Abstract
Shallow lowland lakes undergo long-lasting natural eutrophication processes, which can be studied through the development of communities of aquatic organisms. However, records showing millennial-scale trophic status variability in these water bodies are rare. Two radiocarbon-dated sedimentary profiles from former (now destroyed by brown coal mining) Lake Komořany (Central Europe, Czech Republic) served for a multi-proxy study of biological remains (diatoms, chironomids, pollen) supplemented by X-ray fluorescence (XRF) and loss-on-ignition (LOI). The age–depth model and palynostratigraphy confirm a continuous Late-Glacial to Early-Holocene record. The results suggest consistent in-lake conditions with high nutrient availability since the lake origin in the Late-Glacial period. A distinct shift at the Late-Glacial/Holocene boundary evidenced by an enhancement in diatom valve concentration and a lithological interface was foregone by a qualitative change in diatom and chironomid assemblages along with rise in LOI. It suggests that a major transformation occurred before the onset of the Holocene. As this qualitative change was characterized by a decrease in relative abundance of nutrient-demanding species, we propose an indirect climatic control by means of nutrient availability as the main driver of the aquatic species composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abrupt climatic changes connected with the Late-Glacial/Holocene (LG/H) transition distinctly altered the species composition of terrestrial (e.g. Lotter, 1999; Hošek et al., 2014; Orbán et al., 2018) and freshwater communities (e.g. Goslar et al., 1993; Birks & Ammann, 2000; Heiri et al., 2014) in Europe. Lacustrine sediments serve as sensitive natural archives of these environmental changes (Cohen, 2003). Temperate shallow lakes provide a separate and highly complex category of sedimentary records due to their polymictic regime and distinct influence of macrophyte vegetation to ecosystem dynamics (Scheffer, 2004; Bennion et al., 2010). They can remain in a stable state supported by various mechanisms until crossing the critical nutrient threshold, leading to a change in the lake’s trophic state (Scheffer 1990; Jeppesen et al., 1998; Scheffer & Van Nes, 2007).
The trophic state development of shallow lowland lakes is driven by natural and anthropogenic factors (Cohen, 2003; Nõges et al., 2003). Although great attention is paid to human-induced eutrophication, shallow lowland lakes naturally tend towards a higher trophic state. Limited stratification in shallow basins prevents phosphorus losses to the hypolimnion and furthermore sustains phosphorus recycling at the water–sediment interface (Scheffer, 2004), which is favoured by a large bottom surface-to-water volume area ratio (Wetzel, 2001). Higher mean temperatures in low altitudes may further naturally enhance the input of nutrients due to faster weathering (Cardoso et al., 2007) but simply the large ratio between the catchment and lacustrine area and the terminal position in the hydrological cycle cause natural eutrophy (Cremer et al., 2004). Fortunately, the past trophic state can be estimated from the sedimentary record without directly measuring it. Such reconstructed past environmental conditions are based on multi-proxy studies combining sedimentary characteristics and biotic variables (Birks & Birks, 2006). For trophic state reconstruction, diatom and chironomid indicators are often used for their sensitivity to diverse aspects of trophic conditions (Brodersen & Lindegaard, 1999; Hall & Smol, 2010). Diatom and chironomid species composition is also affected by other factors including the abundance and diversity of macrophytes (Sayer et al., 1999; Brodersen et al., 2001; Sayer et al., 2010; Vermaire et al., 2013; Tarrats et al., 2018), water depth (Bennion, 1995; Barley et al., 2006) and chironomids additionally to the oxygen level (Brodersen & Quinlan, 2006). Despite this, and because of that their paleorecords represent an imprint of various environmental driving factors, diatoms and chironomids have a great potential to elucidate complex shallow-water ecosystem changes.
Although shallow lakes frequently occur in Central Europe, few studies have investigated the response of the lacustrine ecosystem to the LG/H transition in lowland shallow lake basins in this region. Shallow thermokarstic lakes in Poland (Apolinarska et al., 2012; Drzymulska et al., 2015; Gałka et al., 2015; Kołaczek et al., 2015; Pedziszewska et al., 2015; Mendyk et al., 2016; Zawiska et al., 2019) demonstrate lacustrine development along the gradient of glacier influence depending on the position towards the line of glaciation (Mirosław-Grabowska & Zawisza, 2018). German lowlands provide records of Holocene onset from the deeper Sacrower See (Kirilova et al., 2009; Enters et al., 2010) and shallow floodplain lakes of the Jeetzel Valley (Turner et al., 2013, 2014). Late-Glacial sediments were studied in the Pannonian Basin in paleolake Šúr (Petr et al., 2013) and in the largest lake of Central Europe—Balaton (Sümegi et al., 2008). In the Czech Republic, paleolimnological studies of the LG/H transition in shallow lowland lakes are lacking. From Czech non-montane localities at middle altitudes, a detailed record of the LG was found in the former Lake Švarcenberk (Pokorný & Jankovská, 2000; Hošek et al., 2014) and the LG/H transition was documented in the shallow lake Velanská Cesta (Bešta et al., 2009). Our target locality, Lake Komořany, reached the largest surface area (max ~ 25 km2) compared to other shallow lakes in the region of the Czech Republic.
A relatively high trophic state and ingrowth by aquatic plants were recorded in the Early Preboreal in Lake Komořany (Jankovská, 1983; Řeháková, 1983, 1986; Jankovská & Pokorný, 2013) but the initial trophic state of this lake before the climate change at the LG/H transition remains unexplored. As European shallow lowland lakes have undergone long-lasting cultural eutrophication (Anderson, 1995) since the Neolithic Age (Dreßler et al., 2011), scarce opportunities exist to study their dynamics and climatic relations without anthropogenic impact. Therefore, the reconstruction of past climatically induced trophic state changes at the LG/H transition may reveal important mechanisms hardly accessible by the research of more recent records.
The aim of this study is to investigate the response of a very shallow large lowland lake ecosystem to climatic changes at the LG/H transition. By analysing sedimentary diatoms, chironomids, pollen and geochemistry, we specifically aim to (i) detect effects of the climate on the lacustrine biota and (ii) reconstruct possible trophic state changes during the investigated period.
Study site
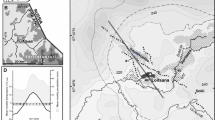
The former Lake Komořany was situated in north-western Bohemia, the Czech Republic, at the foothills of the Krušné Mountains at 230 m a. s. l. (50°32′N, 13°32′E) (Fig. 1). Its origin is likely linked to a Late-Glacial landslide event and the subsequent damming of the Bílina River (Hurník, 1969). This lowland, large (max. surface area ~ 25 km2) and very shallow lake (max. depth ~ 10 m) represented a unique water body type among other localities available in the region (Vondrák et al., 2015). The lake was gradually filled in with sediment until the artificial draining of its last remains (~ 2 km3) and surrounding mires in 1834 (Pokorný, 1963). Unfortunately, the last remnants of the unique lake sediment archive were destroyed in the 1980s by surface coal mining, because of an overlying Tertiary coalfield located in the Most Basin. However, several profiles were taken just before the complete removal of sediments and have been stored for further research (Jankovská, 1983, 1984, 1988; Jankovská & Pokorný, 2013).
Map of the study site with locations of the cores. The location of the former Lake Komořany (Czech Republic, Central Europe) indicated by a red dot. The location of the profiles PK-1-L (black dot) and PK-1-M (red triangle) indicated inside the estimated maximal area of the lake (hatched area) using a 230 m a. s. l. threshold, taken after Bešta et al. (2015)
Although its sediments have been investigated since the 19th century (Wettstein, 1896; Lühne, 1897), most studies have dealt with vegetation succession during the Holocene while the Late-Glacial (LG) record was only considered marginally (Rudolph, 1926; Losert, 1940; Neustupný, 1985). Similarly, the research of diatom succession was systematically conducted only in the Holocene sedimentary sequence (Řeháková, 1983, 1986; Bešta et al., 2015; Houfková et al., 2017). Our study presents the first multi-proxy research of the Lake Komořany record representing the LG/H transition based on the basal part of the profiles PK-1-L and PK-1-M.
Materials and methods
Sampling and lithology
Studied profiles PK-1-L and PK-1-M were sampled from the bottom of the former Dřínov reservoir by V. Jankovská, J. Kyncl, J. Klápště and J. Beneš in 1987 using Kubiena tins (50 × 10 × 8 cm metal boxes with one 50 × 10 cm side open). The profiles were stored at 4°C until 2008 when they were lithologically described and subsampled.
The basal parts of the profiles PK-1-L (128–143.6 cm) and PK-1-M (134–148 cm) were chosen for further analyses due to their supposed LG age. PK-1-L was subsampled at regular intervals of 2 mm (in two sections in depth of 139.4–140 cm and 142–143.6 cm at intervals of 4 mm) and processed for diatom, pollen and X-ray fluorescence (XRF) analyses. PK-1-M was subsampled at regular intervals of 1 cm and provided sufficient material for chironomid analysis. This was done due to low chironomid head capsule concentration in the sediment (see also Houfková et al., 2017) and the resulting need for relatively large samples (> 8 g of dry sediment). Additionally, XRF analysis was also processed on samples from PK-1-M.
The frequency and size of silica grains were subjectively monitored using a light microscope in parallel to diatom analysis in the profile PK-1-L. Four categories of clastic silica quantity were determined using a nominal scale: 0—grains absent or negligible, 1—grains rare, 2—grains common, 3—grains abundant and larger pieces (> 100 μm) present.
