Abstract
This work had two aims related to the diet of brown skuas (Stercorarius antarcticus lonnbergi) breeding at Laurie Island (South Orkney Islands, Antarctic). The first aim was to explore whether there are changes throughout the breeding season. The second aim was to determine whether those changes relate to differences in food resource availability of their main prey, penguins, at different time periods of the penguins’ breeding cycles, or to different moments of the skuas breeding cycle, which may variably restrict the foraging activities of parents. Diet was analyzed from pellet samples grouped in two different ways. They were grouped in three periods defined for the skuas breeding cycle (laying and incubation; early parental care; later parental care), or the pellets were assigned to five periods based on the type of food resources available at the penguin colonies (eggs; eggs and small chicks; small and large chicks; large and fledged chicks; fledged chicks). A temporal variation in diet composition was evident from the analysis of contingency tables for both sample grouping methods. The more represented item in every period for both analyses was adult penguins, which may be related to the proposed cleaning function of the gut of penguin feathers. Both ways of grouping the samples suggest a relationship between the kind of resources available at the penguin colonies and the easiness of delivering them to the skuas chicks, reflected in a successive predominance of use of penguin eggs first and of penguin chicks and other birds later.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diet composition is determined by, among other factors, the availability of food and the ability of animals to obtain it. In this regard, breeding activities such as defense of the breeding territory, incubation, or caring and feeding of nestlings may limit access to food resources by reducing the time parents spend away from the nest and, consequently, the distance they may travel to search for food (Bujoczek and Ciach 2009). Restrictions on food access may change throughout the breeding cycle, depending on the parental care activities required at each stage. During stages needing a great deal of care, animal feeding would be limited to food items closer to the nest and with easier access (Gaston et al. 2007). For example, chicks may require a larger amount of food as they grow and thus force parents to make more foraging trips, which would reduce the distance traveled each time.
The brown skua, Stercorarius antarcticus lonnbergi, breeds in Antarctica and sub-Antarctic islands (Ritz et al. 2008) and feeds mainly on chicks, eggs of other seabirds and carrion during their breeding cycle (Reinhardt et al. 2000). The species is strongly associated with colonies of penguins, which represent its main food resource (Pietz 1987; Young 1994; Hahn and Peter 2003; Hahn et al. 2005; Graña Grilli and Montalti 2012).
Considering the possibility that brown skuas’ diet changes throughout the breeding cycle, the aim of the present study was to determine whether this change really happens, and in that case whether those changes are in relationship to different periods of the breeding cycle (1) of brown skuas and (2) of penguins. The first would restrict parents’ ability to search for food because of different caring requirements of specific periods such as incubation and chick growth, while the latter would have a potential strong effect on food type availability for skuas.
Materials and methods
Study area
Pellets were sampled at Laurie Island (60°45′S, 44°39′W), South Orkney Islands, Antarctic, during the breeding seasons 2000–2001, 2001–2002, 2002–2003, 2003–2004, and 2004–2005. The brown skua population was composed of 200 pairs, most of which were associated with large groups of Adelie penguins (Pygoscelis adeliae) and chinstrap penguins (P. antarcticus). Adelie and chinstrap penguin populations were composed of 80,976 and 143,792 breeding pairs, respectively (Coria et al. 2011). Only one pair of south polar skua (S. maccormicki), a possible competitor for food (Malzof and Quintana 2008), was recorded at the site (Coria et al. 2011).
Pellet collection and analysis
Upon arrival to the study site, the area surrounding the skuas’ nests was cleaned of pellets in order to avoid the collection of pellets belonging to previous seasons. All those pellets were discarded, and the pellets for analysis were then collected near active nests with samples taken on the same day being considered as a set. The food item analysis was performed on pellets collected on 2–3 days per month, uniformly distributed throughout each breeding season (between November and March).
