Abstract
Most freshwater lakes in continental Antarctica are in a paradoxical situation as they are in nutrient-poor conditions despite luxuriant vegetation growth covering the entire lakebed. Although the phytobenthos possibly take up nutrients from inside lakebeds, the amount of nutrients and their utilization by these phytobenthos are unclear. Sediment cores were collected from 17 freshwater lakes in East Antarctica, then dissolved inorganic nitrogen (DIN) and phosphate of the lake waters, and the vertical profiles of the interstitial water in the sediment cores were analyzed. Here we revealed that there are abundant nutrients inside lakebeds surface with 3–220 times the amount of DIN and 2–102 times concentration of phosphate than those in lake water, and the nutrient profile inside the sediment suggested that the phytobenthos can utilize the much nutrients from lakebeds. We also show that nitrogen stable isotope ratios of shallower phytobenthos lying on the small amount of nutrients in a lake are similar to that of terrestrial cyanobacteria possessing N2 fixation ability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antarctica is an ice continent. It has one of the most extreme environments for life in the world. There are very little ice-free regions with life in it, so these regions are sometimes called oases within the polar desert. The ice-free regions are scattered along the coastal regions and around mountainous peaks (nunataks) in continental Antarctica or concentrated in Antarctic Peninsula in maritime Antarctica. The biota is simple due to the lack of remarkable organisms at the top of food webs, and primary producers such as cyanobacteria, algae, lichens, mosses, heterotrophic microorganisms and metazoans dominate the sparse communities. The availability of liquid water is limited; thus, activity is suppressed during most part of the year.
A remarkable diversity of lakes exists in Antarctica, ranging from hypersaline with more than 10 times salinity of seawater, to brackish and freshwater, sub-glacial, permanently ice-covered and seasonally ice-covered lakes (Vincent et al. 2008). These lakes are unproductive with typical photosynthetic levels of 0.1–30 µg-C/L/day (Vincent 1981; Vincent and Vincent 1982; Priscu 1995; Lizotte et al. 1996; Bayliss et al. 1997; Laybourn-Parry et al. 2001; Kaup 2005; Vincent et al. 2008) from phytoplankton. Phytoplankton can hardly survive in the water column during the summer that is most light available period (Tanabe et al. 2008). This results from low annual levels of photosynthetically active radiation and ice cover that attenuate light into the water column or photo-inhibit photosynthetic systems due to continuous low temperatures and the lack of any significant input of inorganic nutrients (Laybourn-Parry 2002). Limited productivity due to limited nutrient availability is an established paradigm for these lake ecosystems (Carpenter 2008; Sterner 2008). Despite the variability in the chemical characteristics of the Antarctica lakes, most water bodies are considered N limited (Hawes et al. 1993; Vincent and Howard-Williams 1994) and P limited (Vincent and Vincent 1982; Kaup 1994; Priscu 1995; Dore and Priscu 2001).
Despite such nutrient-poor situations, lakes in some regions of Antarctica have a few meters of luxuriant vegetation coating the entire lakebed (Kudoh et al. 2009; Tanabe et al. 2010). Under the nutrient-poor conditions, any N input into the ecosystem will be of significance for biota. N2 fixation by cyanobacteria plays an important role in nitrogen budgets of ecosystems in polar regions (Christie 1987; Nakatsubo and Ino 1987; Line 1992; Lennihan et al. 1994; Liengen and Olsen 1997). Cyanobacteria are major components of the Antarctic glacial environment, cyanobacterial mats exist in a wide range of lacustrine habitats (Howard-Williams et al. 1990; Pearce and Galand 2008; Vincent 2000; Tarton et al. 2011; Vincent and Quesada 2012), and this organism is known as a pioneer at the earliest stage of primary succession in this ecosystem (Schmidt et al. 2008). Many studies have been conducted about the cyanobacterium Nostoc (Davey 1983) or cyanobacterial communities dominated by heterocystous N2-fixing species, cyanobacterial N2-fixing activities associated with mosses (Smith and Russell 1982; Christie 1987; Pandey et al. 1992), and the structure and function of microbial communities including cyanobacteria (Lauro et al. 2011; Velázquez et al. 2016), lichens and angiosperms (Nakatsubo and Ino 1987). N2 fixation provides 10 % of the nitrogen required to support benthic cyanobacterial mats in Antarctic glacial melt streams (Howard-Williams et al. 1989). Although most regions in continental Antarctica are dominated by cyanobacteria, most lakes on the Sôya Coast region are dominantly lined not only by cyanobacteria but also by many green algae and two species of mosses coexisting in a community (Imura et al. 1999, 2003; Kudoh et al. 2009; Tanabe et al. 2010).
