Abstract
Knowledge of seabirds’ diet at each breeding site and its temporal variation is key to understanding and evaluating how changes in marine resources affect each seabird population. In this study, we determined the diet of Magellanic penguins (MP, Spheniscus magellanicus) at Martillo Island, accounting for sex, breeding stage and year. We analyzed a total of 144 stomach contents during three consecutive breeding seasons (2006–2007, 2007–2008 and 2008–2009) and stages (incubation, early and late chick-rearing). MP fed mainly on fuegian sprat (Sprattus fuegensis), which represented 75 % of the biomass consumed by birds during the entire study. The next important prey was squat lobster (Munida gregaria), followed by Patagonian squid (Loligo gahi). Both sexes consumed similar prey items. We observed variation in diet relative composition among breeding years and stages. Fuegian sprat consumption decreased throughout the years whereas squat lobster increased. Penguins consumed a higher proportion of squat lobster and Patagonian squid during the incubation stage than in the chick-rearing stages, whereas fuegian sprat was almost the only prey item consumed during the late chick-rearing stage. MPs show certain flexibility in the use of resources probably as a response to changes in prey populations. Variability in the diet among different reproductive stages could be related to changes in the distribution and abundance of their main prey near the colony during the breeding season together with changes in the energy requirements of seabirds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term diet studies of seabirds have been used as indicators of changes in the marine ecosystem, considering that variability in diet composition reflects changes in prey availability (Montevecchi 1993; Montevecchi and Myers 1995, 1996). These changes in prey distribution and availability could be influenced by several factors (e.g., climate change and fisheries) and, in turn, affect seabird populations (Croxall et al. 2002; Boersma 2008).
Seabird responses to changes in food sources or energetic demands related to their parental duties vary depending on the species. Seabirds that have greater flexibility in their diet show a greater ability to exploit alternative prey when main prey availability fluctuates (Montevecchi and Myers 1995, 1996; Thayer and Sydeman 2007). Therefore, variation in the amount and quality (type) of prey may be found in seabirds’ diet throughout the breeding season and/or among seasons (Thompson 1993; Culik 1994; Pütz et al. 2001; Litzow et al. 2002). Additionally, a species can present sexual segregation, where each sex feeds on different prey or at different locations, thus reducing intraspecific competition (González-Solís et al. 2000; Forero et al. 2002, 2005; Raya Rey et al. 2012).
Magellanic penguins (Spheniscus magellanicus) are found in both the Pacific and Atlantic Oceans and nest along the coasts of Argentina and Chile. Breeding colonies are found along the southern coast of South America: from Pájaro Niño Islet (33°21′S, 71°41′W) on the Pacific Ocean to Redondo islet (41°26′S, 65°01′W), including the Malvinas (Falkland) Islands in the Atlantic Ocean (Williams 1995; Gandini et al. 1996; Schiavini et al. 2005). Magellanic penguins have a seasonal breeding, where both sexes defend the nest site, incubate eggs and feed chicks (Boersma et al. 1990). They lay two eggs in October, which hatch approximately 40 days after, between mid-November and early December. Chicks fledge from late January to early February (Boersma et al. 1990; Williams 1995). Magellanic penguins show modest sexual dimorphism in body size (Scolaro et al. 1983; Gandini et al. 1992), males being 5–15 % larger than females (Agnew and Kerry 1995).
Diet of Magellanic penguin has been studied in different colonies along the coast of Argentina (Scolaro and Badano 1986; Frere et al. 1996; Wilson et al. 2005) and Chile (Wilson et al. 1995; Venegas 1998; Radl and Culik 1999) as well as in several colonies of the Malvinas (Falkland) Islands (Thompson 1993; Pütz et al. 2001; Clausen and Pütz 2002). Along the coast of South America Magellanic penguins feed mainly on pelagic school fish such as anchovy (Engraulis spp), sprat (genus Sardinops and Sprattus) and silverside and, to a lesser extent, on squid (genus Loligo, Illex, Todarodes). However, in colonies of the Malvinas (Falkland) Islands squid (mainly the genus Gonatus, Loligo) and crustaceans (represented mainly by squat lobster, Munida gregaria) take more relevance in the diet. In addition to the spatial variation in diet composition of Magellanic penguins, in some colonies temporal variation has also been registered (e.g., among and within breeding seasons; Thompson 1993; Pütz et al. 2001; Clausen and Pütz 2002).
Tierra del Fuego colonies represent the southernmost breeding range of the species breeding at a strong seasonal environment, and to date there is no information on their diet. To evaluate how changes in marine resources affect different populations of Magellanic penguins, it is necessary to know their diet in each breeding site, taking into account temporal variations. Thus, the aims of this study are: (1) to determine the major components and characteristics of the diet of Magellanic penguins at Martillo Island, Beagle Channel, (2) to evaluate the variation among years and breeding stages within a breeding season and (3) to analyze the sexual variation in the diet. Also, we try to broaden the knowledge on biodiversity of the Beagle Channel based on Magellanic penguins’ prey items. Thus, this work is a first attempt to quantify the species diet in the southern limit of its geographical distribution and complements previous studies at other places of the species range distribution.
