Abstract
The current information about the diet composition of the rock shag (Phalacrocorax magellanicus) in the SW Atlantic coast comes mainly from conventional pellet or stomach content analysis from a few locations situated in northern Patagonia (Chubut Province, Argentina). In this work, we studied the diet of breeding rock shags over several years at a colony from southern Patagonia (Ría Deseado, Santa Cruz Province, Argentina) using a combined technique of conventional diet assessment (pellet analysis) and stable isotope analysis of carbon and nitrogen. Our results confirm the importance of benthic prey and the low inter-annual variability in the diet of the rock shag. These results coincide with previous research in relation to the exploitation of slow moving, predictable, but low-energy density prey. The stable isotope mixing models, which was informed with prior data obtained from pellet analysis, allowed for the detection of subtle differences between the diet of adults and chicks, consisting in the incorporation of higher proportions of cephalopods, an energy-rich prey, in the diet of chicks. By comparing our results with the diet of the red-legged cormorant, which breeds in sympatry in the Ría Deseado Estuary and whose diet composition is strongly pelagic, we suspect a certain level of trophic resource partitioning between these rock shag and red-legged cormorant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge of seabirds’ trophic ecology is fundamental to understand the ecosystem functioning and to assess potential impacts of oceanographic changes and anthropic activities on their populations (Weimerkirch et al. 2001; Suryan et al. 2006; Elliot et al. 2015). Furthermore, the diet of a specific seabird species can differ among colonies likely due to the effect of the community structure and the habitat characteristics of prey availability (Karnovsky et al. 2008; Chiaradia et al. 2012; Fernandez et al. 2019). The diet can also vary between years or breeding stages, in response to changes in food availability or individual demands (Bertellotti and Yorio 1999; Weimerskirch et al. 2001; Carscadden et al. 2002; Ibarra et al. 2018).
The analysis of pellet casts (regurgitated indigestible remains of prey) has been widely used to study the diet of seabirds, as the sampling is simple and non-invasive (Barrett et al. 2007). Nevertheless, this approach can present some biases caused by the differential erosion rate of the prey remains that can over or under-represent certain prey types, and some secondary prey remnants can be retained (Barrett et al. 2007). This is why many authors (Barrett et al. 2007; Bond and Jones 2009; Ciancio et al. 2015) recommend the use of this conventional technique in combination with Stable Isotope Analysis (SIA) of carbon and nitrogen. Under the appropriate conditions, the SIA quantifies the relative importance of the main prey consumed with stable isotope mixing models (Parnell et al. 2010; Philips et al. 2014). One of the advantages of the SIA is that it provides integrated information on the diet assimilated over time and not at a punctual moment like conventional techniques (Barrett et al. 2007; Bond and Jones 2009). However, more specific dietary knowledge about potential prey is required to perform mixing models of a predator. That is why the SIA is an interesting tool to estimate prey proportions, especially when used in combination with conventional diet analysis. This becomes even more useful with the integration of prior information obtained from conventional diet in the SIA mixing models (Moore and Semmens 2008; Layman et al. 2012).
The rock shag (Phalacrocorax magellanicus) is endemic to the Atlantic and Pacific coasts of Patagonia, including the Falkland Islands (Malvinas) (Harrison and Peterson 1985; Johnsgard 1993; Yorio et al. 1998). In the Atlantic coast, a small population of around 7000 breeding pairs is distributed in 145 colonies, ranging between 42° 50′ S to 54° 50′ S (Yorio et al. 1998; Frere et al. 2005). Rock shags frequently nest together with imperial shags or red-legged cormorants, in mixed or adjacent colonies (Frere et al. 2005).
Like all shags and cormorants, rock shags are foot-propelled pursuit-divers; they feed mainly in shallow waters (< 10 m) near the coast (< 5 km) (Quintana et al. 2002). Frere et al. (2008) found that rock shags living in sympatry with red-legged cormorant (Ría Deseado, Santa Cruz) had significantly larger foraging ranges and trip durations than in allopatry (without red-legged cormorant; Caleta Malaspina, Chubut). They attributed these differences to possible interspecific competition mechanisms, forcing niche differentiation in areas of sympatry. Rock shag’s diet has been studied through conventional analysis, and in only a few colonies from northern Patagonia (Chubut). It has been described as composed of a high proportion of benthic fish and invertebrates, including polychaetes, cephalopods and crustaceans and, to a lesser extent, demersal-pelagic fish (Malacalza et al. 1997; Punta et al. 2003; Bulgarella et al. 2008; Sapoznikow and Quintana 2009). Sapoznikow and Quintana (2009) found a very low seasonal and inter-annual variability in rock shag’s diet, and suggest that its prey are relatively predictable and stable, although not abundant. These characteristics could help to explain its small population along the Patagonian coast (Quintana et al. 2002; Sapoznikow and Quintana 2005, 2009).