The analysis of four different sets (30–35 pellets) and the resulting accumulation curves for the food items indicated that ten pellets per set were enough to identify 70–100 % of the food items. Therefore, ten pellets were randomly re-sampled from each set for further analyses. As a result, a total of 241 pellets were analyzed, from which 30 pellets were collected in 2000–2001, 48 in 2001–2002, 20 in 2002–2003, 60 in 2003–2004, and 83 in 2004–2005.
Food items were classified to the lowest possible taxonomic level. The availability of reference material of the different items potentially used as food by skuas made it possible to identify penguins and species of flying birds by their bones and feathers as well as to assign them to adult or chick categories according to their level of bone ossification, type of feather and color of down feather. In the same way, eggs were identified as corresponding to penguins or flying birds by comparing the eggshell color with reference material. Fish items were recognized from otoliths, scales and vertebrae in the pellets and cephalopods items from beaks, both using reference material. Mammal items were identified from bones and hair. Other pellet components, such as mosses and pebbles, were excluded from the analysis because they are considered as not representing food resources for skuas.
Since a contingency table analysis, used to explore the similarity in the items used among the different seasons, showed similar results among seasons (χ 2 = 52.45, df = 57, p = 0.354), data from different years were pooled and assigned to three periods according to the breeding cycle of skuas, and to five periods according to the breeding cycle of penguins.
The use of pellets probably hid some results due to an overestimation of food items with indigestible parts and an underestimation of items with soft, more digestible tissues (Votier et al. 2001; Santos et al. 2012), leading to a predominance of adult penguin remains. However, it is expected that the degree of overestimation would be similar among individuals and periods. Therefore, it would have no effect when comparing different breeding cycle stages of the same species and at the same study site.
Classification of pellet samples according to the breeding cycle of skuas
The breeding cycle periods of brown skuas were defined from the breeding chronology dates reported for ten eggs of this species on Laurie Island during the breeding season 1993–1994 (Montalti 2005) (Table 1). The dates recorded correspond to: the start of laying (when the first egg was laid), the peak of laying (when 50 % of the total eggs during the season were laid), the start of hatching (when the first chick hatched), the peak of hatching (when 50 % of the total chicks hatched), the start of slowing down in growth rate of chicks, at the inflexion point of the weight gaining curve around the age of 30 days old, and emancipation (when parents stopped feeding their chicks around the age of 70 days old). The dates recorded for laying and hatching start are in complete coincidence with those reported by Hemmings (1984) on brown skuas at Signy Island—ca. 50 km away from the study site—during the seasons 1981–1982 and 1982–1983.
On this basis, the breeding cycle was divided into the following periods: laying and incubation (27/11–25/12), early parental care (26/12–01/02), and later parental care (02/02–13/03). Due to the breeding asynchrony previously observed among pairs (Peter et al. 1990), samples from one period could be wrongly assigned to the next one. To avoid this, pellets collected on the five previous and five subsequent days of each transitional date between periods were excluded from the analysis. Therefore, periods were defined as follows: laying and incubation, 27/11–20/12 (24 days, n = 68 pellets); early parental care, 31/12–28/01 (29 days, n = 90); later parental care, 08/02–13/03 (34 days, n = 80).
Classification of pellet samples according to the breeding cycle of penguins
The penguin breeding cycle periods were defined from the breeding chronology reported on Adelie (Coria and Santos—unpub. data) and chinstrap (Rombolá—unpub. data) penguins on Laurie Island by the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR), Ecosystem Monitoring Programme (CEMP) during the breeding season 2004–2005 (Table 2). The recorded dates correspond to: the start and peak of laying, the start and peak of hatching, the beginning of crèche stage—when the first chick at that stage was found, and the start of fledging (Table 2). However, the breeding cycles of the two penguin species are out of phase: Adelie penguins start breeding approximately 30 days earlier than chinstrap penguins. In order to solve this problem, the above-mentioned dates of the breeding cycles of the two species were overlapped to define periods during which both colonies provide skuas with the same penguin food items (Fig. 1). As a result, the breeding cycle was divided into five 15-day periods, which were named according to the food items present in each one: eggs (E) 09/11–23/11 (n = 27), eggs and small chicks (E + SC) 07/12–21/12 (n = 40), small chicks and large chicks (SC + LC) 06/01–20/01 (n = 52), large chicks and fledged chicks (LC + FC) 06/02–20/02 (n = 38), and fledged chicks (FC) 07/03–21/03 (n = 50). Chicks were regarded as small if they were intensively cared by their parents during the whole day, and as large if they were at the crèche stage under the care of a few adults while others were involved in foraging trips (Williams 1995).