Benthic phototrophs may utilize more nutrient resources from the lakebed where they live than from the water column. Although nutrient concentrations in benthic mats have been measured in Arctic ponds and lakes (Villeneuve et al. 2001; Bonilla et al. 2005; Rautio and Vincent 2006) and a few lakes in Antarctic peninsula (Hansson 1992), in Antarctic continent that is just a hypothesis and the actual nutrient deposits that exist and nutrients availability by phototrophs have not been clearly investigated. Our study aimed to reveal the amount of nutrients in lake sediments and possible utilization by the phytobenthic communities living on top of the lakebeds. Then, we tested a hypothesis that the benthic phototrophs are using more nutrient resources from the lakebed than they derive from N2 fixation. To do so, we collected lake sediments and compared dissolved inorganic nutrients in interstitial waters of benthic mats with that in lake waters from 17 oligotrophic freshwater lakes in Sôya Coast, Lützow-Holm Bay, East Antarctica. To reveal possible nutrients uptake by phytobenthic communities, we measured the amount of dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphorus (DIP) and investigated the relationship between the carbon/nitrogen (C/N) ratio of the phytobenthos as an indicator of N limitation and the vertical profile of DIN in sediments. Finally, to assess the contribution of N2 fixation, we measured nitrogen stable isotope ratios (δ15N) of terrestrial cyanobacteria living near the lakeshore and the phytobenthos on different thicknesses of sediment at different water depths.
Methods
Study site
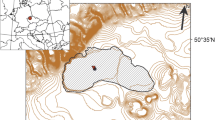
The Skarvsnes (69°20′S, 39°36′E), Langhovde (69°14′S, 39°40′E) and Skallen (69°40′S, 39°25′E) are ice-free areas of the Sôya Coast near Syowa station in East Antarctica and are located on the eastern shore of Lützow-Holm Bay (Fig. 1). More than 100 lakes diverse in size, shape and limnological characteristics occur in these areas (Nakatsubo and Ino 1987; Tanabe et al. 2008, 2010), including freshwater lakes affected by continental glaciers and saline lakes that have evaporated following their isolation from the ocean during the Holocene glacio-isostatic uplift (Miura et al. 1998; Takano et al. 2012). Figure 1 shows the 17 studied lakes in Langhovde, Skarvsnes, Skallen and Breidvågnipa (an ice-free area indicating a lake no. 16 in left map of Fig. 1). The number, name, limnological parameters and the other information are summarized in Table 1.
Map showing the distribution of ice-free areas along the Sôya Coast near Syowa station, East Antarctica. Detailed location maps of the studied lakes are shown with the numbers 1–17 at Skarvsnes, Langhovde and Skallen. Only lake number 16 is shown in the large area map. Table 1 lists the lake numbers and names. The maps were created using Adobe Illustrator CS6 based on topographic maps issued by Geospatial Information Authority of Japan
Sampling and sample procedure
Exact depths of the sampling sites were checked using an ultrasonic depth meter (PS-7, Hondex) whenever the boat drifted significantly from the lake center. Lake sediments were collected from the 17 lakes using a gravity corer (diameter 5 cm, length ~50 cm) during the 51st Japanese Antarctic Research Expedition (JARE) in 2009–2010 and 53rd JARE in 2011–2012. All sampling and measurement were carried out in lake ice-free period. The water depths and GPS positions of the sampling points in each lake are shown in Table 1. Six sediment cores were collected at different water depths of 1, 2, 4, 7, 8 and 10 m along a line from the southwestern lakeshore to the deepest lake center using transparent acrylic pipe (diameter 5 cm, length 50 cm at 1, 2, 4, 8 and 120 cm at 7 and 10 m) by scuba diving in Lake Naga Ike (Fig. 2, and lake no. 8 in Table 1). The thickness of the sediments was determined with a metal stick (diameter 2 cm, length 150 cm) at each water depth of 7, 8 and 10 m. The thickness formed by detritus at 1, 2 and 4 m was determined from the collected sediment cores. A 100-mL aliquot of lake water was collected using a cylindrical water sampler (volume 500 mL) from mid-depth of the water column at the same position that the sediments were collected because the water column in all lakes revealed vertically well-mixed properties in the summer after assessing the vertical limnological profile using a multi-water quality logger (YSI-6600V2, YSI). Terrestrial cyanobacteria, Nostoc commune, colonies were collected on the ground approximately 50–100 cm away from the shoreline of Lake Naga Ike on January 28, 2011.