In this work, we studied the diet of breeding rock shags over several years at a colony from southern Patagonia using a combined technique of a conventional diet assessment (pellet analysis) and stable isotope analysis of carbon and nitrogen. Our objectives were to describe the diet of the rock shag at this colony and to assess the differences in diet composition among breeding stages (pellets) and between adults and chicks (SIA). Finally, we were also interested in discussing and contrasting the diet of this colony in which the rock shag breeds in sympatry with the red-legged cormorant with the diet of the rock shag from northern Patagonia in which it breeds in allopatry (without the red-legged cormorant).
Materials and methods
Study area
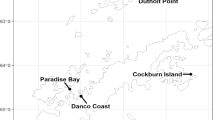
The Ría Deseado Estuary is found in southern Patagonia, near the town of Puerto Deseado, on the northern coast of the Santa Cruz Province in Argentina (47° 45′ S, 65° 53′ W). This estuary, or ria, is a long (> 40 km), narrow inlet formed by the partial submergence of a river valley, and belongs to the protected area Reserva Provincial Ría Deseado. The fieldwork was carried out at Isla Elena (47° 45′ S, 65° 56′ W), an island situated 5 km from the entrance of the ria, hosting the only breeding colony of rock shags from the Ría Deseado (124 breeding pairs, Gandini and Frere 1998; Frere et al. 2005).
Conventional diet sampling
Pellets were collected at Isla Elena during four breeding seasons (2009, 2011, 2012 and 2013) by fixing collecting bags (2 × 0.7 × 0.5 m) on the cliffs, underneath two groups of three to five nests each. The bags containing the pellets were emptied every 1 to 2 weeks between November and February. The pellets were collected during three breeding stages: incubation (approximately early-November to mid-December), early chick-rearing (chicks up to 4 weeks old; mid-December to mid-January), and late chick-rearing (chicks between approximately 4 weeks old and fledglings; mid-January to end-February). The pellets were analysed with a binocular microscope and hard prey remains were used to quantify and identify prey at the lowest taxonomic level possible. Identification was carried out by using our own collections and available literature and catalogues (Lombarte et al. 1991; Boschi et al. 1992; Gosztonyi and Kuba 1996; Pineda et al. 1996; Piacentino 1999; Volpedo and Echeverría 2000; Tombari et al. 2010). The frequency of occurrence (%FO) and the number of occurrence (%N) were calculated for all prey items, and expressed as percentages. Published or our own allometric regressions were used to estimate the average total length (TL) and wet weight (W) of different prey types (Pineda et al. 1996; Koen-Alonso et al. 2000). The length of fish otoliths and cephalopod mandibles from the 2009 season pellets were used for TL and W calculations. Finally, the Shannon–Weaver diversity index was calculated for the whole time period (all years together) and for each separate year (Tramer 1969).
SIA sample collection and processing
Whole blood samples of adult and chick rock shags were collected during three consecutive breeding seasons (2011 to 2013) at Isla Elena (overall N = 53) for SIA. Adults were captured from their nests during late-incubation to early chick-rearing stage (mid-December), corresponding to the period of time when they were less prone to fly away (Frere et al. 2002). Each manipulation lasted less than 5 min, and on release, birds flew directly to the water and returned to their nests shortly afterwards. The 2- to-4 week old chicks were captured from their nests in early January, and put back promptly after manipulation. Approximately, 0.5 mL of blood was extracted from the brachial vein of adults and chicks, and preserved in 70% ethanol before processing in the laboratory (Hobson et al. 1997).
Samples of the main prey sources for SIA were collected from 2011 to 2013 spring–summer seasons. Four potential prey sources were chosen according to our preliminary dietary results and unpublished information of the same area (Gandini and Frere, unpublished data). These prey were two demersal-benthic fish groups: the rock cods (Patagonotothen cornucola) and the eelpouts (zoarcidae fish), and two different cephalopods: the benthic octopus (Enteroctopus megalocyathus, known as red octopus) and the demersal-pelagic squid (Doryteuthis gahi, known as Patagonian squid). Fish samples were collected under intertidal rocks, squid samples were found from regurgitates in the collecting bags or around nests, and octopus’ samples were obtained from local fishermen. All prey samples were identified using the available literature and catalogues (Menni et al. 1984; Pineda et al. 1998; Bovcon et al. 2007) and were stored frozen until processing in the laboratory.