Chronology
Five bulk samples from the PK-1-L profile were dated by 14C AMS at the Center for Applied Isotope Studies (CAIS), University of Georgia, USA (Table 1). A Poisson-process deposition model (Bronk Ramsey, 2008) with k = 2.5 (Bronk Ramsey & Lee, 2013) based on the IntCal 13 calibration curve (Reimer et al., 2013) was constructed using the OxCal 4.2.4 application (Bronk Ramsey, 2013) with a boundary at the depth of 134 cm where sedimentation rate changed considerably. The basal bulk sample of the PK-1-M profile was dated using the same methods to date the start of sedimentation. The profiles were correlated by the fitting of Rb/Sr ratio curves acquired using XRF (Fig. 2).
Lithology and correlation of the profiles PK-1-L and PK-1-M. Clastic silica quantity from profile PK-1-L quoted in four categories: 0—grains absent or negligible, 1—grains rare, 2—grains common, 3—grains abundant and larger pieces (> 100 μm) present. Rb/Sr ratios plotted for both profiles. Clayey gyttja represented by a grey background, layered gyttja represented by a white background
Diatom analysis
The diatom record was analysed from 2-mm-thick layers in 0.4–0.6-mm intervals from the core PK-1-L. In total, 32 samples were processed from the depth interval of 128–143.6 cm. Laboratory processing and the subsequent quantitative analysis of diatom content followed standard procedures (Battarbee et al., 2001). After boiling in 30% hydrogen peroxide, the subsamples were carefully rinsed with distilled water. Purified solutions were thinned and an accurate volume gauged onto cover slides. Permanent mounts were created using Naphrax® mounting resin. The concentration of valves per dry weight (DW) of sediment was estimated using divinylbenzene microspheres as an internal standard.
At least 500 diatom valves were counted in each sample excluding dominant fragilarioid taxa (i.e. > 30%: Staurosira construens f. construens Ehrenberg, Staurosira construens f. venter (Ehrenberg) Bukhtiyarova). The dominants were enumerated separately in the resolution of 100 valves per sample. A similar approach has been already adopted in several studies (e.g. Battarbee, 1986; Heinsalu et al., 2008; Demiddele et al., 2016) in the case of a high abundance of fragilarioid taxa. Diatom slides were observed under × 1,000 magnification using the light microscope Nikon Eclipse E400 with a Canon EOS650D camera. Determination followed Krammer & Lange-Bertalot (1986, 1988, 1991a, b), Krammer (2000, 2002, 2003) and Lange-Bertalot (2001). Nomenclature was updated according to AlgaeBase (Guiry & Guiry, 2019) and unified with the approach of last diatom analyses from Lake Komořany (Bešta et al., 2015; Houfková et al., 2017). Autecology of constituent taxa was specified using mainly Denys (1991) and Van Dam et al. (1994) and complemented with information from determination literature where necessary.
Chironomid and other Diptera analyses
For chironomid analysis, 14 1-cm-thick layers from the core PK-1-M were used (depth interval of 130–144 cm according to PK-1-L profile’s scale). A known weight of dry sediment (8.6–22.3 g) was deflocculated in 1% potassium hydroxide at 70°C for 20 min and washed with distilled water onto a sieve with 100-μm mesh size. The remaining material was transferred from the sieve into a modified Sedgewick-Rafter counting chamber and all head capsules were picked with a needle using a stereomicroscope ( ×40 to ×50 magnification). After dehydration in 80% ethyl alcohol, head capsules were mounted on slides in Euparal® mounting medium and identified using a light microscope (125 to ×250 magnification). Taxonomical and autecological determination mainly follows Wiederholm (1983) and Brooks et al. (2007). When mentum was damaged or worn and mandibles or premandibles were not available, a higher taxonomic group was identified. Chironomid head capsule concentration was estimated by counting all head capsules in the samples.
Pollen analysis
Pollen analysis was conducted on the same samples as diatom analysis, i.e. from 2 mm-thick layers in 0.4–0.6-mm intervals from the core PK-1-L (128–143.6 cm depth). Sediment samples were boiled in potassium hydroxide, sieved, acetolysed and incubated in fluoric acid according to Faegri & Iversen (1989). A known quantity of Lycopodium spores (three tablets) was added to each sample prior to the treatment to determine the absolute pollen concentration (Stockmarr, 1971). Pollen was counted under a Nikon Eclipse 80i optical microscope at magnifications of × 400 to × 1000. At least 500 pollen determinations were conducted per sample. Taxonomic identifications followed Punt (1976), Eide (1981), Punt & Clarke (1980, 1981, 1984), Punt et al. (1988, 1995, 2003), Punt & Blackmore (1991), Beug (2004) and Punt & Hoen (2009). Reworked pollen grains of presumably the Tertiary period were identified according to Stuchlík (2001).
Numerical analysis
Significant diatom (DAZ), pollen (PAZ) and chironomid (CAZ) assemblage zones were based on CONISS (Constrained Incremental Sums of Squares) (Grimm, 1987). From chironomid and diatom dataset, uncertain gathering categories were excluded for further clustering using R 3.2.4 (R Core Team, 2016). From the pollen dataset, unidentified objects and Tertiary and aquatic plants’ pollen were removed. Relative species abundances were processed by Hellinger transformation prior to clustering by CONISS and testing by the Broken-Stick model (MacArthur, 1957; Legendre & Legendre, 1998). Diatom assemblage zones (DAZ) were calculated both from the entire assemblage and from dominant-free species data (without taxa > 30%: Staurosira construens f. construens, Staurosira construens f. venter).
Geochemistry
Dry weights of sediment samples were determined by drying them at 105°C for six hours during diatom analysis and at 50°C for 24 h during chironomid analysis (Boyle, 2001) in order to obtain standardized sediment weights for quantitative analysis of the biological proxies.
Loss-on-ignition (LOI) analysis was conducted at 550°C for 4 h according to Heiri et al. (2001) to quantify the relative proportion of organic matter in the sediment.
For the X-ray fluorescence measurements (XRF), a Delta Professional Handheld XRF analyser with 9-mm (uncollimated) spot size was used. Every 1-cm-thick layer was exposed three times for 3 min with two beams in “Geochem mode”—the first beam of up to 11 keV for lighter elements for 90 s and a second beam of up to 50 keV for heavier elements for 90 s. Presented data (%) are arithmetic averages from the three measurements.
Results
Lithology
A sharp lithological interface between clayey and layered gyttja was observed at the depth of 135 cm in the PK-1-L profile (Fig. 2). The PK-1-M profile was created by clayey gyttja without remarkable lithological change. The concentration of clastic silica decreased around the lithological boundary towards the Holocene and larger grains disappeared (Fig. 2). The Rb/Sr ratio increased 1.5 times at the lithological boundary in the PK-1-L profile (Fig. 2).
Chronology
A Poisson-process deposition model (Fig. 3) based on five radiocarbon data (Tab. 1) demonstrated a change of sedimentation rate at the lithological interface in the profile PK-1-L. The studied depth interval covered period ~ 15,600–10,800 cal year BP. The sedimentation rate of the clayey gyttja (depth 143.6–135 cm) was 430 year/cm on average, while the average sedimentation rate of layered gyttja (depth 135–128 cm) was 160 year/cm.
Diatom record
Altogether, 105 diatom taxa were observed in the 32 studied samples. The diatom valve concentration allowed to achieve the intended total counts in all samples with the exception of the most basal one (143.2–143.6 cm). The layered gyttja provided generally higher concentrations of diatom valves than the clayey gyttja from the basal part of the profile (Fig. 4).
Stratigraphical diagram of diatom relative abundances from the profile PK-1-L. Diatom concentration curve expressed as number of valves per 1 g of dry sediment. Relative abundances represented by a black silhouette, lines corresponding to 10 times of exaggeration. Significant diatom assemblage zones (DAZ 1, DAZ 2) and non-significant subzones (DAZ 2a, DAZ 2b) defined by CONISS (stratigraphically constrained cluster analysis) and Broken-Stick model. Clayey gyttja indicated by a grey background, layered gyttja indicated by a white background
Tiny fragilarioid taxa (Staurosira Ehrenberg, Staurosirella Williams & Round, Pseudostaurosira Williams & Round) predominated along the whole studied interval. The diatom relative abundance record was divided into two significant diatom assemblage zones, (DAZ 1, DAZ 2) and the second zone was divided into two non-significant subzones (Fig. 4).
DAZ 1 (143.6–136.2 cm, 15,600–12,600 cal year BP) was characterized by the dominance of Staurosira construens f. venter, accompanied by subdominant Staurosira construens var. binodis (Ehrenberg) Hamilton and Fragilaria heidenii Østrup. Pseudostaurosira polonica (Witak & Lange-Bertalot) Morales & Edlund, Gyrosigma acuminatum (Kützing) Rabenhorst and Planothidium joursacense (Héribaud-Joseph) Lange-Bertalot reached a considerable abundance (> 0.5%) within this zone only. Aulacoseira ambigua (Grunow) Simonsen was the only noticeable representative of euplanktonic taxa in DAZ 1.
DAZ 2a (136.2–131.2 cm, 12,600–11,100 cal year BP) started with a gradual increase in the total concentration of diatom valves. S. construens f. venter declined, and consequently Staurosira construens f. construens became dominant after the distinct increase in its relative abundance. Staurosirella pinnata (Ehrenberg) Williams & Round rose slightly at the beginning of the zone, whereas S. construens var. binodis decreased in abundance at the end of the zone. Two other euplanktonic taxa Lindavia cf. balatonis (Pantocsek) Nakov et al. and Staurosirella berolinensis (Lemmermann) Bukhtiyarova reached noticeable relative abundance beside A. ambigua being continuously present. The upper subzone boundary was defined using CONISS with excluded dominant taxa only.
DAZ 2b (131.2–128 cm, 11,100–10,800 cal year BP) reflected growth of less abundant motile benthic taxa Geissleria schoenfeldii (Hustedt) Lange-Bertalot & Metzeltin, Navicula laterostrata Hustedt, Navicula radiosa Kützing and Sellaphora vitabunda (Hustedt) Mann, accompanied by Fragilaria capucina s. l. Desmazières. Prevailing S. construens f. construens remained without any significant change. S. construens f. venter rose slightly, whereas relative abundance of S. construens var. binodis and all euplanktonic species decreased.