Periods of the breeding cycles of the brown skua (Stercorarius antarcticus lonnbergi) (Montalti 2005) and Adelie (Pygoscelis adeliae) (Coria and Santos, unpub. data) and chinstrap penguins (P. antacticus) (Rombolá, unpub. data). Numbers indicate the peak dates for each period (laying, early parental care, crèche, and fledging in gray scale) and species of penguin. The types of food resources present in the penguin colonies during each period are also indicated (E eggs, SC small chicks, LC large chicks, FC fledged chicks)
Statistical analysis
Data from the different periods were compared in order to look for similarity between them using analysis of contingency tables. For both sample grouping methods, more than 20 % of the expected frequencies were lower than five (Quinn and Keough 2002). Hence, the food items corresponding to adult skuas, sheathbills (Chionis albus), cape petrels (Daption capense), and non-identified birds were grouped into the category ‘adult bird,’ while the items corresponding to cape petrel chicks and non-identified bird chicks were grouped into the category ‘bird chick.’ Fish and cephalopods were also joined in a single category. The Pearson residuals were calculated for each category of the contingency tables in order to analyze the residuals pattern (Quinn and Keough 2002) which were considered as different from expected when they were at least ± 1 and to have a strong influence on the deviation of the diet from the expected when they were ± 2.
Results
A total of 14 food items were identified, among which penguin resources predominated, followed by eggs of flying birds (Table 3). Mosses and pebbles were found in 7 and 27 of the 241 pellets analyzed, respectively.
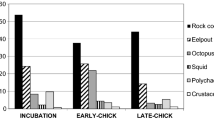
On the one hand, the contingency table analysis showed significant differences in the consumption of food resources by skuas among the three periods of their breeding cycle (χ 2 = 33.88, df = 16, p = 0.006). The results of the Pearson residuals analysis suggest that those differences were due to a high use of penguin eggs during the incubation period of skuas compared to their use in the following periods. This analysis also shows that skuas are more likely to use chicks of penguins and other birds during the period when they are feeding their own large chicks (Fig. 2).
On the other hand, the contingency table analysis showed significant differences in the use of food resources by skuas among the five periods defined for the breeding cycle of penguins (χ 2 = 49.02, df = 32, p = 0.028). The analysis of the Pearson residuals indicated a variable pattern of item consumption in the five periods (Fig. 3). The use of penguin eggs was greater than expected when colonies had eggs and chicks, while the consumption of adult penguins was lower when colonies had small and large chicks. Finally, chicks of other birds were highly consumed by skuas when penguin chicks were unavailable, with a considerable decrease in their use in the following periods when penguin chicks were available at the colonies.
Discussion
Some degree of temporal variation in diet was shown by both sample grouping methods. However, a clear pattern explaining those changes is difficult to see from our results. Based on the fact that the access of an animal to food resources is determined by the interaction between the resource availability and the animals’ ability to obtain them, the variation of both factors at different temporal scales would result in a poorly defined temporal pattern. This may also be the result of the opportunistic feeding habit of the brown skuas (Reinhardt et al. 2000; Phillips et al. 2004; Malzof and Quintana 2008), which may make up for changes in the availability of food items by allowing the use of a variety of resources over the study period.