The samples were dried at 60 °C for > 24 h for whole blood, and for > 48 h for fish muscle and cephalopod mantle samples, and ground to a fine, homogenized powder. Carbon and nitrogen isotope ratios were measured in the Center for Stable Isotopes at the University of New Mexico, USA, by Elemental Analyser Continuous Flow Isotope Ratio Mass Spectrometry using a Costech ECS 4010 Elemental Analyser coupled to a Thermo Fisher Scientific Delta V Advantage mass spectrometer via a CONFLO IV interface. Isotope ratios were reported using the standard delta (δ) notation relative to AIR and Vienna Pee Dee Belemnite (V-PDB), respectively, and expressed in units per thousand (‰) as follows: δ = (Rsample/Rstandard − 1), where Rsample and Rstandard are the molar ratios of the heavy to light isotopes (13C/12C or 15N/14N) of the sample and standard, respectively. Average analytic precision based on routine analysis of a laboratory protein standard was < than 0.1‰ (1σ). The laboratory standard was calibrated against IAEA-N-1, IAEA-N-2, USGS 42 and USGS 43 for nitrogen and NBS 21, NBS 22 and USGS 24, USGS 42 and USGS 43 for carbon.
Posterior inspection of C/N mass ratio showed that the eelpout (zoarcidae fish) presented several C/N values > 4, indicating possible bias in δ13C due to high lipid content (Post et al. 2007; Bond and Jones 2009). Therefore, δ13C values of the eelpout were corrected with a mathematical model according to Logan et al. (2008). Rock cod and Patagonian squid SIA values used in this study were previously published by Morgenthaler et al. (2016).
SIA mixing models
The relative contribution of the potential prey to the diet of the shags was estimated using Bayesian mixing models from the ‘simmr’ package (Parnell 2016) in R 3.6.0 (R Core Team 2019). Due to inter-annual differences of shag and prey isotopic data, models were run for each year separately, with adults and chicks considered as two different groups. The prey sources data used for each model (year) were from the same year as the consumers’ data, when available (see Table 2). Two different models were run for each year: (1) an initial model, with no prior information and (2) an informed model with the following priors obtained from conventional diet: rock cod: 0.4; eelpout: 0.25; octopus: 0.25; squid: 0.1.
Up to the researchers’ knowledge, no diet to whole blood discrimination factor, a key parameter for stable isotope mixing models, has been experimentally determined for the rock shag. Only an experimentally determined value is known for a species of shag/cormorant: the double-crested cormorant (Phalacrocorax auritus) which was fed with catfish (Ictalurus punctatus), a freshwater farm fish (Craig et al. 2015). In our study, the whole blood discrimination factor the Magellanic penguin (Spheniscus magellanicus) a seabird species that coexists with the rock shag in the Ría Deseado, was used (Ciancio et al. 2016). Although this factor was obtained from a taxonomically different bird family, the feeding trials were made with anchovy (Engraulis anchoita), a prey found in the Patagonian Sea which is ecologically and isotopically more similar to the prey of rock shag than the freshwater farm-raised catfish fed to the double-crested cormorant. The Δ discrimination factor values used were: + 2.31‰ for Δ15N and + 0.41‰ for Δ13C (Ciancio et al. 2016); a standard deviation of ± 1 ‰ was included to take into account the uncertainty due to possible differences in the discrimination factors between penguins and shags (Votier et al. 2010). The simulation method for inspection of mixing polygons from Smith et al. (2013) confirmed that the consumer data were situated within the 95% mixing regions for each model (probability ranges for each model: 2011: 0.55 to 0.69, 2012: 0.35 to 0.65, 2013: 0.23 to 0.66).
Statistical analyses
For conventional diet, multivariate similarity analyses (ANOSIM) using the R 'vegan' package were used to test for differences in the biomass estimates of the main prey types among years and among breeding stages (Oksanen et al. 2016). The isotopic centroid positions were examined using nested linear models and residual permutation procedures (Turner et al. 2010). Centroid locations were compared among each pair of years (adults and chicks together), and between adults and chicks within each year, and were considered different if the Euclidean Distance (ED) between centroid locations was significantly greater than zero (Turner et al. 2010).
Results
Conventional diet
Out of 70 analysed pellets, 26 different prey items belonging to seven different taxa (teleost fishes, cephalopods, crustaceans, polychaetes, gasteropods, ascidians and algae) were identified (Table 1). Overall prey diversity was high (Shannon–Weaver Index: 1.43) and did not vary much among years (2009: 1.16, 2011: 1.45, 2012: 1.35 and 2013: 1.57) and among breeding stages (mean value of all 4 years: incubation: 1.34, early chick rearing: 1.58 and late chick rearing: 1.38). The dietary composition, considering the biomass estimates of the main prey types, showed a high level of overlap and no significant differences among breeding stages (R = 0.031, p = 0.175), and a high level of overlap but significant differences among years (R = 0.050, p = 0.044).