Chironomid and other Diptera records
A total number of 35 chironomid and two other dipteran morphotaxa were identified at the studied depth interval separated into 14 1-cm-thick layers (Fig. 5, Table 2). The concentration of head capsules was extremely low and oscillated between two and six individuals per gram of dry sediment. To produce statistically robust reconstructions and associated interpretations, large samples of dry weight from 9 to 23 g were analysed. The subsequent picking of head capsules yielded 29–110 individuals per sample. Chironomid abundance decreased temporarily at the depth of 140.5 cm and from 135.5 cm started to decrease continually towards the onset of the Holocene. Due to poor preservation, some of the findings were identified only to genus or subfamily level. Chironomus plumosus-type, C. anthracinus-type, and Procladius were the most abundant taxa along the studied profile. Two significant chironomid assemblage zones were defined (CAZ 1 and 2).
Stratigraphical diagram of chironomid relative abundances from the profile PK-1-M. Depth expressed in PK-1-L depth scale. Chironomid concentration curve expressed as number of HC (head capsules) per 1 g of dry sediment. Relative abundances represented by a black silhouette, lines corresponding to 10 times of exaggeration. Significant chironomid assemblage zones (CAZ 1, CAZ 2) defined by CONISS (stratigraphically constrained cluster analysis) and Broken-Stick model. Not-studied depth interval indicated by hatched area
CAZ 1 (143.5–136 cm, 15,600–12,500 cal year BP) was characterized by the dominance of C. plumosus-type, particularly in the basal part (> 80%) and decreasing towards 24% at the depth of 140.5 cm. This temporary drop in C. plumosus-type relative abundance coincided with the decrease in total abundance of chironomid head capsules (number of individuals/head capsules per 1 g of dry sediment), C. anthracinus-type establishment and an increase in Procladius. Then the relative abundance of dominant C. plumosus-type recovered to ~ 30–40% and the total chironomid abundance also increased. The dominant taxa (> 15% at least in one layer) were accompanied by several subdominant taxa (4–15% at least in one layer)—the Cladotanytarsus mancus-type, Einfeldia dissidens/natchitocheae-type, Microchironomus, Stictochironomus rosenschoeldi-type and Tanytarsus lugens-type. Several head capsules of the T. lugens-type had typical mandibles with three inner and two dorsal teeth but their surficial tooth was strongly reduced. These findings are presented as the T. cf. lugens-type.
CAZ 2 (136–130.5 cm, 12,500–11,000 cal year BP) showed a gradual decline of C. plumosus-type as well as a decline in the total abundance of head capsules in the sediment. Procladius increased distinctly in reverse and became the most abundant taxon. Besides these two taxa, only Einfeldia natchitocheae-type exceeded the relative abundance of 15% in the uppermost layer. C. anthracinus-type and T. (cf.) lugens-type progressively disappeared from the record. Stictochironomus rosenschoeldi-type was completely missing, Microchironomus remained present, Cladotanytarsus mancus-type reappeared in the lower half of the CAZ 2 and disappeared again at 133 cm and the Einfeldia dissidens/natchitocheae-type increased. In addition, several new subdominants (relative abundance of 4–15%) appeared—the Einfeldia dissidens-type, Polypedilum nubeculosum-type and Glyptotendipes pallens-type.
Besides the chironomid remains, the presence of Chaoborus flavicans (Meigen, 1830) mandibles in both CAZ 1 and CAZ 2, and one head capsule of family Simuliidae in CAZ 2 was documented.
Pollen record
Three significant pollen assemblage zones (PAZ) were determined (Fig. 6) with a total of 107 pollen and spore types recorded. Pollen types of Tertiary origin numbering 15 were recorded mainly in PAZ 1. The recovered pollen grains were well preserved. Total pollen concentration reached ca 1.7–5 × 105 grains g−1 in PAZ 1 and amplified to ca 1–2.5 × 106 grains g−1 in PAZ 2 and 3. Pinus sylvestris-type and Betula pubescens-type pollen were the most abundant (~ 70–80%) in PAZ 1 (143.3–135.7 cm, 15,600–12,300 cal year BP). Pollen of shrubs (Juniperus, Salix and Alnus alnobetula) reached ~ 5% abundance and non-arboreal pollen (NAP) ~ 20–30%. Aquatic species were above all represented by pollen of the Myriophyllum spicatum-type, Alisma plantago-aquatica-type and Oenanthe fistulosa-type. Some rare and rather thermophilous species such as Butomus umbellatus were recorded. The pollen of light demanding species (the Corylus avellana-type), the deciduous species of the Quercetum mixtum (the Quercus robur-group, Tilia cordata-type, Ulmus-type and Fraxinus excelsior-type) and Picea-type were of extremely low abundance. By the end of the zone, spores of Sphagnum and pollen of the Elatine-type were recorded. An increase in the abundance of the pollen of trees and shrubs of up to 90% proceeded in PAZ 2 (135.7–130.2 cm, 12,300–11,000 cal year BP). In PAZ 3 (130.2–128 cm, 11,000–10,800 cal year BP), pollen of the Corylus avellana increased in abundance above 20% and was succeeded by pollen of deciduous species of the Quercetum mixtum. Hygrophilous and water species were above all represented by pollen of the Typha angustifolia and the Sparganium emersum-type.
Stratigraphical diagram of pollen relative abundances from the profile PK-1-L. Pollen concentration curve expressed as number of particles per 1 g of dry sediment. Relative abundances of AP (arboreal pollen) represented by a black silhouette, relative abundances of NAP (non-arboreal pollen) represented by a dark grey silhouette. Relative species abundances represented by black silhouettes, lines corresponding to 10 times of exaggeration. Significant pollen assemblage zones (PAZ 1, PAZ 2, PAZ 3) defined by CONISS (stratigraphically constrained cluster analysis) and Broken-Stick model. Clayey gyttja indicated by a grey background, layered gyttja indicated by a white background
Trophic state indication
The succession of diatom and chironomid ecological groups suggested a decreasing trend of nutrient availability during the LG/H transition (Fig. 7). The most abundant taxa of each ecological group are listed in Table 2. Diatom ecological groups are presented without dominant fragilarioid taxa; however, they remained significant owing to the exclusion of dominants since the phase of diatom valves’ counting. Eutraphentic diatom species abundance decreased at the DAZ 1/DAZ 2 boundary. Similarly, eutraphentic chironomids became less abundant after the CAZ 1/CAZ 2 boundary. While meso-eutraphentic diatoms decreased later at the DAZ 2a/DAZ 2b boundary inversely to the increase of mesotraphentic and oligotraphentic diatom species, meso-eutraphentic chironomids increased at the CAZ 1/CAZ 2 boundary. Higher relative abundances of oligotraphentic chironomids in CAZ 1 were driven by Tanytarsus lugens-type presence (Table 2).
Stratigraphical diagram of diatom and chironomid ecological groups. Chironomid relative abundances shown for whole assemblage, diatom relative abundances shown for assemblage counted without two dominant fragilarioid taxa. Significant diatom assemblage zones (DAZ) and chironomid assemblage zones (CAZ) defined by CONISS (stratigraphically constrained cluster analysis) and Broken-Stick model. Not-studied depth interval indicated by a hatched area
Diatom valve concentration increased at the LG/H boundary. Similarly, the pollen concentration of aquatic macrophytes increased slightly with the onset of the Holocene during PAZ 2 and later distinctly at PAZ 3 (Fig. 8). Both variables are in more or less direct proportion to the primary productivity of diatoms and aquatic macrophytes respectively.
Stratigraphical diagram of variables representing lacustrine trophic state from the profile PK-1-L. LOI (loss-on-ignition) represents percentages of organic matter. Diatom and pollen concentration curves expressed as number of particles per 1 g of dry sediment. Fe/Mn ratio based on XRF analysis. Significant diatom assemblage zones (DAZ 1, DAZ 2) and non-significant subzones (DAZ 2a, DAZ 2b) defined by CONISS (stratigraphically constrained cluster analysis) and Broken-Stick model. Clayey gyttja indicated by a grey background, layered gyttja indicated by a white background
Loss-on-ignition (LOI) had a distinct rising trend ranging between 1.5 and 10.4% in the LG and between 13 and 16.5% in the Holocene but it remained at a relatively low level along the whole studied interval of the PK-1-L profile (max. 16.5% at 129–129.8 cm) (Fig. 8). Fe/Mn ratio decreased twice at the lithological boundary, when average values for the LG (91.1) and for the Holocene were compared (41.2) (Fig. 8).