The six sediment cores collected from Lake Naga Ike were cut into 0.5–1 cm each in the order of depth from top to bottom, and the sediment cores collected from the 17 lakes were cut into 1 and 4 cm intervals repeatedly in the order of depth from top to bottom and then separated into a solid portion and water portion using a centrifuge for 10 min at 1700×g under ambient temperature (3–8 °C) in a field laboratory as soon as possible (usually within a few hours after sample collection). The separated interstitial water and lake water were passed through cellulose acetate syringe filters (pore size = 0.2 µm, DISMIC, Advantec). All solid and water samples were stored at −20 °C for further chemical analysis.
Analysis of water, element and stable isotopes
For all sediment core samples, dissolved inorganic nutrients (PO4 −, NH4 +, NO2 − and NO3 −) were determined colorimetrically using an autoanalyzer, AACS III autoanalyzer system (Bran + Luebbe, Japan) for PO4 − and QuAAtro-Marine 5ch autoanalyzer system (SEAL Analytical, UK) for NH4 +, NO2 − and NO3 −.
The solid portion of the sediments was freeze-dried and ground with an agate mortar and pestle and then treated with 1 N HCl in a silver cup to remove the CaCO3 −. Samples were combusted at 1020 °C in an elemental analyzer to determine organic CN content and nitrogen stable isotope ratios (δ15N) simultaneously (EA-1108 CHNS-O element analyzer, Fisons). The combustion products (N2) were introduced into an isotope ratio mass spectrometer (Delta Plus, Finnigan) under continuous flow using a He carrier. The ratio of 15N:14N was expressed relative to N2 in air for nitrogen. The ratio of 15N:14N was calculated as:
where R = 15N/14N.
Isotope analysis reproducibility was determined with L-a-alanine (standard deviation, ±0.11 with n = 11 for δ15N). Accuracy was determined using interlaboratory-determined nitroarginine following the method of IAEA-N1 for δ15N (0.54 ‰). The amount of nitrogen in the standard and samples was 11–86 µg.
The DIN accumulation (µmol/L) in the sediment was the sum of DIN concentration in 0–8 cm which is the maximum thickness of the detritus at 1 and 2 m, and in 0–25 cm at 4, 7, 8 and 10 m.
The decrease in the DIN concentration on the top of each core (characterized by ∆DINsurf) to estimate nutrient availability by phototrophs was calculated as:
where C ′surf (µmol/L) is the DIN concentration at 0–1 cm depth of interstitial water in the sediment cores calculated by linear regression of DIN vertical profiles from 5 to 50 cm as diffusive flux, C surf is the DIN concentration at 0–1 cm depth of interstitial water in the sediment core (µmol/L).
Results
The surfaces of the sediment cores collected in this study consisted of dozens species of phototrophs, dominated by cyanobacteria (Leptolyngbya perelegans, Leptolyngbya tenuis and Nostoc spp.), green algae (Oedogonium sp., Thorakochloris sp., Kentrosphaera grandis and Chlamydomonas sp.), and a few diatoms in all of the freshwater lakes around the Sôya Coast region, and one or two species of aquatic moss [mainly Leptobryum willsonii (Mitt.) and Bryum sp.] in a few lakes. The dominant vegetation composition of the each benthic mat is indicated in Table 1.
Nutrient concentrations of the water in all lakes were 0.026–0.262 and 0.41–1.14 µmol/L for phosphate and DIN, respectively, which is an oligotrophic level (Table 2). The surfaces (upper sections, 0–1 cm) of sediment cores contained a large amount of phosphate (0.11–4.70 µmol/L) and ammonium as the major DIN component (1.6–208.0 µmol/L), with a concentration of 2–102 times higher phosphate and 3–220 times higher DIN than those of the lake water. Nitrate and nitrite were negligible in the interstitial water of all sediment cores. N/P ratios calculated from DIN and phosphate range from 2.9 to 24.8 (mean value 13.8) in the lake waters and from 6.8 to 347.8 (mean value 72.6) in the surface pore waters (Table 2).