The most important prey, based on the biomass estimates, were the rock cods (Patagonotothen spp.), followed by eelpouts (zoarcidae fishes) and by the red octopus (Enteroctopus megalocyathus) (Fig. 1). This later prey showed higher estimates during the early chick rearing stage (Fig. 1). The most frequent prey (highest frequency of occurrence) were the rock cod and the polychaete worms (Table 1). Polychaetes were also the most numerically abundant prey. Although their individual size and weight is small, their overall estimated biomass (%W) during the incubation stage accounted for 9.7% (Fig. 1). All the main prey of the rock shag are considered demersal-benthic or benthic, except the demersal—pelagic Patagonian squid (Doryteuthis gahi) (mean W%: 3.1%).
Percent estimated wet weight (%W) of the main prey for each breeding stage in the diet of rock shag based on pellet analysis. The prey presented in the figure are: Rock cod, Patagonotothen spp. in black; Eelprout (zoarcidae fishes) with black-white diagonal lines; Red octopus, Enteroctopus megalocyathus, in dark grey; Patagonian squid, Doryteuthis gahi, with black-white vertical lines; Polychaetes worms in light grey and Crustaceans in white. Values are shown for each of the three breeding stages: incubation, early chick-rearing and late chick-rearing from Isla Elena colony, all years together (number of pellets analyzed: N incubation = 31, N early- chick = 28, N late chick = 12)
Stable isotope values and diet estimated from the mixing models
The stable isotope values of rock shag whole blood ranged between − 17.1 and − 13.7‰ for δ13C, and between + 17.8 and + 19.6‰ for δ15N (Table 2). Although the distances of the mean isotopic positions (adults and chicks together) among all pairs of years were small (ED = 0.3–0.5‰), the positions were significantly different between 2011 and 2012 (ED = 0.5‰, p = 0.05). The mean isotopic position of adults and chicks differed significantly within years in 2011 and 2012, with adults presenting higher δ13C values than chicks (Table 2, Fig. 2). The mean isotopic position of the rock cod (Patagonotothen cornucola), the main prey of shags, differed significantly between 2011 and 2012 (ED = 2.2‰, p = 0.001), and between 2011 and 2013 (ED = 1.5‰, p = 0.03), but not between 2012 and 2013 (ED = 0.7‰, p = 0.3; Table 3).
Stable isotope mixing diagram of adults (AD) and chicks (CHICKS) of rock shag (black) from Isla Elena and their potential prey (white), presented by year. Values are mean +-s.d.. Stable isotope values of the prey were corrected by adding the Diet Tissue Discrimination Factor (see methods). Prey items: Rock cod, Patagonotothen spp., Eelprout (zoarcidae fishes), Red octopus, Enteroctopus megalocyathus, and Patagonian squid, Doryteuthis gahi
The results of the initial mixing models (model 1) of adult shags showed similar proportions of the three benthic prey (rock cod, eelpout and octopus) for 2011, 2012 and 2013, which ranged between 21 and 33% each, and lower proportions of the pelagic prey (squid: 13–18%). All proportion estimates showed high credibility intervals (Fig. 3). The models for the chicks showed proportions for all four prey varying between 17 and 35%, with high credibility intervals, and variable order of importance of prey among years (Fig. 3). The results of the informed mixing models (model 2) reduced the credibility intervals in all proportion of prey estimates, restricting the results to a range of proportions more similar to those obtained with conventional diet analysis, with the rock cod found as the main prey in all the models (Fig. 4). Despite the fact that the adult and chick models were informed with identical priors, chicks showed higher proportions of squid than adults for every year (Fig. 4).
Estimated prey contributions to the diet of adult rock shags obtained from initial (left) and informed (right) stable isotope mixing models. Box plots display the range between 25 and 75% credibility quantiles, with error bars extending to the maximum and minimal values (97.5% and 2.5%, respectively), and the median represented by the bold line. Prior data of informed models: Rock cod: 0.4; Eelpout: 0.25; Octopus: 0.25; Squid: 0.1
Estimated prey contributions to the diet of chick rock shags obtained from initial (left) and informed (right) stable isotope mixing models. Box plots display the range between 25 and 75% credibility quantiles, with error bars extending to the maximum and minimal values (97.5% and 2.5%, respectively), and the median represented by the bold line. Prior data of informed models: Rock cod: 0.4; Eelpout: 0.25; Octopus: 0.25; Squid: 0.1
Discussion
Our diet results from southern Patagonia are in accordance with previous diet studies of Rock shag from northern Patagonia (Malacalza et al. 1997; Punta et al. 2003; Bulgarella et al. 2008; Sapoznikow and Quintana 2009) showing that benthic species of fish are the most frequent and/or abundant prey. Rock cod (Patagonotothen spp.) is the most abundant prey for both adult and chick shags in our study, followed by another benthic fish group, the eelpout (zoarcidae). Both rock cods and eelpouts live in similar habitat, and are usually associated with rocky bottoms and holdfasts of kelp (Macrocystis pyrifera) forest (Vanella et al. 2007).The benthic rock cod is considered a low-energy content prey, contrary to pelagic fish (Ciancio et al. 2007; Fernández et al. 2009). Punta et al. (2003) and Malacalza et al. (1997) found some pelagic or demersal fish species (Engraulis anchoita and Merluccius hubbsi respectively) in the diet of rock shags, although in much smaller proportions than benthic species. No such prey are found in our study, despite the presence of pelagic or demersal-pelagic small fish (Sprattus fuegensis and Odontesthes spp.) in the Ría Deseado (Frere et al. 2002; Nasca et al. 2004; Millones et al. 2005). Nevertheless, the benthic eelpouts might be considered a higher energy content prey than benthic rock cods due to the high lipid content found in this study (C:N > 4), and could therefore play an important role in the diet of breeding rock shags at Ría Deseado.