Discussion
The effect of climate on lacustrine biota
At the Late-Glacial/Holocene transition (LG/H), a gradual succession of aquatic and terrestrial biota without any sharp interface was recorded in Lake Komořany. The remarkable feature of this transition is the asynchronous change in diatom species composition and their productivity indicated by diatom valve concentration (Fig. 4). While diatom productivity abruptly increased and changed the character of sediment at the supposed LG/H boundary (135–134.5 cm, 12,000–11,700 cal year BP) (Figs. 2, 4), the main shift in both dominant and rarer diatom taxa along with change in chironomid assemblage and rise in LOI had taken place slightly before (136.5–136 cm, 12,800–12,500 cal year BP) this lithological interface (Fig. 4). Therefore, there must have been two, qualitatively different, breakthroughs in ecosystem response. The chronologically first change could have resulted from ecosystem response to the warmer second half of the Younger Dryas period (Stuiver et al., 1995) which is of considerable influence on the lowland lakes (Goslar et al., 1993; Zawiska et al., 2015) and was detected also in a diatom record from a shallow lake situated in the southern part of the Czech Republic (Bešta et al., 2009). Although the climatic forcing of the first discussed event is far behind the resolution of our sedimentary record, we did not find another possible trigger of such changes. Therefore, we put it here as a provisional explanation, since a rather gradual process without the detection of generally accepted LG periods (e.g. Allerød) was observed in the profile. This is consonant with the view that neither a particular event nor even a biostratigraphic episode (cf. Rasmussen et al., 2014) can be distinguished for the LG terrestrial record from Lake Komořany. The second event, characterized by the abrupt rise in diatom productivity, is synchronous with the LG/H boundary (Fig. 2). It offers a bold conclusion that the first recorded change preceding the LG/H boundary was more prominent, since there was a shift in both chironomid and diatom species composition (quality), whereas only diatom valve concentration (quantity) changed during the second shift. The second quantitative shift in diatom assemblage, i.e. the increase in productivity of dominant fragilarioids, could be connected with spread of suitable littoral habitats with emergent aquatic macrophytes as the fragilarioids possibly prefer emergent substrates to colonize (Sayer et al., 1999). Whatever the case, the shift in productivity could not be biassed by enhanced sedimentation rate (allochthonous material input), since than the valve concentration had to drop.
Terrestrial vegetation showed gradual change with the continual disappearance of steppe and tundra indicators (e.g. Artemisia, Helianthemum, Potentilla, Juniperus, Salix, Betula nana, Alnus viridis-type) and a successive increase in wood’s proportion. Pinus and Betula trees were present in the region perhaps in the form of a patchy woodland during the LG (16,300/15,200–11,700 cal year BP). Such scattered patches of hemiboreal forest, further indicated by the presence (< 1.5%) of Pinus cembra-type pollen (Fig. 6), were reconstructed at low altitudes in the Bohemian Massif (Kuneš et al., 2008). The spread of mixed deciduous forest starts very early with the scattered occurrence of pollen of some thermophilous trees (Quercus robur-type, Tilia cordata-type) (Fig. 6). Such early findings of these taxa are also usually thought to originate in a long-distance transport or reworked material (Pokorný, 2002; Ralska-Jasiewiczowa et al., 2004). Even though the steppe vegetation probably persisted during the Holocene in the adjacent thermophilous hilly region of České Středohoří (Pokorný et al., 2015), the pollen of the steppe elements almost disappeared from the Lake Komořany record until 5,500 cal year BP (Houfková et al., 2017).
The recorded gradual development of terrestrial vegetation documents a very limited climatic forcing of the studied lowland environment at the LG/H transition transferring to a less pronounced response of the lacustrine ecosystem. We found a distinct shift in the relative abundances of diatoms and chironomids but only minor changes in species compositions (Figs. 4, 5). An addition of several new diatom and chironomid taxa to the persisting Late-Glacial species composition rather than their complete substitution generally characterizes the changes in Lake Komořany aquatic assemblages. A similar pattern was also recorded in other lowland eutrophic lakes in central Europe at the Holocene onset (Kirilova et al., 2009; Turner et al., 2014).
Low water depth is considered as one of the driving factors causing relatively stable aquatic assemblage composition in Lake Komořany because it determines the frequency of mixing in the whole water column (Scheffer, 2004). The long-lasting shallow character of Lake Komořany could explain the predominance of diatom fragilarioid taxa known to thrive in polymictic shallow waters (Bennion, 1995). Observed diatom assemblages represent a typical pioneer species composition well known from other Late-Glacial lakes (Haworth 1976; Denys et al. 1990) and large shallow lakes in general (Heinsalu et al., 2008; Buczkó et al., 2019). This is consistent with the dominance of fragilarioid tychoplanktonic taxa throughout the existence of Lake Komořany (Bešta et al., 2015; Houfková et al., 2017). Observed invariable diatom composition indicates a shallow lacustrine character without extensive phases of deep water during the LG and weak climate forcing of the lake ecosystem. Nevertheless, subtle water-level fluctuations can be inferred from the pollen of macrophytes typical of disturbed wet stands (among others Sphagnum or Elatine, Fig. 6). The water-level rise caused by humification at the onset of the Holocene was proven by the start of sedimentation in the more elevated parts of the basin (Houfková et al., 2017). This is further supported by the outset of diatom euplanktonics Staurosirella berolinensis and Lindavia cf. balatonis (Fig. 4). An analogous water-level rise was also described in other European records (Magny, 2004; Bos et al., 2007). The shift to the lake with more stable littoral parts is further indicated by a presence of pollen of Typha angustifolia or Humulus lupulus ~ 11,200 cal year BP (PAZ 3) (Fig. 6).
Although the water level strongly influenced the general character of observed aquatic assemblages, it fails to explain species composition changes during the LG in Lake Komořany. Therefore, the change in trophic state was tracked as a potential prominent device in how climate indirectly influenced the lacustrine ecosystem.
Trophic state reconstruction
Our results document mesotrophic to eutrophic conditions already since the lake formation in the LG (Figs. 7, 8). Various aspects of the lacustrine trophic state were reconstructed, through aquatic macrophytes, primary productivity and nutrient availability.
Aquatic macrophytes shape the regime of shallow lakes (Jeppesen et al., 1998) and affect species composition of chironomid and diatom assemblages (Sayer et al., 1999; Brodersen et al., 2001; Vermaire et al., 2013; Tarrats et al., 2018). Even in the LG, Myriophyllum spicatum was a dominant component of aquatic macrophyte assemblage (Fig. 6) but absolute concentrations of aquatic macrophytes’ pollen were distinctly lower than later on in the Early Holocene (Fig. 8). Nevertheless, the lower volume of water in the LG and submergent character of M. spicatum could have made its vegetation an important habitat for small fragilarioids and Gyrosigma acuminatum (Fig. 4), a diatom frequently observed on aquatic macrophytes (Dong et al., 2008). The first evidence of heightened macrophyte growth at the onset of the Holocene is documented by an increase in Einfeldia dissidens-type and Glyptotendipes pallens-type (Fig. 5), two chironomid taxa associated with aquatic macrophytes (Brodersen et al., 2001). The subsequent increase in the pollen concentration of aquatic macrophytes and the spread of broad-leaved species (Potamogeton natans-type, Sparganium emersum-type, Typha angustifolia) are linked with the onset of the Holocene (Fig. 6). This littoral vegetation provided more stable bottom habitats enabling the establishment of an epipelic diatom community including Navicula radiosa and other motile benthic diatoms (Geissleria schoenfeldii, Navicula laterostrata, Sellaphora vitabunda) (Fig. 4). Moreover, we must consider not only the substantial influence of aquatic macrophytes on nutrient availability by bottom stabilization but also the direct consumption of phosphorus during enhanced growth under the Holocene favourable climate. Aquatic macrophytes can affect the lacustrine trophic state as they serve as effective phosphorus sink and mitigate sediment resuspension (Canfield & Jones, 1984; Jeppesen et al., 1998). Furthermore, the lower abundance of macrophytes during the LG could strengthen the sensitivity of benthic diatoms including small fragilarioid taxa to the concentration of epilimnetic phosphorus (Werner & Smol, 2005).
Diatom and chironomid ecological groups in our record show a clear trend from more towards less nutrient-demanding taxa (Fig. 7). We attempted to reduce the influence of poor fragilarioid indicators (Bennion, 1995; Sayer, 2001; Bennion et al., 2010) by excluding the dominant Staurosira spp. from our assessment. Moreover, the nutrient concentration signal of remaining fragilarioid taxa (F. heidenii, Pseudostaurosira brevistriata (Grunow) Williams & Round, P. polonica, S. berolinensis, S. pinnata, S. construens var. binodis) was considered with caution. Such an approach provides a clear signal of decreasing nutrient availability towards the Holocene through decreasing eutraphentic and increasing meso- and oligotraphentic diatom species (Fig. 7). Additionally, the substitution of two diatom dominants, S. construens f. venter and S. construens f. construens (Fig. 4), could be explained by nutrient availability since S. construens f. venter tolerates higher concentrations of organically bound nitrogen compared to S. construens f. construens (Van Dam et al., 1994). Although the coenococcal algae Pediastrum kawraiskyi Schmidle, used typically as an indicator of cold and oligotrophic conditions, was found in Late-Glacial sediments of Lake Komořany (Komárek & Jankovská, 2001; Jankovská & Pokorný, 2013), its presence during cold periods in a naturally eutrophic lake documented by Turner et al. (2014) questioned the indicative value for nutrient levels. A more probable connection of P. kawraiskyi to low temperatures rather than low nutrient level supports our interpretation of there being relatively high nutrient levels since the LG.
Chironomid ecological preferences follow a similar trend showing a substitution of eutraphentic dominants for meso-eutraphentic taxa (Fig. 7). The most abundant chironomids of our record, namely Chironomus anthracinus-type, C. plumosus-type and Procladius (Fig. 5, Table 2), often produce very abundant populations in highly productive lakes with low clarity and oxygen depletion or anoxia near the bottom (Nagell & Landahl, 1978; Matěna, 1989; Hamburger et al., 1994). Characteristic cold stenothermic taxa inhabiting oligotrophic and well-oxygenated waters (e.g. the Heterotrissocladius grimshawi-type or Micropsectra spp.) are missing, although reported in many European records, including LG sediments of lowland shallow lakes (Brooks et al., 1997; Brodersen & Lindegaard, 1999; Płóciennik et al., 2011; Bos et al., 2017) or Czech LG lakes situated in elevations of above 900 m a. s. l. (Kletetschka et al., 2018). Furthermore, mesotraphentic species (e.g. the Derotanypus or Corynocera ambigua-type) present in regional mid-altitude lakes (400–900 m a. s. l.) (Hošek et al., 2014; Hájková et al., 2016) are also absent from Lake Komořany records. The only exception are rare findings of Tanytarsus lugens-type, a morpho-taxon typical of the profundal in oligotrophic lakes (Brooks et al., 2007).