Figures 3 and 4 show the vertical profiles of the DIN and phosphate concentration in the sediment cores from Lake Naga Ike at different water depths (Fig. 2). The DIN concentrations increased from the surface toward deeper section and peaks occurred near the deepest section in all sediments (Fig. 3). The gradients of the DIN concentration were large at the deeper water depths (>4 m) in Fig. 3c–f, although it was small at the shallow area (1 and 2 m) in Fig. 3a, b; each peak value was 22–1700 times higher than that of the surface in the deeper water depths (>4 m). The DIN concentration gradient changed drastically in the surface sections (<ca. 5 cm depth) compared with the underlying section (deeper than ca. 5 cm). The phosphate concentration profiles showed similar patterns to the DIN profiles that increase from surface toward the deepest section at the deeper water depths (>4 m) in Fig. 4c–f although low concentration overall, and the phosphate could not be detected at 1 and 2 m deep in Fig. 4a, c. However, the phosphate concentrations drop to almost 0 µmol/L at ca. 5 cm deep in contrast to DIN.
Figure 5 shows DIN accumulations in the sediments and δ15N of the sediment surfaces consisting of live organisms at each sampling water depth along the lakeshore to the deepest point in Lake Naga Ike, and Fig. 5 shows the sediment thickness derived mainly from detritus at each water depths. There were a large amount of DIN accumulations at the deeper areas than 4 m depth (1396–1853 µmol/L), but the amount was quite small at the shallower areas 1 and 2 m (182 and 38 µmol/L) in Fig. 5a. δ15N values were similar at deeper areas than 4 m depth (−0.3 to 0.2 ‰), but the values were large and negative toward the lakeshore (−0.8 ‰ at 2 m and −1.4 ‰ at 1 m). Terrestrial cyanobacterial colony, N. commune, living near the lakeshore of Lake Bosatsu Ike had a more negative δ15N value (−2.2 ‰). The thicknesses of the benthic detritus were >150 cm at deeper areas such as 7, 8 and 10 m, but the thicknesses were only 8 cm at 1 and 2 m, and the bedrock lay under the bottom of 8 cm sediments (Fig. 5). The thickness was judged to be >50 cm at 4 m, as the sediment core (50 cm in length) used for the sampling was fully filled with detritus.
The DIN accumulation in the sediments (filled circle), 0–8 cm for 1 and 2 m depth, and 0–25 cm for depth >4 m (a). The nitrogen stable isotope ratio δ15N of the sediment surfaces (open circle) and terrestrial cyanobacteria, N. commune (open square) indicated in the gray area (a). The thickness of benthic microbial and detritus mat at each water depth (b). The thicknesses indicated by black circle (filled circle) were maximum thickness of benthic mats at 1 and 2 m depth, and white circle (open circle) at a depth >4 m showed the core length collected in this study; the actual maximum thicknesses of the benthic mat were thicker than the value indicated in the figure
The carbon and nitrogen content in the solid portion of the sediment core surface (0–1 cm depth) consisting of live phototrophs is shown in Table 2, and the values varied widely within each lake with 7.3–30.0 % carbon, 0.3–2.5 % nitrogen and a 8.9–22.6 C/N ratio. The ∆DINsurf as estimation of nutrient availability in the top was negatively correlated with the C/N ratio of the sediment surfaces from 17 lakes in Fig. 6 (r = −0.61, p = 0.009).
Discussion
Antarctic ecosystems are either oligotrophic or ultraoligotrophic, and many studies have been conducted with a view to study this unproductive condition in Antarctic lakes (Laybourn-Parry 2002). In the Arctic and Antarctic regions the cyanobacterial communities are widespread in lakes, ponds, streams, glaciers and ice shelves, and they often dominate total biomass and biological productivity by adapting to the severe polar environment (Paerl et al. 2000; Vincent 2000; Hodgson et al. 2004). Under these circumstances, any N input in the ecosystem will be of significance for the biota, because most of the water bodies are usually N limited in freshwater ponds and shallow lakes in Antarctica (Davey 1993; Hawes et al. 1993) if the lakes do not locate near marine animal colonies. Not only nitrogen but also the phosphorus availability may limit microbial growth in almost polar lakes (Dore and Priscu 2001; Laybourn-Parry and Wadham 2014).
Biological N2 fixation sometimes provides up to 10 % of the nitrogen required to support benthic cyanobacterial mats in Antarctica (Howard-Williams et al. 1989). Therefore, many studies have been conducted on the ecological importance of N2 fixation in cyanobacterial mats in a polar lacustrine habitat, particularly Antarctica (Fernández-Valiente et al. 2001).