The diet of the rock shag at Ría Deseado is very diverse and includes several invertebrate groups. The most frequent and numerically abundant (%FO and %N) are the polychaete worms, which, despite their small size, contribute to a non-negligible percentage of biomass (%W: 6.2). Although some authors consider the polychaetes as secondary prey (Casaux et al. 1995), Sapoznikow and Quintana 2009 considered them as primary prey because of their high frequency and abundance (some pellets only contained polychaetes’ mandibles and chaeta), which is similar to our study. Polychaetes are interesting from an energetic perspective because of their energy content, comparable or superior to that of benthic fish (Ciancio et al 2007; Sapoznikow and Quintana 2009).
The most abundant (%W and mixing models proportions) invertebrate group are the cephalopods, composed of one octopus and one squid species. Interestingly, the mixing models allowed to determine that cephalopods proportion estimates were higher in chicks than in adults. Seabirds sometimes feed their chicks with different prey items from what the adults eat for themselves (Davoren and Burger 1999). The incorporation of cephalopods during chick rearing period is likely to be an interesting strategy from an energetic perspective, as both the octopus and the squid have higher energy content than benthic fishes, like rock cods (Ciancio et al 2007; Sapoznikow and Quintana 2009). The Patagonian squid is the only demersal-pelagic prey found in the diet of the rock shag. It is an abundant species on the coast of northern Santa Cruz, and it is often preyed upon by other colonial seabird nesting in the Ría Deseado estuary (Frere et al. 1996; Millones et al. 2005; Morgenthaler et al. 2016; Barrionuevo et al. 2018).
Frere et al. (2008) found that rock shags living in sympatry with red-legged cormorants at Ría Deseado showed a different foraging behavior (feeding trip distance and duration) than in a colony from northern Patagonia where rock shag breeds in allopatry (without red-legged cormorants). Despite the differences in the foraging patterns between these colonies, we found no notable difference in the diet of rock shags from Ría Deseado compared to that from northern Patagonia (Malacalza et al. 1997; Punta et al. 2003; Bulgarella et al. 2008; Sapoznikow and Quintana 2009). We found some taxonomic differences in the prey composition, likewise related to their distribution and availability, but no important ecological differences in prey types, indicating a consistent benthic diving behavior.
The comparison of the feeding behaviour of the rock shag and the red-legged cormorant at Ría Deseado colony yields little overlap in their feeding locations and shows that red-legged cormorants feed significantly closer to the colony and undertake shorter foraging trips than rock shags do (Frere et al. 2008). The diet of breeding red-legged cormorants, studied through a similar dietary combined framework, show a low diversity and a strong pelagic component (Morgenthaler et al. 2016). Patagonian sprats and Patagonian squid are the two main prey (Morgenthaler et al. 2016). Therefore, the Patagonian squid seems to be the only important prey that both rock shags and red-legged cormorants are sharing, suggesting little trophic overall overlap. The interspecific differences in the feeding areas and the diet composition of these two species belonging to the same guild could be indicating a certain level of trophic resources partitioning. Furthermore, two more species belong to the Phalacrocoracidae guild resort to the Ría Deseado estuary for feeding: the neotropic cormorant (Phalacrocorax brasilianus) and the imperial shag (Phalacrocorax atriceps). Therefore, this estuary is one of the very few locations in the world with four sympatric species of shags and cormorants, and constitute an ideal location to study the trophic segregation within this guild. Further knowledge on the diet composition of these four species is necessary.
The combination of pellet and stable isotope analyses offered a complementary framework to study the diet of this shag, in a much more robust manner than deploying each technique separately. The pellet analysis provided comprehensive trophic information regarding the prey spectrum, as well as a prior set of information to incorporate in the mixing models. The stable isotope analysis of blood and an adequate isotopic prey sampling design, allow for the quantification of assimilated prey through mixing models (Phillips et al. 2014). The incorporation of previous dietary information in the mixing model, particularly when the isotopic values of the prey types are not different enough or their position in the bivariate space is not geometrically adequate like in our study help to reduce the credibility intervals and orientate the models towards the most appropriate dietary proportion combinations (Phillips et al. 2014).