Driving mechanisms of nutrient availability
We make the case that nutrient availability was a probable factor in the Lake Komořany ecosystem change at the LG/H transition. Similar decreases in the nutrient level at the time of the climate amelioration are known from Scandinavian (Björk, 2010), Polish (Apolinarska et al., 2012) and German lowland lakes (Kirilova et al., 2009; Turner et al., 2014). In case of Lake Komořany record, this reduction in nutrient availability was likely accompanied by changes in the catchment erosion intensity, duration of seasonal anoxia and shift in primary productivity.
- 1.
More intensive catchment erosion during the colder and drier period could have enhanced nutrient availability in the LG. A higher input of inorganic material into Lake Komořany is clearly indicated by an increased proportion of clastic particles in the LG parts of the profiles (Fig. 2). Although a decrease of Rb at the LG/H transition corresponds with more intensive erosion from catchment during the LG (cf. Hošek et al., 2014), our lower values of Rb/Sr ratio in LG (Fig. 2) contradict trends usually observed for colder periods (Jin et al., 2006). This is caused by a distinct decrease of Sr at the LG/H transition, most likely linked to the affinity of Sr to Ca (Jin et al., 2006) and a decrease of alkalinity towards the Holocene.
- 2.
Seasonal anoxia under prolonged winter stratification causes an internal recycling of sedimentary phosphorus (Wetzel, 2001). The presence of Chironomus anthracinus-type, C. plumosus-type and Stictochironomus rosenschoeldi-type during the LG (Fig. 5) suggests at least seasonally anoxic conditions near the lake bottom (Nagell & Landahl, 1978; Int Panis et al.,1995), whereas Tanytarsus lugens-type presence indicates well-oxygenated conditions (Brooks et al., 2007). This discrepancy could be explained by the presence of a mosaic of different habitats in the lake and a possible restriction of T. lugens-type (Fig. 5) to a better oxygenated environment near the inflow of the Bílina River. The above-mentioned taxa are characteristic of our LG record and disappeared or declined with the onset of the Holocene. Higher Fe/Mn ratios (Fig. 8) also suggest at least seasonal anoxic conditions leading to higher nutrient concentrations during the LG. On the other hand, we cannot rule out the possibility that anoxic conditions affected Early Holocene chironomid assemblages as well. Chironomid concentration became very low (Fig. 5) documenting unfavourable conditions. Profundal taxa decreased and were partially replaced by littoral taxa. Therefore, the effect of oxygen depletion caused by the enhanced decomposition of biomass produced after climate amelioration cannot be fully excluded.
- 3.
Higher primary productivity in the Early Holocene was able to enhance competition for available nutrients and made them a limiting factor in contrast to the LG. Both growing terrestrial and aquatic vegetation serve as a phosphorus sink and can make nutrients unavailable for algal primary producers (Canfield & Jones, 1984; Tabacchi et al., 2000). While primary productivity rises, a phosphorus turnover in the lake increases but its instantaneous concentrations often decrease or fluctuate (Wetzel, 2001). In Lake Komořany, a higher biomass production of terrestrial and/or aquatic vegetation was suggested by an increasing proportion of organic matter in sediment (Fig. 8) and higher pollen concentration (Fig. 6). An increase of algal biomass in the Early Holocene is documented by higher diatom valve concentration (Fig. 4). Additionally, the limitation of biomass production by harsh conditions during the LG possibly allowed a higher availability of nutrients for surviving organisms. Light availability and mechanical disturbances are supposed key limiting factors for primary productivity during the LG in Lake Komořany: (a) Severe light attenuation by turbid inorganic particles is expected during the LG as a consequence of the potentially stronger input of minerogenic material by the tributary (the Bílina River). As we found only indications of a slightly higher input of particles in the LG (Fig. 2) and the sedimentation rate remained low (Fig. 3), the turbidity of particles by water column mixing seems to be a more important factor than the absolute amount of washed-in material. The potential input of particles could also fertilize benthic algae surviving in shallower parts where physical conditions were more stable and light more available (Stevenson et al., 1996). This could thus amplify the discrepancy between the low primary productivity and the presence of the nutrient-demanding taxa. Restricted favourable habitats did not allow heightened biomass production. (b) Mechanical disturbances by turbulent water conditions or prolonged ice-cover could impede the biomass growth of algal mats and aquatic macrophytes as frequent disturbance reduces benthic biomass (Stevenson et al., 1996).
These processes could cause either lower absolute nutrient level towards the Holocene or at least raise relative nutrient availability in the LG. Although absolute nutrient concentration in the lacustrine ecosystem could be higher in the Holocene, the instantaneous nutrient availability was lower due to the fast nutrient turnover. In Lake Komořany, the presence of nutrient-demanding diatoms and chironomids in the Late-Glacial period and their subsequent decrease towards the Holocene agrees with this hypothesis. Furthermore, the same timing of main species compositional change in diatom and chironomid assemblages with the increase in organic matter proportion supports the influence of changes in catchment and/or in-lake primary productivity to ecosystem structuring. Other enriching mechanisms such as erosion from the catchment and anoxia seem to be of less importance. Primary productivity is considered the main driving factor of change in lacustrine nutrient availability in Lake Komořany at the LG/H transition.
Limits of the sedimentary record
Interpretation of the large shallow lowland lakes’ records brings various constrictions particularly due to the specific features of in-lake sedimentation such as the low inflow of allochthonous material, the possible resuspension of sediments by waves in polymictic regime or the effective oxidation of deposited organic matter (Blais & Kalff, 1995; Scheffer, 2004). The aforementioned factors result in a low sedimentation rate, which reduces stratigraphic resolution of the natural archive. Our study faced an absence of terrestrial macrofossils for radiocarbon dating and very low time resolution of the record. Bulk sediment was used for AMS 14C dating with reference to previous successful application to Holocene material from Lake Komořany (Bešta et al., 2015; Houfková et al., 2017). Although negligible bias was supposed, possible errors obliged us to take the modelled chronology with reserve.
Our results provided a clear evidence of LG record from both studied profiles. The continuous development of assemblages and geochemical variables suggested undisturbed sedimentation without more extensive reworking. The presence of minor hiatuses cannot be excluded but they do not threaten the aims of this study. The depth–age model was supported by palynostratigraphy. In particular, the observed peak of Corylus avellana (24%, ~ 10,800 cal year BP) was seen to be identical to the peak recorded in the PK-1-C profile from Lake Komořany (26%, ~ 11,000 cal year BP) (Houfková et al., 2017), which is in accordance with data from other sites in Central Europe (Theuerkauf et al., 2014).
Conclusion
A relatively stable ecosystem with gradual changes in aquatic and terrestrial communities was recorded in Lake Komořany at the Late-Glacial/Holocene transition. Two major, almost certainly climatically driven, shifts were recorded in the studied profiles: (i) the qualitative one with changes in species composition of the diatom and chironomid communities foregoing the LG/H boundary and (ii) the quantitative one at the LG/H boundary distinguishable by the enhanced diatom valve concentrations and the lithological change. These shifts were linked with two opposite trends: decreasing inferred trophic status and increasing primary productivity towards the Holocene. This discrepancy between nutrient availability and primary productivity can be best explained by a sink of nutrients in biomass produced after the climatic amelioration. In other words, nutrients became a limiting factor only after the onset of Holocene favourable climate. The species composition and primary productivity were therefore shaped by the climate change indirectly by means of nutrient availability.
References
Anderson, N. J., 1995. Naturally eutrophic lakes: reality, myth or myopia? Trends in Ecology and Evolution 10: 137–138.
Apolinarska, K., M. Woszczyk & M. Obremska, 2012. Late Weichselian and Holocene palaeoenvironmental changes in northern Poland based on the Lake Skrzynka record. Boreas 41: 292–307.
Barley, E. M., I. R. Walker, J. Kurek, L. C. Cwynar, R. W. Mathewes, K. Gajewski & B. P. Finney, 2006. A northwest north American training set: distribution of freshwater midges in relation to air temperature and lake depth. Journal of Paleolimnology 36: 295–314.
Battarbee, R. W., 1986. Diatom Analysis. In Berglund, B. E. (ed.), Handbook of Holocene Palaeoecology and Palaeohydrology. Wiley, Chichester: 527–570.
Battarbee, R. W., V. J. Jones, R. J. Flower, N. G. Cameron, H. Bennion, L. Carvalho & S. Juggins, 2001. Diatoms. In Smol, J. P., H. J. B. Birks & W. M. Last (eds), Tracking Environmental Change Using Lake Sediments, Vol. 3. Terrestrial, Algal and Siliceous Indicators. Kluwer Academic Publishers, Dordrecht: 155–202.
Bennion, H., 1995. Surface-sediment diatom assemblages in shallow, artificial, enriched ponds, and implications for reconstructing trophic status. Diatom Research 10: 1–19.
Bennion, H., C. D. Sayer, J. Tibby & H. J. Carrick, 2010. Diatoms as Indicators of Environmental Change in Shallow Lakes. In Smol, J. P. & E. F. Stoermer (eds), The Diatoms: Applications for the Environmental and Earth Sciences. Cambridge University Press, Cambridge: 152–173.
Bešta, T., J. Šafránková, M. Pouzar, J. Novák & K. Nováková, 2009. Late Pleistocene-Early Holocene transition recorded in the sediments of a former shallow lake in the Czech Republic. Hydrobiologia 631: 107–120.
Bešta, T., J. Novák, D. Dreslerová, V. Jankovská, A. Bernardová, L. Lisá & D. Valentová, 2015. Mid-Holocene history of a central European lake: lake Komořany, Czech Republic. Boreas 44: 563–574.