Contrary to expectations, the consumption of penguin chicks by skuas was lower than expected when penguin colonies had eggs and small chicks, and at the same time, use of penguin eggs was higher than expected. This was possibly due to a constant parental care by penguins, which may make it difficult for skuas to access the chicks and may suggest a stronger defense of chicks than of eggs by penguins, possibly due to the fact that an already borne and fed chick means a higher investment than eggs to the penguin parents.
This high consumption of penguin eggs occurred also in the incubation period of brown skuas’ breeding cycle (Fig. 2), when they may use this item for self-feeding instead of using it when they are feeding chicks, as eggs are not an easy food to deliver to chicks. Therefore, the high use of penguin eggs during the incubation period of skuas may be related to their high availability and weak defense by parents added to the usefulness as food for self-feeding. By contrast, penguin chicks and other birds may be a food that is easier to deliver to chicks, which may explain their use when skuas are feeding their own chicks during the later care period. In this case, both the ease of delivery and the higher energy content of penguin chicks older than 2 weeks compared to that of eggs (Young 1994) may explain the higher use of penguin chicks when skuas are feeding their own chicks.
Older penguin chicks at the crèche stage are still protected by their parents, but may be attacked if they move away from the group (Burton 1968). However, from the Pearson residuals, the consumption of penguin chicks was not higher than expected when they are left unguarded and supposedly more accessible to skuas [small and large chicks (SC + LC), large and fledged chicks (LC + FC), and fledged chicks (FC)]. This is in line with observations that state that the intensity of skua predation on penguin colonies decreases at the penguin crèche stage, both because the size of penguin chicks at this stage makes it more difficult to capture them and because they mean a bigger food contribution that satisfies skuas for longer periods (Young 1994; Emslie et al. 1995).
Both penguin eggs and chicks of other birds had a low representation in our samples, as revealed by their low occurrence frequencies (Table 3; Figs. 2, 3). It is important to highlight, as can be seen from the combined analysis of the occurrence frequency of food items and the Pearson residuals, that those items with more variation have a low occurrence frequency in the diet of skuas. By the opposite, the item more represented in the pellets—adult penguin remains—remained essentially unchanged throughout the three periods of the skua breeding cycle.
Those adult penguin remains were mostly constituted by feathers, which is caused by the predominance of indigestible items (Barrett et al. 2007), but also suggests the possibility that its high occurrence in pellets may be related not only to the use of adult penguin carcasses as food but also to the feathers’ suggested gut cleaning function, as proposed for south polar skuas (Santos et al. 2012). Because of the high availability of penguin carcasses, penguin feathers may be selected for this function over mosses, which are also supposed to perform the same function and, despite their high availability at the site of nesting of brown skuas at the study site, were not found in as high amount as the penguin feathers were.
Despite the limitations imposed by the technique of analysis of pellets used in this work (Votier et al. 2001; Barrett et al. 2007), our results show the existence of a variation in the diet of brown skuas throughout the breeding season that could be related to the availability of resources and to the chronology of their breeding cycle. Both grouping methods indicate an initial predominance of use of penguin eggs as food, followed by a change to penguin chicks and other birds, and suggest that those changes would be regulated by both the availability of prey items and the requirements of nutrition and parental care in different periods in the skuas’ breeding cycle.