The dietary results obtained through this study for the rock shag at Ría Deseado framework are in agreement with previous studies from locations situated further North along the Patagonian coast. This study confirms the importance of benthic prey, and particularly of benthic fish species, and the low inter-annual variability in the diet of the shag. These results coincide with the previous research in relation to the exploitation of slow moving, predictable, but not abundant and low-energy density prey (Sapoznikow and Quintana 2009). The stable isotope approach allowed for the detection of subtle differences between the diet of adults and chicks, like the incorporation of higher proportions of cephalopods, an energy-rich prey, in the diet of chick rock shags.
References
Barrett RT, Camphuysen K, Anker-Nilssen T et al (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691. https://doi.org/10.1093/icesjms/fsm152
Barrionuevo M, Ciancio J, Marchisio N, Frere E (2018) Parental body condition and high energy value of fish determine nestling success in Magellanic penguin (Spheniscus magellanicus). Mar Biol 165:105
Bertellotti M, Yorio P (1999) Spatial and temporal patterns in the diet of the kelp gull in Patagonia. Condor 101:790–798
Bond AL, Jones IL (2009) A practical introduction to stable-isotope analysis for seabird biologists: approaches, cautions and caveats. Mar Ornithol 37:183–188
Boschi E, Fischbach C, Iorio M (1992) Catálogo ilustrado de los crustáceos estomatópodos y decápodos marinos de Argentina. Frente Marítimo 10:7–94
Bovcon ND, Cochia PD, Gosztonyi AE (2007) Guía para el reconocimiento de los peces capturados por buques pesqueros monitoreados con observadores a bordo. Publicación especial de la Secretaría de Pesca de la Provincia del Chubut, Rawson, Argentina. Secretaría de Pesca de la Provincia del Chubut, Rawson, Argentina
Bulgarella M, Pizarro LC, Quintana F et al (2008) Diet of imperial cormorants (Phalacrocorax atriceps) and rock shags (P. magellanicus) breeding sympatrically in Patagonia. Argentina Ornitol Neotrop 19:553–563
Carscadden JE, Montevecchi WA, Davoren GK, Nakashima BS (2002) Trophic relationships among capelin (Mallotus villosus) and seabirds in a changing ecosystem. ICES J Mar Sci 59:1027–1033
Casaux RJ, Favero M, Barrera-Oro ER, Silva P (1995) Feeding trial on an imperial Cormorant Phalacrocorax atriceps: preliminary results of fish intake and otolith digestion. Mar Ornithol 23:101–106
Chiaradia A, Forero MG, Hobson KA, Swearer SE, Hume F, Renwick L, Dann P (2012) Diet segregation between two colonies of little penguins Eudyptula minor in southeast Australia. Austral Ecol 37:610–619
Ciancio JE, Pascual M, Beauchamp D (2007) Energy density of patagonian aquatic organisms and empirical predictions based on water content. Trans Am Fish Soc 136:1415–1422. https://doi.org/10.1577/T06-173.1
Ciancio J, Botto F, Frere E (2015) Combining a geographic information system, known dietary, foraging and habitat preferences, and stable isotope analysis to infer the diet of Magellanic Penguins in their austral distribution. Emu 115:237–246. https://doi.org/10.1071/MU14032
Ciancio JE, Righi C, Faiella A, Frere E (2016) Blood-specific isotopic discrimination factors in the Magellanic penguin (Spheniscus magellanicus). Rapid Commun Mass Spectrom 30:1865–1869. https://doi.org/10.1002/rcm.7661
Craig EC, Dorr BS, Hanson-Dorr KC et al (2015) Isotopic discrimination in the double-crested cormorant (Phalacrocorax auritus). PLoS ONE 10:1–7. https://doi.org/10.1371/journal.pone.0140946
Davoren GK, Burger AE (1999) Differences in prey selection and behaviour during self-feeding and chick provisioning in rhinoceros auklets. Anim Behav 58:853–863. https://doi.org/10.1006/ANBE.1999.1209
Elliott ML, Bradley RW, Robinette DP, Jahncke J (2015) Changes in forage fish community indicated by the diet of the Brandt's cormorant (Phalacrocorax penicillatus) in the central California current. J Mar Syst 146:50–58
Fernández DA, Lattuca ME, Boy CC et al (2009) Energy density of sub-Antarctic fishes from the Beagle channel. Fish Physiol Biochem 35:181–188. https://doi.org/10.1007/s10695-008-9234-1
Fernandez SJ, Yorio P, Ciancio JE (2019) Diet composition of expanding breeding populations of the Magellanic Penguin. Mar Biol Res 15:1–13
Frere E, Gandini P, Lichtschein V (1996) Variación latitudinal en la dieta del pingüino de Magallanes (Spheniscus magellanicus) en la costa patagónica, Argentina. Ornitol Neotrop 7:35–41