Beug, H. J., 2004. Leitfaden der Pollenbestimmung fur Mitteleuropa und angrenzende Gebiete. Friedrich Pfeil, München.
Birks, H. H. & B. Ammann, 2000. Two terrestrial records of rapid climatic change during the glacial–Holocene transition (14,000–9,000 calendar years B. P.) from Europe. Proceedings of the National Academy of Sciences of the USA 97: 1390–1394.
Birks, H. H. & H. J. B. Birks, 2006. Multi-proxy studies in palaeolimnology. Vegetation History and Archaeobotany 15: 235–251.
Björk, S., 2010. The Evolution of Lakes and Wetlands. In Eiseltová, M. (ed.), Restoration of Lakes, Streams, Floodplains, and Bogs in Europe Wetlands: Ecology, Conservation and Management, Vol. 3. Springer, Dordrecht: 25–35.
Blais, J. M. & J. Kalff, 1995. The influence of lake morphometry on sediment focusing. Limnology and Oceanography 40: 582–588.
Bos, J. A. A., B. Van Geel, J. Van Der Plicht & S. J. P. Bohncke, 2007. Preboreal climate oscillations in Europe: wiggle-match dating and synthesis of Dutch high-resolution multi-proxy records. Quaternary Science Reviews 26: 1927–1950.
Bos, J. A. A., P. De Smedt, H. Demiddele, W. Z. Hoek, R. Langohr, V. Marcelino, N. Van Asch, D. Van Damme, T. Van der Meeren, J. Verniers, P. Boeckx, M. Boudin, M. Court-Picon, P. Finke, V. Gelorini, S. Gobert, O. Heiri, K. Martens, F. Mostaert, L. Serbruyns, M. Van Strydonck & P. Crombé, 2017. Multiple oscillations during the Lateglacial as recorded in a multi-proxy, high-resolution record of the Moervaart palaeolake (NW Belgium). Quaternary Science Reviews 162: 26–41.
Boyle, J. F., 2001. Inorganic geochemical methods in palaeolimnology. In Last, W. M. & J. P. Smol (eds), Tracking environmental Change Using Lake Sediments. Volume 2: Physical and Geochemical Methods. Kluwer Academic Publishers, Dordrecht: 83–142.
Brodersen, K. P. & C. Lindegaard, 1999. Classification, assessment and trophic reconstruction of Danish lakes using chironomids. Freshwater Biology 42: 143–157.
Brodersen, K. P. & R. Quinlan, 2006. Midges as palaeoindicators of lake productivity, eutrophication and hypolimnetic oxygen. Quaternary Science Reviews 25: 1995–2012.
Brodersen, K. P., B. V. Odgaard, O. Vestergaard & N. John Anderson, 2001. Chironomid stratigraphy in the shallow and eutrophic Lake Søbygaard, Denmark: chironomid-macrophyte co-occurrence. Freshwater Biology 46: 253–267.
Bronk Ramsey, C., 2008. Deposition models for chronological records. Quaternary Science Reviews 27: 42–60.
Bronk Ramsey, C., 2013. OxCal 4.2.4 online program. https://c14.arch.ox.ac.uk/oxcal/OxCal.html. OxCal Project web.
Bronk Ramsey, C. & S. Lee, 2013. Recent and planned developments of the program Oxcal. Radiocarbon 55: 720–730.
Brooks, S. J., J. J. Lowe & F. E. Mayle, 1997. The late devensian lateglacial palaeoenvironmental record from Whitrig Bog, SE Scotland. 2. Chironomidae (Insecta: Diptera). Boreas 26: 297–308.
Brooks, S.J., P.G. Langdon & O. Heiri, 2007. The identification and use of palaearctic chironomidae larvae. Palaeoecology, Technical Guide No. 10. Quaternary Research Association, London.
Buczkó, K., É. Ács, K. Báldi, V. Pozderka, M. Braun, T. Kiss & J. Korponai, 2019. The first high resolution diatom record from Lake Balaton, Hungary in Central Europe. Limnetica 38: 417–430.
Canfield Jr., D. E. & J. R. Jones, 1984. Assessing the trophic status of lakes with aquatic macrophytes. Lake and Reservoir Management 1: 446–451.
Cardoso, A. C., A. Solimini, G. Premazzi, L. Carvalho, A. Lyche & S. Rekolainen, 2007. Phosphorus reference concentrations in European lakes. Hydrobiologia 584: 3–12.
Cohen, A. S., 2003. Paleolimnology: The History and Evolution of Lake Systems. Oxford University Press, New York.
Cremer, H., A. D. Buijse, A. F. Lotter, W. Oosterberg & M. Staras, 2004. The palaeolimnological potential of diatom assemblages in floodplain lakes of the Danube Delta, Romania: a pilot study. Hydrobiologia 513: 7–26.
Demiddele, H., P. Finke & P. Crombé, 2016. Diatom-based palaeoecology of a late-glacial palaeolake in the Moervaart area (northwestern Belgium) in relation to its prehistoric occupation. Notae Praehistoricae 36: 29–46.
Denys, L., 1991. A checklist of the diatoms in the Holocene deposits of the western Belgian coastal plain with a survey of their apparent ecological requirements. I. Introduction, ecological code and complete list. Professional Paper Belgische Geologische Dienst 246.
Denys, L., C. Verbruggen & P. Kiden, 1990. Palaeolimnological aspects of a late-Glacial shallow lake in Sandy Flanders, Belgium. Hydrobiologia 214: 273–278.
Dong, X., H. Bennion, R. Battarbee, X. Yang, H. Yang & E. Liu, 2008. Tracking eutrophication in Taihu lake using the diatom record: potential and problems. Journal of Paleolimnology 40: 413–429.
Dreßler, M., A. Schwarz, T. Hübener, S. Adler & B. W. Scharf, 2011. Use of sedimentary diatoms from multiple lakes to distinguish between past changes in trophic state and climate: evidence for climate change in northern Germany during the past 5,000 years. Journal of Paleolimnology 45: 223–241.
Drzymulska, D., M. Fiłoc, M. Kupryjanowicz, K. Szeroczyńska & P. Zieliński, 2015. Postglacial shifts in lake trophic status based on a multiproxy study of a humic lake. Holocene 25: 495–507.
Eide, F., 1981. Key for northwest european Rosaceae pollen. Grana 20: 101–118.
Enters, D., E. Kirilova, A. F. Lotter, A. Lücke, J. Parplies, S. Jahns, G. Kuhn & B. Zolitschka, 2010. Climate change and human impact at Sacrower See (NE Germany) during the past 13,000 years: a geochemical record. Journal of Paleolimnology 43: 719–737.
Faegri, K. & J. Iversen, 1989. Textbook of Pollen Analysis, 4th ed. Wiley, Chichester.
Gałka, M., K. Tobolski & I. Bubak, 2015. Late Glacial and Early Holocene lake level fluctuations in NE Poland tracked by macro-fossil, pollen and diatom records. Quaternary International 388: 23–38.
Goslar, T., T. Kuc, M. Ralska-Jasiewiczowa, K. Rózánski, M. Arnold, E. Bard, B. Van Geel, M. E. Pazdur, K. Szeroczyńska, B. Wicik, K. Wieckowski & A. Walanus, 1993. High-resolution lacustrine record of the Late Glacial/Holocene transition in Central Europe. Quaternary Science Reviews 12: 287–294.
Grimm, E. C., 1987. CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Computers & Geosciences 13: 13–35.
Guiry, M.D. & G.M. Guiry, 2019. AlgaeBase. World-wide electronic publication. National University of Ireland. Galway. http://www.algaebase.org. Accessed 7 July 2019
Hájková, P., P. Pařil, L. Petr, B. Chattová, T. M. Grygar & O. Heiri, 2016. A first chironomid-based summer temperature reconstruction (13–5 ka BP) around 49 N in inland Europe compared with local lake development. Quaternary Science Reviews 141: 94–111.
Hall, R. I. & J. P. Smol, 2010. Diatoms as Indicators of Lake Eutrophication. In Smol, J. P. & E. F. Stoermer (eds), The Diatoms: Applications for the Environmental and Earth Sciences. Cambridge University Press, Cambridge: 122–151.
Hamburger, K., P. C. Dall & C. Lindegaard, 1994. Energy metabolism of Chironomus anthracinus (Diptera: Chironomidae) from the profundal zone of Lake Esrom, Denmark, as a function of body size, temperature and oxygen concentration. Hydrobiologia 294: 43–50.
Haworth, E. Y., 1976. Two Late-Glacial (Late Devensian) diatom assemblage profiles from northern Scotland. The New Phytologist 77: 227–256.
Heinsalu, A., H. Luup, T. Alliksaar, P. Nõges & T. Nõges, 2008. Water level changes in a large shallow lake as reflected by the plankton: periphyton-ratio of sedimentary diatoms. Hydrobiologia 599: 23–30.
Heiri, O., A. F. Lotter & G. Lemcke, 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology 25: 101–110.
Heiri, O., S. J. Brooks, H. Renssen, A. Bedford, M. Hazekamp, B. Ilyashuk, E. S. Jeffers, B. Lang, E. Kirilova, S. Kuiper, L. Millet, S. Samartin, M. Toth, F. Verbruggen, J. E. Watson, N. Van Asch, E. Lammertsma, L. Amon, H. H. Birks, H. J. B. Birks, M. F. Mortensen, W. Z. Hoek, E. Magyari, C. Munõz Sobrino, H. Seppä, W. Tinner, S. Tonkov, S. Veski & A. F. Lotter, 2014. Validation of climate model-inferred regional temperature change for late-glacial Europe. Nature Communications 5: 1–7.