Although every lake around the present study area was oligotrophic level in lake water body, a dissolved inorganic nutrient analysis from the 17 lakes revealed the presence of a large amount of nutrients in the interstitial water of the lake sediments, with DIN concentrations 3–220 times higher and phosphate concentration 2–102 times higher than that of lake water below the sediment surface at 1 cm (Table 1). The DIN and phosphate accumulated much more in whole lakebeds (from the surface of the lakebed to the bedrock) because the concentration increased toward the deeper section of the sediment cores (Figs. 3, 4). Lake Skallen Ô-ike contained 208.0 µmol/L of DIN in the sediment surface, which was the highest of the studied lakes, and this concentration was more than that of Lake Shinji (55–180 µmol/L), a eutrophic lake in Japan (Yamamuro and Koike 1998), and other eutrophic lakes in temperate area (50–120 µmol/L in Switzerland and 200 µmol/L in USA) (Wersin et al. 1991; Urban et al. 1997; Koretsky et al. 2006), and nearly one order of magnitude more than that of the other oligotrophic lake in Switzerland (20–50 µmol/L) (Müller et al. 2007).
The gradient of vertical DIN and phosphate profiles changed clearly between the deeper layer and the surface layer at ca. 5 cm depth (Figs. 3, 4). According to the concept of molecular diffusion, concentration decreases linearly in the direction from the deep layer to the upper layer in the lake sediment. These benthic mats mainly consisted of photosynthetic organisms in this region, which are aerobic environments in the surface layer because solar radiation penetrates into the benthic mats approximately 2 cm under the surface, and photosynthetic activity occurs from the surface to 2–3 cm depth (Tanabe et al. 2010). As is the case in nitrogen, the phosphate concentration was quite low, but 2–102 times higher in benthic surfaces than in lake waters, and phosphate accumulated at higher concentration toward the deeper section. However, the phosphate concentrations drastically dropping at ca. 5 cm deep in the sediments, and the average of N/P ratio 72.6 with almost lakes having more than 16 according to Redfield ratio, suggest that phosphorus is limited more than nitrogen.
Little nitrate exists on the sediment surface, but denitrification from nitrate to N2 by denitrifying bacteria is not expected due to the aerobic conditions on the surface. The anammox bacteria may be key players in the nitrogen cycle in natural marine environments (Devol 2003), as they produce N2 by anaerobic oxidation of ammonia. Although there was a large amount of ammonium in the sediments of the studied lakes, it is reasonable that the anammox reaction does not occur in this environment in which only a small amount of nitrite was detected in only a few lakes, as this reaction requires nitrite as an electron donor (Strous and Jetten 2004). Therefore, the decrease in DIN on the sediment surface in our data indicates that the active parts of phytobenthos on the sediment surfaces may utilize these nutrient sources and are remineralized by the bacteria supplied from the lakebeds. The negative correlation between D surf and C/N ratio may have indicated that the present relatively N-rich phytobenthic communities among the 17 lakes have appeared as a result of utilizing larger N resources (Fig. 6).
The δ15N of the phytobenthic communities was higher near the deeper section than that of the shallow section near shore in the same lake (Fig. 5). N2 fixation was considered to not be so necessary for the phytobenthic communities in the deeper area because much nutrients accumulate beneath the thick mats formed by detritus. However, it is thought that the shallow phytobenthic communities may not obtain enough nutrients due to their thinness but are dependent on terrestrial cyanobacteria possessing N2 fixation ability which cover the lake shoreline and streams in a patchy fashion on the water catchment area as is suggested by their similar δ15N values.
This study revealed that there are high N and P budgets inside the present Antarctic lakebeds extending well beyond the amount inside temperate lakes. Our study area is thought to contain a relatively high amount of benthic phototrophic organisms in freshwater habitats contrasting with other areas of continental Antarctica. Rich green algae often dominate more than cyanobacteria (Tanabe and Kudoh 2012), and algal-coated large moss pillars up to 60 cm high are found in only two areas of continental Antarctica, the Sôya Coast region (Imura et al. 1999) and Maitri station region at Schirmacher Oasis (Tewari and Pant 2004). The diverse vegetation may be the result of the abundant nutrients that have accumulated in the lake sediments although it is not clear how much amount of interstitial nutrients exist inside lakebeds in the other oases of continental Antarctica. Input of nutrients from the water catchment of Antarctic freshwater lakes is considerably low, and cyanobacterial N2 fixation would have been especially an important source for these lake ecosystems at the first stage after glacier retreat such as thin benthic mat situation at the shallow area of the present lake; however, the continuous input even if very little for long time from birth of the Antarctic lakes after Last Glacial Maximum and activity of organisms during such long time in closed system are thought to provide abundant accumulation of nutrients inside lakebeds with developing benthic vegetation until present. Also, this abundant accumulation in lakebeds possibly may give important nutrient source to the growth of phytoplankton community which constantly exposed to nutrient-poor situation in the water column.