Frere E, Quintana F, Gandini P (2002) Diving behavior of the red-legged cormorant in Southeastern Patagonia, Argentina. Condor 144:440–444
Frere E, Quintana F, Gandini P (2005) Cormoranes de la costa patagónica: estado poblacional, ecología y conservación. Hornero 20:35–52
Frere E, Quintana F, Gandini P, Wilson RP (2008) Foraging behaviour and habitat partitioning of two sympatric cormorants in Patagonia, Argentina. Ibis 150:558–564. https://doi.org/10.1111/j.1474-919X.2008.00824.x
Gandini PA, Frere E (1998) Seabird and shorebird diversity and associated conservation problems in Puerto Deseado, Patagonia, Argentina. Ornitol Neotrop 9:13–22
Gosztonyi AE, Kuba L (1996) Atlas de huesos craneales y de la cintura escapular de peces costeros patagónicos. Fund Patagon Nat Inf Técnico 4:1–29
Harrison P, Peterson R (1985) Seabirds: an identification guide. Houghton Mifflin, Boston
Hobson KA, Gloutney ML, Gibbs HL (1997) Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Can J Zool 75:1720–1723. https://doi.org/10.1139/z97-799
Ibarra C, Marinao C, Suárez N, Yorio P (2018) Differences between colonies and chick-rearing stages in imperial Cormorant (Phalacrocorax atriceps) diet composition: implications for trophic studies and monitoring. Wilson J Ornithol 130:224–234. https://doi.org/10.1676/16-184.1
Johnsgard P (1993) Cormorants, darters, and pelicans of the world. Smithsonian Inst Pr, Washington, D.C.
Karnovsky NJ, Hobson KA, Iverson S, Hunt GL (2008) Seasonal changes in diets of seabirds in the North Water Polynya: a multiple-indicator approach. Mar Ecol Prog Ser 357:291–299
Koen Alonso M, Crespo EA, Pedraza SN, Garcia NA, Coscarella MA (2000) Food habits of the South American sea lion, Otaria flavescens, off Patagonia, Argentina. Fish Bull 98:250–263
Layman CA, Araujo MS, Boucek R et al (2012) Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol Rev 87:545–562. https://doi.org/10.1111/j.1469-185X.2011.00208.x
Logan JM, Jardine TD, Miller TJ et al (2008) Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. J Anim Ecol 77:838–846. https://doi.org/10.1111/j.1365-2656.2008.01394.x
Lombarte A, Rucabado J, Matallanas J, Lloris D (1991) Taxonomía numérica de Nototheniidae en base a la forma de los otolitos. Sci Mar 55:413–418
Malacalza VE, Bertellotti NM, Poretti TI (1997) Variación estacional de la dieta de Phalacrocorax magellanicus (Aves: Phalacrocoracidae) en Punta Loma (Chubut, Argentina). Neotrópica 43:35–38
Menni RC, Ringuelet RA, Arámburu RA (1984) Peces marinos de la Argentina y Uruguay. Editorial Hemisferio Sur
Millones A, Frere E, Gandini P (2005) Dieta del cormorán gris (Phalacrocorax gaimardi) en la Ría Deseado, Santa Cruz, Argentina. Ornitol Neotrop 16:519–527
Moore JW, Semmens BX (2008) Incorporating uncertainty and prior information into stable isotope mixing models. Ecol lett 11:470–480
Morgenthaler A, Millones A, Gandini P, Frere E (2016) Pelagic or benthic prey? Combining trophic analyses to infer the diet of a breeding South American seabird, the Red-legged Cormorant, Phalacrocorax gaimardi. Emu 116:360–369. https://doi.org/10.1071/MU15101
Nasca PB, Nasca PB, Gandini PA et al (2004) Caracterización de las asociaciones de alimentación multiespecíficas de aves marinas en la ría deseado, Santa Cruz, Argentina. Hornero 19:29–36
Oksanen J, Blanchet F, Kindt R, et al (2016) Vegan: community ecology package. R package 2.3-3
Parnell A (2016). Simmr: a stable isotope mixing model. R package version 0.3. R
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:1–5. https://doi.org/10.1371/journal.pone.0009672
Phillips DL, Inger R, Bearhop S et al (2014) Best practices for use of stable isotope mixing models in food-web studies. Can J Zool 92:823–835. https://doi.org/10.1139/cjz-2014-0127
Piacentino GL (1999) Osteología craneana de Odontesthes nigricans (Richardson 1845) y Odontesthes smitti (Lahille 1929) de la Ría de Puerto Deseado (Santa Cruz, Argentina) (Teleostei, Atherinopsidae). Boletim do Lab de Hidrobiol 12:23–47
Pineda S, Aubone A, Brunetti N (1996) Identificación y morfometría de las mandibulas de Loligo gahi y Loligo sanpaulensis (Cephalopoda, Loliginidae) del Atlántico Sudoccidental. Rev Investig y Desarro Pesq 10:85–99
Pineda SE, Brunetti NE, Scarlato N (1998) Calamares Loliginidos (Cephalopoda, Loliginidae) Tomo 2. In. ‘El mar argentino y sus recursos pesqueros.’ (Ed E.E. Boschi) pp. 13–36 (INIDEP, Mar del Plata, Argentina)