Hošek, J., P. Pokorný, V. Kubovčík, I. Horáček, P. Žáčková, J. Kadlec, F. Rojik, L. Lisá & S. Bučkuliaková, 2014. Late glacial climatic and environmental changes in eastern-central Europe: correlation of multiple biotic and abiotic proxies from the Lake Švarcenberk, Czech Republic. Palaeogeography, Palaeoclimatology, Palaeoecology 396: 155–172.
Houfková, P., T. Bešta, A. Bernardová, D. Vondrák, P. Pokorný & J. Novák, 2017. Holocene climatic events linked to environmental changes at Lake Komořany Basin, Czech Republic. The Holocene 27: 1–14.
Hurník, S., 1969. Příspěvek ke geologické problematice tzv. Komořanského jezera [Contribution to the geology of so-called Lake Komořany]. Mostecko-Litvínovsko, Regionální Studie 6: 5–14.
Int Panis, L., B. Goddeeris & R. F. Verheyen, 1995. On the Relationship Between the Oxygen Microstratification in a Pond and the Spatial Distribution of the Benthic Chironomid Fauna. In Cranston, P. (ed.), From Genes to Ecosystems. Canberra, C.S.I.R.O.: 482.
Jankovská, V., 1983. Palynologische Forschung am ehemaligen Komořany-See (Spätglazial bis Subatlantikum). Věstník Ústředního ústavu geologického 58: 99–107.
Jankovská, V., 1984. Radiokarbondatierung der Sedimente aus dem ehemaligen Komořany-See (NW-Böhmen). Věstník Ústředního ústavu geologického 59: 235–236.
Jankovská, V., 1988. Palynologische Erforschung archäologischer Proben aus dem Komořanské jezero-See bei Most (NW-Böhmen). Folia Geobotanica et Phytotaxonomica 23: 45–77.
Jankovská, V. & P. Pokorný, 2013. Reevaluation of the palaeoenvironmental record of the former Komořanské jezero lake: Late-Glacial and Holocene palaeolimnology and vegetation development in north-western Bohemia, Czech Republic. Preslia 85: 265–287.
Jeppesen, E., M. Sondergaard, M. Sondergaard & K. Christoffersen, 1998. The Structuring Role of Submerged Macrophytes in Lakes. Ecological Studies Vol. 131. Springer, LLC: 1–423.
Jin, Z., J. Cao, J. Wu & S. Wang, 2006. A Rb/Sr record of catchment weathering response to Holocene climate change in inner Mongolia. Earth Surface Processes and Landforms 31: 285–291.
Kirilova, E., O. Heiri, D. Enters, H. Cremer, A. F. Lotter, B. Zolitschka & T. Hübener, 2009. Climate-induced changes in the trophic status of a Central European lake. Journal of Limnology 68: 71–82.
Kletetschka, G., D. Vondrák, J. Hruba, V. Prochazka, L. Nabelek, H. Svitavská-Svobodová, P. Bobek, Z. Horicka, J. Kadlec & M. Takac, 2018. Cosmic-impact event in lake sediments from central Europe postdates the laacher see eruption and marks onset of the younger dryas. Journal of Geology 126: 561–575.
Kołaczek, P., J. Mirosław-Grabowska, M. Karpińska-Kołaczek & R. Stachowicz-Rybka, 2015. Regional and local changes inferred from lacustrine organic matter deposited between the Late Glacial and mid-Holocene in the Skaliska Basin (north-eastern Poland). Quaternary International 388: 51–63.
Komárek, J. & V. Jankovská, 2001. Review of the green algal genus Pediastrum; Implication for pollen-analytical research. Bibliotheca Phycologica 108.
Krammer, K., 2000. Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats. The Genus Pinnularia, Vol. 1. A.R.G. Gantner Verlag K.G., Ruggell.
Krammer, K., 2002. Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats. Cymbella, Vol. 3. A.R.G. Gantner Verlag K.G., Ruggell.
Krammer, K., 2003. Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella, Vol. 4. A.R.G. Gantner Verlag K.G., Ruggell.
Krammer, K. & H. Lange-Bertalot, 1986. Bacillariophyceae, 1. Teil: Naviculaceae. In H. Ettl, J. Gerloff, H. Heynig, D. Mollenhauer (eds), Süßwasserflora von Mitteleuropa (Band 2/1). Gustav Fischer Verlag, Jena.
Krammer, K. & H. Lange-Bertalot, 1988. Bacillariophyceae, 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In H. Ettl, J. Gerloff, H. Heynig, D. Mollenhauer (eds), Süßwasserflora von Mitteleuropa (Band 2/2). Gustav Fischer Verlag, Jena.
Krammer, K. & H. Lange-Bertalot, 1991a. Bacillariophyceae, 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süßwasserflora von Mitteleuropa (Band 2/3). Gustav Fischer Verlag, Jena.
Krammer, K. & H. Lange-Bertalot, 1991b. Bacillariophyceae, 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. In H. Ettl, J. Gerloff, H. Heynig, D. Mollenhauer (eds), Süßwasserflora von Mitteleuropa (Band 2/4). Gustav Fischer Verlag, Jena.
Kuneš, P., B. Pelánková, M. Chytrý, V. Jankovská, P. Pokorný & L. Petr, 2008. Interpretation of the last-glacial vegetation of eastern-central Europe using modern analogues from southern Siberia. Journal of Biogeography 35: 2223–2236.
Lange-Bertalot, H., 2001. Diatoms of Europe. Diatoms of Europeaen Inland Waters and Comparable Habitats. Navicula sensu stricto, 10 Genera Separated from Navicula sensu lato, Frustulia, Vol. 2. A.R.G. Gantner Verlag K.G., Ruggell.
Legendre, P. & L. Legendre, 1998. Numerical Ecology. Elsevier, Amsterdam.
Losert, H., 1940. Beiträge zur spät- und nacheiszeitlichen Vegetationsgeschichte Innerböhmens, I. Der Kommerner See. Beihefte zum Botanischen Centralblatt 60B: 346–394.
Lotter, A. F., 1999. Late-Glacial and Holocene vegetation history and dynamics as shown by pollen and plant macrofossil analyses in annually laminated sediments from Soppensee, central Switzerland. Vegetation History and Archaeobotany 8: 165–184.
Lühne, V., 1897. Über ein subfossiles Vorkommen von Diatomaceen in Böhmen. Österreichische botanische Zeitschrift 9: 316–319.
MacArthur, R. H., 1957. On the relative abundance of bird species. Proceedings of the National Academy of Sciences 43: 293–295.
Magny, M., 2004. Holocene climate variability as reflected by mid-European lake-level fluctuations and its probable impact on prehistoric human settlements. Quaternary International 113: 65–79.
Matěna, J., 1989. Seasonal dynamics of a Chironomus plumosus (L.) (Diptera, Chironomidae) population from a fish pond in southern Bohemia. Internationale Revue der gesamten Hydrobiologie und Hydrographie 74: 599–610.
Mendyk, Ł., M. Markiewicz, R. Bednarek, M. Świtoniak, W. W. Gamrat, I. Krześlak, M. Sykuła, L. Gersztyn & A. Kupniewska, 2016. Environmental changes of a shallow kettle lake catchment in a young glacial landscape (Sumowskie Lake catchment), North-Central Poland. Quaternary International 418: 116–131.
Mirosław-Grabowska, J. & E. Zawisza, 2018. Reaction of the lake environment to the Holocene warming depending on the distance to the maximum extent of the Vistulian ice sheet. Catena 171: 494–504.
Nagell, B. & C.-C. Landahl, 1978. Resistance to Anoxia of Chironomus plumosus and Chironomus anthracinus (Diptera) Larvae. Holarctic Ecology 1: 333–336.
Neustupný, E., 1985. On the Holocene period in the Komořany Lake area. Památky archeologické 76: 9–70.
Nõges, P., T. Nõges, L. Tuvikene, H. Smal, S. Ligeza, R. Kornijów, W. Peczula, E. Bécares, F. Garcia-Criado, C. Alvarez-Carrera, C. Fernandez-Alaez, C. Ferriol, R. M. Miracle, E. Vicente, S. Romo, E. Van Donk, W. Van De Bund, J. P. Jensen, E. M. Gross, L. A. Hansson, M. Gyllström, M. Nykänen, E. De Eyto, K. Irvine, D. Stephen, S. Collings & B. Moss, 2003. Factors controlling hydrochemical and trophic state variables in 86 shallow lakes in Europe. Hydrobiologia 506–509: 51–58.
Orbán, I., H. H. Birks, I. Vincze, W. Finsinger, I. Pál, E. Marinova, G. Jakab, M. Braun, K. Hubay, T. Bíró & E. K. Magyari, 2018. Treeline and timberline dynamics on the northern and southern slopes of the Retezat Mountains (Romania) during the late glacial and the Holocene. Quaternary International 477: 59–78.
Pedziszewska, A., W. Tylmann, M. Witak, N. Piotrowska, E. Maciejewska & M. Latałowa, 2015. Holocene environmental changes reflected by pollen, diatoms, and geochemistry of annually laminated sediments of Lake Suminko in the Kashubian Lake District (N Poland). Review of Palaeobotany and Palynology 216: 55–75.
Petr, L., P. Žácková, T. M. Grygar, A. Píšková, M. Krížek & V. Treml, 2013. Šúr, a former late-glacial and Holocene lake at the westernmost margin of the Carpathians. Preslia 85: 239–263.
Płóciennik, M., A. Self, H. J. B. Birks & S. J. Brooks, 2011. Chironomidae (Insecta: Diptera) succession in Żabieniec bog and its palaeo-lake (central Poland) through the Late Weichselian and Holocene. Palaeogeography, Palaeoclimatology, Palaeoecology 307: 150–167.
Pokorný, O., 1963. Několik poznámek k historickému vývoji Komořanského jezera [Some remarks to the historical development of Lake Komořany]. Sborník Československé společnosti zeměpisné 1: 52–57.