References
Bayliss P, Ellis-Evans JC, Laybourn-Parry J (1997) Temporal patterns of primary production in a large ultraoligotrophic Antarctic freshwater lake. Polar Biol 18:363–370. doi:10.1007/s003000050201
Bonilla S, Villeneuve V, Vincent WF (2005) Benthic and planktonic algal communities in a high arctic lake: pigment structure and contrasting responses to nutrient enrichment. J Phycol 41:1120–1130
Carpenter SR (2008) Phosphorus control is critical to mitigating eutrophication. Proc Natl Acad Sci USA 105:11039–11040. doi:10.1073/pnas.0806112105
Christie P (1987) Nitrogen in two contrasting Antarctic bryophyte communities. J Ecol 75:73–93. doi:10.2307/2260537
Davey A (1983) Effects of abiotic factors on nitrogen fixation by blue-green algae in Antarctica. Polar Biol 2:95–100. doi:10.1007/BF00303174
Davey MC (1993) Carbon and nitrogen dynamics in a small pond in the maritime Antarctic. Hydrobiologia 257:165–175. doi:10.1007/BF00765009
Devol AH (2003) Solution to a marine mystery. Nature 422:575–576. doi:10.1038/422575a
Dore JE, Priscu JC (2001) Phytoplankton phosphorus deficiency and alkaline phosphatase activity in the McMurdo Dry Valley lakes, Antarctica. Limnol Oceanogr 46(6):1331–1346. doi:10.4319/lo.2001.46.6.1331
Fernández-Valiente E, Quesada A, Howard-Williams C, Hawes I (2001) N2-fixation in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Microb Ecol 42:338–349. doi:10.1007/s00248-001-1010-z
Hansson L-A (1992) Factors regulating periphytic algal biomass. Limnol Oceanogr 37(2):322–328. doi:10.4319/lo.1992.37.2.0322
Hawes I, Howard-Williams C, Pridmore RD (1993) Environmental control of microbial biomass in the ponds of the McMurdo Ice Shelf, Antarctica. Arch Hydrobiol 127:271–287
Hodgson DA, Vyverman W, Verleyen E, Sabbe K, Leavitt PR, Taton A, Squier AH, Keely BJ (2004) Environmental factors influencing the pigment composition of in situ benthic microbial communities in east Antarctic lakes. Aquatic Microb Ecol 37:247–263. doi:10.3354/ame037247
Howard-Williams C, Priscu J, Vincent WF (1989) Nitrogen dynamics in two Antarctic streams. Hydrobiologia 172:51–61. doi:10.1007/BF00031612
Howard-Williams C, Pridmore R, Broady P, Vincent WF (1990) Environmental and biological variability in the McMurdo ice Shelf Ecosystem. In: Kerry KR, Hempel G (eds) Antarctic ecosystems: ecological change and conservation. Springer, Berlin, pp 23–31
Imura S, Bando T, Saito S, Seto K, Kanda H (1999) Benthic moss pillars in Antarctic lakes. Polar Biol 22:137–140. doi:10.1007/s003000050401
Imura S, Bando T, Seto K, Ohtani S, Kudoh S, Kanda H (2003) Distribution of aquatic mosses in the Sôya Coast region, East Antarctica. Polar Biosci 16:1–10
Kaup E (1994) Annual primary production of phytoplankton in Lake Verkhneye, Schirmacher Oasis, Antarctica. Polar Biol 14:433–439. doi:10.1007/BF00239045
Kaup E (2005) Development of anthropogenic eutrophication in lakes of the Schirmacher Oasis, Antarctica. Proc Int Assoc Theor Appl Limnol 29(2):678–682
Koretsky CM, Haas JR, Miller D, Ndenga NT (2006) Seasonal variations in pore water and sediment geochemistry of littoral lake sediments (Asylum Lake, MI, USA). Geochem Trans 7:11. doi:10.1186/1467-4866-7-11
Kudoh S, Tanabe Y, Matsuzaki M, Imura S (2009) In situ photochemical activity of the phytobenthic communities in two Antarctic lakes. Polar Biol 32(11):1617–1627. doi:10.1007/s00300-009-0660-z
Lauro FM, DeMaere MZ, Yau S, Brown MV, Ng C, Wilkins D, Raftery MJ, Gibson JA, Andrews-Pfannkoch C, Lewis M, Hoffman JM, Thomas T, Cavicchioli R (2011) An integrative study of a meromictic lake ecosystem in Antarctica. ISME J 5:879–895. doi:10.1038/ismej.2010.185
Laybourn-Parry J (2002) Survival mechanisms in Antarctic lakes. Philos Trans R Soc Lond B 357:863–869. doi:10.1098/rstb.2002.1075