References
Barrett RT, Camphuysen CJ, Anker-Nilssen T, Chardine JW, Furness RW, Garthe S, Hüppop O, Leopold MF, Montevecchi WA, Veit RR (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691
Bujoczek M, Ciach M (2009) Seasonal changes in the avian diet of breeding Sparrowhawks Accipiter nisus: how to fulfill the offspring’s food demands? Zool Stud 48:215–222
Burton RW (1968) Breeding biology of the brown skua, Catharacta skua lönnbergi (Mathews), at Signy Island, South Orkney Islands. Br Antarct Surv Bull 15:9–28
Coria NR, Montalti D, Rombola EF, Santos MM, Garcia Betoño MI, Juares MA (2011) Birds at Laurie Island, South Orkney Islands, Antarctica: breeding species and their distribution. Mar Ornithol 39:207–213
Emslie SD, Karnovsky N, Trivelpiece W (1995) Avian predation at penguin colonies on King George Island, Antarctica. Wilson Bull 107:317–327
Gaston AJ, Ydenberg RC, Smith GEJ (2007) Ashmole’s halo and population regulation in seabirds. Mar Ornithol 35:119–126
Graña Grilli M, Montalti D (2012) Trophic interactions between brown and south polar skuas at deception island, Antarctica. Polar Biol 35:299–304
Hahn S, Peter H-U (2003) Feeding territoriality and the reproductive consequences in brown skuas Catharacta antarctica lonnbergi. Polar Biol 26:552–559
Hahn S, Peter H-U, Bauer S (2005) Skuas at penguin carcass: patch use and state-dependent leaving decisions in a top-predator. Proc R Soc B 272:1449–1454
Hemmings AD (1984) Aspects of the breeding biology of McCormick’s skua Catharacta maccormicki at Signy island, South Orkney islands. Br Antarct Surv Bull 65:65–79
Malzof SL, Quintana RD (2008) Diet of the south polar skua Catharacta maccormicki and the brown skua C. antarctica lonnbergi at Cierva Point, Antarctic Peninsula. Polar Biol 31:827–835
Montalti D (2005) Morfología y biología reproductiva de las especies del género Catharacta (Aves, Stercorariidae) de la Antártida. Dissertation, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata
Peter H-U, Kaiser M, Gebauer A (1990) Ecological and morphological investigations on South Polar Skuas (Catharacta maccormicki) and Brown Skuas (Catharacta skua lonnbergi) on Fildes Peninsula, King George Island, South Shetland Islands. Zool Jahrb Syst 117:201–218
Phillips RA, Phalan B, Forster IP (2004) Diet and long-term changes in population size and productivity of brown skuas Catharacta antarctica lonnbergi at Bird Island, South Georgia. Polar Biol 27:555–561
Pietz PJ (1987) Feeding and nesting ecology of sympatric south polar and brown skuas. Auk 104:617–627
Quinn G, Keough M (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Reinhardt K, Hahn S, Peter H-U, Wemhoff H (2000) A review of the diets of Southern Hemisphere skuas. Mar Ornithol 28:7–19
Ritz MS, Millar C, Miller GD, Phillips RA, Ryan P, Sternkopf V, Liebers-Helbig D, Peter H-U (2008) Phylogeography of the southern skua complex-rapid colonization of the southern hemisphere during a glacial period and reticulate evolution. Mol Phylogenet Evol 49:292–303
Santos MM, Juares MA, Rombolá EF, García ML, Coria NR, Donkaster CP (2012) Over-representation of bird prey in pellets of South Polar Skuas. J Ornithol 153:979–983
Votier SC, Bearhop S, Ratcliffe N, Furness RW (2001) Pellets as indicators of diet in Great Skuas Catharacta skua. Bird Study 48:373–376
Williams TD (1995) The penguins: spheniscidae. Oxford University Press, New York
Young E (1994) Skua and penguin: predator and prey. Cambridge University Press, Cambridge
Acknowledgments
The fieldwork was supported by the Instituto Antártico Argentino. We thank E. Rombolá and N. Coria for providing data on the breeding chronology of penguins at Laurie Island and to four anonymous referees who improved the manuscript with their comments. We are grateful to D. Brooks and the Program of Editorial Assistance of the Association of Field Ornithologists and to Bruce Peterson, who through the program improved the English of the manuscript, and to A. Vilas for further help with the language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graña Grilli, M., Montalti, D. Variation in diet composition during the breeding cycle of an Antarctic seabird in relation to its breeding chronology and that of its main food resource. Polar Biol 38, 643–649 (2015). https://doi.org/10.1007/s00300-014-1627-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1627-2