Pokorný, P., 2002. A high-resolution record of Late-Glacial and Early-Holocene climatic and environmental change in the Czech Republic. Quaternary International 91: 101–122.
Pokorný, P. & V. Jankovská, 2000. Long-term vegetation dynamics and the infilling process of a former lake (Švarcenberk, Czech Republic). Folia Geobotanica 35: 433–457.
Pokorný, P., M. Chytrý, L. Juřičková, J. Sádlo, J. Novák & V. Ložek, 2015. Mid-Holocene bottleneck for central European dry grasslands: did steppe survive the forest optimum in northern Bohemia, Czech Republic? The Holocene 25: 716–726.
Punt, W., 1976. The Northwest European Pollen Flora: Parts 1–7. Elsevier, Amsterdam.
Punt, W. & S. Blackmore, 1991. The Northwest European Pollen Flora: Parts 44–51. Elsevier, Amsterdam.
Punt, W. & G. Clarke, 1980. The Northwest European Pollen Flora: Parts 8–20. Elsevier, Amsterdam.
Punt, W. & G. Clarke, 1981. The Northwest European Pollen Flora: Parts 21–28. Elsevier, Amsterdam.
Punt, W. & G. Clarke, 1984. The Northwest European Pollen Flora: Parts 29–37. Elsevier, Amsterdam.
Punt, W. & P. Hoen, 2009. The Northwest European Pollen Flora: Part 70. Elsevier, Amsterdam.
Punt, W., S. Blackmore & G. Clarke, 1988. The Northwest European Pollen Flora: Parts 38–43. Elsevier, Amsterdam.
Punt, W., S. Blackmore & P. Hoen, 1995. The Northwest European Pollen Flora: Parts 52–56. Elsevier, Amsterdam.
Punt, W., S. Blackmore, P. Hoen & P. J. Stafford, 2003. The Northwest European Pollen Flora: Parts 57–68. Elsevier, Amsterdam.
R Core Team, 2016. R: A Language and Environment for Statistical Computing.
Ralska-Jasiewiczowa, M., M. Latalowa, K. Wasylikowa, K. Tobolski, E. Madeyska, H. E. Wright & C. Turner (eds), 2004. Late Glacial and Holocene History of Vegetation in Poland Based on Isopollen Maps. W. Szafer Institute of Botany, Polish Academy of Sciences, Krakow.
Rasmussen, S. O., M. Bigler, S. P. Blockley, T. Blunier, S. L. Buchardt, H. B. Clausen, I. Cvijanovic, D. Dahl-Jensen, S. J. Johnsen, H. Fischer, V. Gkinis, M. Guillevic, W. Z. Hoek, J. J. Lowe, J. B. Pedro, T. Popp, I. K. Seierstad, J. Peder, A. M. Svensson, P. Vallelonga, B. M. Vinther, M. J. C. Walker, J. J. Wheatley & M. Winstrup, 2014. A stratigraphic framework for abrupt climatic changes during the Last Glacial period based on three synchronized Greenland ice-core records: refining and extending the INTIMATE event stratigraphy. Quaternary Science Reviews 106: 14–28.
Řeháková, Z., 1983. Diatom succession in the post-glacial sediments of the Komořany Lake, North-West Bohemia, Czechoslovakia. Hydrobiologia 103: 241–245.
Řeháková, Z., 1986. The Postglacial history of diatom-bearing sediments of the former Lake Komorany (North-West Bohemia). Antropozoikum 17: 87–134.
Reimer, P. J., E. Bard, A. Bayliss, J. W. Beck, P. G. Blackwell, C. Bronk, R. Caitlin, E. B. Hai & R. L. Edwards, 2013. Intcal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon 55: 1869–1887.
Rudolph, K., 1926. Pollenanalytische Untersuchungen im thermophilen Florengebiet Böhmens: der ‘Kommerner See’ bei Brüx. Berichten der Deutschen Botanischen Gesellschaft 4: 239–248.
Sayer, C. D., 2001. Problems with the application of diatom-total phosphorus transfer functions: examples from a shallow English lake. Freshwater Biology 46: 743–757.
Sayer, C. D., N. Roberts, J. Sadler, C. David & P. M. Wade, 1999. Biodiversity changes in a shallow lake ecosystem: a multi-proxy palaeolimnological analysis. Journal of Biogeography 26: 97–114.
Sayer, C. D., T. A. Davidson, J. I. Jones & P. G. Langdon, 2010. Combining contemporary ecology and palaeolimnology to understand shallow lake ecosystem change. Freshwater Biology 55: 487–499.
Scheffer, M., 1990. Multiplicity of stable staes in freshwater systems. Hydrobiologia 200: 475–486.
Scheffer, M., 2004. The Story of Some Shallow Lakes. In Usher, M. B. (ed.), Ecology of Shallow Lakes. Kluwer Academic Publishers, Dordrecht.
Scheffer, M. & E. H. Van Nes, 2007. Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584: 455–466.
Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), 1996. Algal Ecology. Freshwater Benthic Ecosystems. Academic Press, San Diego.
Stockmarr, J., 1971. Tablets with spores used in absolute pollen analysis. Pollen et Spores 13: 615–621.
Stuchlík, L., 2001. Atlas of Pollen and Spores of the Polish Neogene. W. Szafer Institute of Botany, Krakow.
Stuiver, M., P. M. Grootes & T. F. Braziunas, 1995. The GISP2 δ18O climate record of the past 16,500 years and the role of the sun, ocean, and volcanoes. Quaternary Research 44: 341–354.
Sümegi, P., S. Gulyás & G. Jakab, 2008. Holocene paleoclimatic and paleohydrological changes in Lake Balaton as inferred from a complex quantitative environmental historical study of a lacustrine sequence of the Szigliget embayment. Documenta Praehistorica 35: 33–44.
Tabacchi, E., L. Lambs, H. Guilloy, A.-M. Planty-Tabacchi, E. Muller & H. Decamps, 2000. Impacts of riparian vegetation on hydrological processes. Hydrological Processes 14: 2959–2976.
Tarrats, P., M. Cañedo-Argüelles, M. Rieradevall & N. Prat, 2018. The influence of depth and macrophyte habitat on paleoecological studies using chironomids: Enol Lake (Spain) as a case study. Journal of Paleolimnology 60: 97–107.
Theuerkauf, M., J. A. A. Bos, S. Jahns, W. Janke, A. Kuparinen, M. Stebich & H. Joosten, 2014. Corylus expansion and persistent openness in the early Holocene vegetation of northern central Europe. Quaternary Science Reviews 90: 183–198.
Turner, F., J. F. Tolksdorf, F. Viehberg, A. Schwalb, K. Kaiser, F. Bittmann, U. von Bramann, R. Pott, U. Staesche, K. Breest & S. Veil, 2013. Lateglacial/Early Holocene fluvial reactions of the Jeetzel river (Elbe valley, northern Germany) to abrupt climatic and environmental changes. Quaternary Science Reviews 60: 91–109.
Turner, F., R. Pott, A. Schwarz & A. Schwalb, 2014. Response of Pediastrum in German floodplain lakes to Late Glacial climate changes. Journal of Paleolimnology 52: 293–310.
Van Dam, H., A. Mertens & J. Sinkeldam, 1994. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherlands Journal of Aquatic Ecology 28: 117–133.
Vermaire, J. C., M.-H. Greffard, É. Saulnier-Talbot & I. Gregory-Eaves, 2013. Changes in submerged macrophyte abundance altered diatom and chironomid assemblages in a shallow lake. Journal of Paleolimnology 50: 447–456.
Vondrák, D., J. Prach & P. Houfková, 2015. Sediments of postglacial lakes in the Czech Republic-unique natural archives overlooked by (Czech) limnology. In V. Rádková & J. Bojková (eds), XVII. konference České limnologické společnosti a Slovenskej limnologickej spoločnosti ‘Voda-věc veřejná’ – Sborník příspěvků. Brno: ČLS and Masarykova univerzita v Brně: 162–167.
Werner, P. & J. P. Smol, 2005. Diatom-environmental relationships and nutrient transfer functions from contrasting shallow and deep limestone lakes in Ontario, Canada. Hydrobiologia 533: 145–173.
Wettstein, R., 1896. Über ein subfossiles Vorkommen von Trapa natans in Böhmen. Lotos 44: 252–258.
Wetzel, R. G., 2001. Limnology. Lake and River Ecosystems. Academic Press, San Diego.
Wiederholm, T., 1983. Chironomidae of the Holarctic region. Keys and diagnoses. Part 1. Larvae. Entomologica Scandinavica 19: 1–457.
Zawiska, I., M. Słowiński, A. Correa-Metrio, M. Obremska, T. Luoto, L. Nevalainen, M. Woszczyk & K. Milecka, 2015. The response of a shallow lake and its catchment to Late Glacial climate changes – a case study from eastern Poland. Catena 126: 1–10.
Zawiska, I., K. Apolinarska & M. Woszczyk, 2019. Holocene climate vs. catchment forcing on a shallow, eutrophic lake in eastern Poland. Boreas 48: 166–178.
Acknowledgements
The authors would like to thank Petr Kuneš, Jan Hošek, Linda Nedbalová and Hana Rajdlová for consultation and important comments. We also thank two anonymous reviewers whose comments substantially improved the manuscript. This project was supported by the Czech Science Foundation (GAČR 17-05935S, GAČR 19-05791S) and the Ministry of Education Youth and Sports of the Czech Republic (Project No. CZ.1.07/2.3.00/20.0289–PAPAVER).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Jasmine Saros
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tichá, A., Bešta, T., Vondrák, D. et al. Nutrient availability affected shallow-lake ecosystem response along the Late-Glacial/Holocene transition. Hydrobiologia 846, 87–108 (2019). https://doi.org/10.1007/s10750-019-04054-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04054-7