Laybourn-Parry J, Wadham J (2014) Antarctic lakes. Oxford University Press, Oxford. doi:10.1093/acprof:oso/9780199670499.001.0001
Laybourn-Parry J, Quayle WC, Henshaw T, Ruddel A, Marchant HJ (2001) Life on the edge: the plankton and chemistry of Beaver Lake an ultra-oligotrophic epishelf lake, Antarctica. Freshw Biol 46:1205–1217. doi:10.1046/j.1365-2427.2001.00741.x
Lennihan R, Chapin DM, Dickson LG (1994) Nitrogen fixation and photosynthesis in high arctic forms of Nostoc commune. Can J Bot 72:940–945. doi:10.1139/b94-119
Liengen T, Olsen RA (1997) Nitrogen fixation by free-living cyanobacteria from different coastal sites in a high Arctic tundra, Spitsbergen. Arct Alp Res 4:470–477
Line MA (1992) Nitrogen fixation in the sub-Antarctic Macquire Island. Polar Biol 11:601–606. doi:10.1007/BF00237954
Lizotte MP, Sharp TR, Priscu JC (1996) Phytoplankton dynamics in the stratified water column of Lake Bonney Antarctic. 1. Biomass and productivity during the winter–spring transition. Polar Biol 16:155–162. doi:10.1007/BF02329203
Miura H, Maemoku H, Igarashi A, Moriwaki K (1998) Late quaternary raised beach deposits and radiocarbon dates of marine fossils around Lützow-Holm Bay. Special map series of NIPR 6
Müller B, Finger D, Sturm M, Prasuhn V, Haltmeier T, Bossard P, Hoyle C, Wüest A (2007) Present and past bio-available phosphorus budget in the ultra-oligotrophic Lake Brienz. Aquatic Sci 69:227–239. doi:10.1007/s00027-007-0871-8
Nakatsubo T, Ino Y (1987) Nitrogen cycling in an Antarctic ecosystem 2. Estimation of the amount of nitrogen fixation in a moss community on East Ongul Island. Ecol Res 2:31–40. doi:10.1007/BF02348617
Paerl HW, Pinckney JL, Steppe TF (2000) Cyanobacterial-bacterial mat consortia: examining the functional unit of microbial survival and growth in extreme environments. Environ Microbiol 2(1):11–12. doi:10.1046/j.1462-2920.2000.00071.x
Pandey KD, Kashyap AK, Gupta RK (1992) Nitrogen fixation by cyanobacteria associated with moss communities in Schirmacher Oasis, Antarctica. Israel J Bot 41:187–198
Pearce DA, Galand PE (2008) Microbial biodiversity and biogeography. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers. Oxford University Press, Oxford, pp 213–230
Priscu JC (1995) Phytoplankton nutrient deficiency in lakes of the McMurdo Dry Valleys, Antarctica. Freshw Biol 34:215–227
Rautio M, Vincent WF (2006) Benthic and pelagic food resources for zooplankton in shallow high-latitude lakes and ponds. Freshw Biol 51:1038–1052
Schmidt SK, Reed SC, Nemergut DR, Grandy AS, Cleveland CC, Weintraub MN, Hill AW, Costello EK, Meyer AF, Neff JC, Martin AM (2008) The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proc R Soc B 275:2793–2802. doi:10.1098/rspb.2008.0808
Smith VR, Russell S (1982) Acetylene reduction by bryophite cyanobacteria associations on a Subantarctic island. Polar Biol 1:153–157. doi:10.1007/BF00287001
Sterner RW (2008) On the phosphorus limitation paradigm for lakes. Int Rev Hydrobiol 93:433–445. doi:10.1002/iroh.200811068
Strous M, Jetten MSM (2004) Anaerobic oxidation of methane and ammonium. Annu Rev Microbiol 58:99–117. doi:10.1146/annurev.micro.58.030603.123605
Takano Y, Tyler JJ, Kojima H, Yokoyama Y, Tanabe Y, Sato T, Ogawa NO, Ohkouchi N, Fukui M (2012) Holocene lake development and glacial-isostatic uplift at Lake Skallen and Lake Oyako; Lützow-Holm Bay; East Antarctica: based on biogeochemical facies and molecular signatures. Appl Geochem 27(12):2546–2559. doi:10.1016/j.apgeochem.2012.08.009
Tanabe Y, Kudoh S (2012) Possible ecological implications of floating microbial assemblages lifted from the lakebed on an Antarctic lake. Ecol Res 27:359–367. doi:10.1007/s11284-011-0907-3
Tanabe Y, Kudoh S, Imura S, Fukuchi M (2008) Phytoplankton blooms under dim and cold conditions in freshwater lakes of East Antarctica. Polar Biol 31:199–208. doi:10.1007/s00300-007-0347-2
