiders and orthopterans respond to intra-seasonal and spatial environmental changes. J Insect Conserv 21, 531–543 (2017). https://doi.org/10.1007/s10841-017-9993-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-017-9993-z