Post D, Layman C, Arrington D, Takimoto G, Quattrochi J, Montaña C (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189
Punta G, Yorio P, Herrera G (2003) Temporal patterns in the diet and food partitioning in imperial cormorants (Phalacrocorax atriceps) and rock shags (P. magellanicus) breeding at Bahia Bustamante, Argentina. Wilson Bull 115:307–315. https://doi.org/10.1676/02-119
Quintana F, Morelli F, Benedetti Y (2002) Buceo eficiente en aguas poco profundas: comportamiento de buceo y patrón de alimentación del Cormorán Cuello Negro, Phalacrocorax magellanicus, en dos colonias de la costa patagónica. Ecol Austral 12:19–28
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Sapoznikow A, Quintana F (2005) Patrón de presencia en la colonia del cormorán cuello negro (Phalacrocorax magellanicus): una evidencia del uso de fuentes de alimento predecibles y de baja estacionalidad. Ornitol Neotro 17:95–103
Sapoznikow A, Quintana F (2009) Asincronía de puesta y reposición de la nidada en el cormorán cuello negro (Phalacrocorax magellanicus): ¿Evidencias de las características de su fuente de alimento? Hornero 24:21–30
Smith JA, Mazumder D, Suthers IM, Taylor MD (2013) To fit or not to fit: evaluating stable isotope mixing models using simulated mixing polygons. Methods Ecol Evol 4:612–618. https://doi.org/10.1111/2041-210X.12048
Suryan RM, Irons DB, Brown ED et al (2006) Site-specific effects on productivity of an upper trophic-level marine predator: Bottom-up, top-down, and mismatch effects on reproduction in a colonial seabird. Prog Oceanogr 68:303–328. https://doi.org/10.1016/j.pocean.2006.02.006
Tombari AD, Gosztonyi A, Echeverría DD, Volpedo AV (2010) Morfología de los otolitos y las vértebras de especies de aterínidos marinos (Atheriniformes, Atherinopsidae) que coexisten en el Océano Atlántico sudoccidental. Cienc Mar 36:213–223
Tramer EJ (1969) Bird species diversity: components of shannon’s formula. Ecology 50:927–929. https://doi.org/10.2307/1933715
Turner TF, Collyer ML, Krabbenhoft TJ (2010) A general hypothesis-testing framework for stable isotope ratios in ecological studies. Ecology 91:2227–2233. https://doi.org/10.1890/09-1454.1
Vanella FA, Fernández DA, Carolina Romero M, Calvo J (2007) Changes in the fish fauna associated with a sub-Antarctic Macrocystis pyrifera kelp forest in response to canopy removal. Polar Biol 30:449–457. https://doi.org/10.1007/s00300-006-0202-x
Volpedo A, Echeverría D (2000) Catálogo y claves de otolitos para la identificación de peces del Mar Argentino. Editorial Dunken
Votier SC, Bearhop S, Witt MJ et al (2010) Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems. J Appl Ecol 47:487–497. https://doi.org/10.1111/j.1365-2664.2010.01790.x
Weimerskirch H (2001) Seabird demography and its relationship with the marine environment. In: Biology of marine birds. CRC press, pp 128–149
Yorio P, Frere E, Gandini P, Harris G (1998) Atlas de la distribución reproductiva de aves marinas en el litoral Patagónico Argentino. Fundación Patagonia Natural, Puerto Madryn
Acknowledgements
This research was funded by the Universidad Nacional de la Patagonia Austral (UNPA) and the Wildlife Conservation Society. We want to thank Fundación Temaikén for providing field and laboratory assistants. Special thanks to Lucas Garbin, Camila MacLaughlin and Evangelina Laztra for their help in the field and in the laboratory. We would also like to thanks the following researchers from CENPAT-CONICET: Nestor García, Atila Gosztonyi and Cyntia Ibarra, for their help in the identification of fish otoliths and bones. All samples were collected under permission of the competent authority from the Santa Cruz Province. We are also grateful to Carolina Mirallas from the Scientific English Laboratory of the UNPA for reviewing the English text. Finally, we would like to thank Dra. Andrea Raya Rey, and an anonymous referee, for reviewing an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and handling of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morgenthaler, A., Millones, A., Gandini, P. et al. The diet of adult and chick rock shags (Phalacrocorax magellanicus) inferred from combined pellet and stable isotope analyses. Polar Biol 43, 511–521 (2020). https://doi.org/10.1007/s00300-020-02653-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02653-y