Tanabe Y, Ohtani S, Kasamatsu N, Fukuchi M, Kudoh S (2010) Photophysiological responses of phytobenthic communities to the strong light and UV in Antarctic shallow lakes. Polar Biol 33(1):85–100. doi:10.1007/s00300-009-0687-1
Tarton A, Wilmotte A, Šmarda J, Elster J, Komárek J (2011) Plectolyngbya hodgsonii: a novel filamentous cyanobacterium from Antarctic lakes. Polar Biol 34(2):181–191. doi:10.1007/s00300-010-0868-y
Tewari SD, Pant G (2004) Some moss collections from Dakshin Gangotri, Antarctica. Bryol Times 91:7
Urban N, Dinkel C, Wehrli B (1997) Solute transfer across the sediment surface of a eutrophic lake: I. Porewater profiles from dialysis samplers. Aquat Sci 59:1–25. doi:10.1007/BF02522546
Velázquez D, López-Bueno A, de Cárcer DA, de Los Ríos A, Alcamí A, Quesada A (2016) Ecosystem function decays by fungal outbreaks in Antarctic microbial mats. Sci Res 6:22954. doi:10.1038/srep22954
Villeneuve V, Vincent WF, Komarek J (2001) Community structure and microhabitat characteristics of cyanobacterial mats in an extreme high Arctic environment: Ward Hunt Lake. In: Elster J, Seckbach J, Vincent WF, Lhotsky O (eds) Algae and extreme environments. Nova Hedwigia Beihefte 123:199–224
Vincent WF (1981) Production strategies in Antarctic inland waters: phytoplankton eco-physiology in a permanently ice-covered lake. Ecology 62(5):1215–1224. doi:10.2307/1937286
Vincent WF (2000) Cyanobacterial dominance in the polar regions. In: Whitton B, Potts M (eds) Ecology of the cyanobacteria: their diversity in space and time. Kluwer Academic, Dordrecht, pp 321–340
Vincent WF, Howard-Williams C (1994) Nitrate-rich inland waters of the Ross Ice Shelf region, Antarctica. Antarct Sci 6:339–346. doi:10.1017/S0954102094000519
Vincent WF, Quesada A (2012) Cyanobacteria in high latitude lakes, rivers and seas. In: Whitton BA (ed) Ecology of cyanobacteria II—their diversity in space and time. Springer, Dordrecht, pp 371–3853. doi:10.1007/978-94-007-3855-3_13
Vincent WF, Vincent CL (1982) Factors controlling phytoplankton production in Lake Vanda (77S). Can J Fish Aquat Sci 39:1602–1609. doi:10.1139/f82-216
Vincent WF, Hobbie JE, Laybourn-Parry J (2008) Introduction to the limnology of high-latitude lake and river ecosystems. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers. Oxford University Press, Oxford, pp 1–23. doi:10.1093/acprof:oso/9780199213887.003.0001
Wersin P, Höhener P, Giovanoli R, Stumm W (1991) Early diagenetic influences on iron transformations in a freshwater lake sediment. Chem Geol 90:232–252. doi:10.1016/0009-2541(91)90102-W
Yamamuro M, Koike I (1998) Concentrations of nitrogen in sandy sediments of a eutrophic estuarine lagoon. Hydrobiologia 386:37–44. doi:10.1023/A:1003414028040
Acknowledgments
This study was supported by a research grant of a project under the Centre for the Promotion of Integrated Sciences (CPIS) of the Graduate University for Advanced Studies (SOKENDAI) and by JSPS KAKENHI Grant Numbers 26310213 and 21810035. The authors acknowledge the members of the 51st and 53rd Japanese Antarctic Research Expedition for their field support, particularly the summer party leaders, Prof. Y. Motoyoshi and Prof. H. Yamagishi, for their logistic support. We also thank Ms. C. Ôuchida and Prof. J. Kanda of Tokyo University of Marine Science and Technology for assistance and for providing the nutrient analysis facilities and Dr. E. Cooper of University of Tromsø for English proofreading.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanabe, Y., Yasui, S., Osono, T. et al. Abundant deposits of nutrients inside lakebeds of Antarctic oligotrophic lakes. Polar Biol 40, 603–613 (2017). https://doi.org/10.1007/s00300-016-1983-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-1983-1