Materials and methods
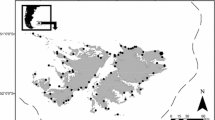
Our study was conducted at the Magellanic penguin breeding colony on Martillo Island, Beagle Channel, Tierra del Fuego, Argentina (54°53′S, 67°34′W, Fig. 1). The colony holds 3,000 breeding pairs (Raya Rey et al. 2014).
Location of the study site, Magallanic penguin colony at Martillo Island, Beagle Channel, southern tip of South America. Also, locations of other Magellanic penguin colonies at which diet studies have been previously contucted: 1 Pingüino Island, Algarrobo; 2 Pinhuil Island, west from Chiloé Island; 3 San Lorenzo; 4 Punta Clara; 5 Punta Loberia; 6 Dos Bahías Cape; 7 Chaffers Island; 8 Pingüino Island, Santa Cruz (Argentine); 9 Laura Bay; 10 San Julián; 11 Monte León; 12 Westponit Island/Malvinas (Falkland) Island; 13 New Island/Malvinas (Falkland) Island; 14 Volunteer Point/Malvinas (Falkland) Island; 15 Cabo Vírgenes; 16 Punta Dungeness; 17 Seno Otway; 18 Magdalena Island (Venegas and Sielfeld 1981; Scolaro and Badano 1986; Thompson 1993; Wilson et al. 1995; Frere et al. 1996; Radl and Culik 1999; Scolaro et al. 1999; Wilson et al. 2005). For more details on colonies from Malvinas (Falkland) Island, see Pütz et al. 2001 and Clausen and Pütz 2002
We studied the diet of male and female Magellanic penguins during incubation (late October, early November), early (chicks 1–30 days old, late November–December) and late chick-rearing (chicks >30 days old, early January) in the 2006–2007, 2007–2008 and 2008–2009 breeding seasons (hereafter referred to as the 2006, 2007 and 2008 years, respectively).
Sample collection and sorting
We captured reproductive adult birds as they returned to the colony on their way to the nests, after foraging at sea, using a hoop-shaped net with a long handle. We weighed (to the nearest 100 g) and sexed penguins by measuring bill depth and length (Gandini et al. 1992) using a caliper (to the nearest 0.02 mm). Afterwards, stomach contents were collected using the water-offloading technique (Wilson 1984). We flushed birds with sea water until we obtained clear water. After sampling, we marked each bird on the breast feathers with picric acid to ensure that no bird was sampled more than once. A total of 121 females and 105 males were sampled over the 3 years (see Table 1 for details). We drained diet samples and preserved them with 70 % ethanol.
We filtered each sample to remove excess liquid and weighted it to obtain stomach load mass (wet weight). Samples less than 25 g (32 % of the captured birds) were not used for analysis. We separated large and entire prey items from the whole sample, then took a subsample at random and extrapolated the results to determine the overall composition. We subsampled only samples heavier than 25 g by taking one half for those between 25 and 50 g, one quarter for samples between 50 and 100 g, and one-eighth for those over 100 g. We then sorted samples into the main components and identified prey items to the lowest taxonomic level possible. We identified fish from whole specimens, otoliths and cranial bones using our reference collection, and following Gosztonyi and Kuba (1996) and Collm (2002). We counted all cranial bones and otoliths for each fish species; for paired bones (e.g., maxilla) the total number was divided by two. The number of individuals per species per sample was then estimated from the most represented bone or otoliths. We measured cranial bones using a magnifier fitted with an eyepiece graticule. We estimated the size and biomass of fuegian sprat, Sprattus fuegensis, (main fish species, see Results) using functional linear regressions for the different cranial bones measured and the total length (TL) (Online Resource 1, Collm 2002). For the rest of the fish species that could not be identified to species level and for which there is no functional relationship in the bibliography, we calculated mean size and biomass in relation to entire specimens found in the samples. Since bone sizes for these species were similar, we reconstituted biomass as: number of individuals in each sample × mean biomass.
We identified cephalopods and assessed their number from lower beaks. We estimated the original biomass and size of ingested cephalopods using functional regressions for the lower rostral length (LRL) of the beak (Online Resource 1, Clarke 1986; Pineda et al. 1996; Jackson et al. 1997). Whenever regressions for any of the species found were not available, we used related species (e.g., Octopus vulgaris for Enteroctopus megalocyathus and Rossia macrosoma for Semirossia sp., Clarke 1986). These estimates, as well as those for small fish, were made to account at least partially for the real contribution of each prey species/taxon to the diet.
We counted entire crustacean bodies and single broken carapaces that conserved the rostrum to estimate the number of individuals consumed. We estimated the original biomass by using functional regressions for carapace length (Online Resource 1, Tapella and Lovrich 2006).
We described penguins’ diet in terms of frequency of occurrence (FO%) and percentage by mass (%M). Percentage by mass is the relative biomass and was calculated as the sum of reconstituted biomass of each prey item divided by the total reconstituted biomass of all prey items by sex and/or breeding stage and/or year. We did not include rare and unidentified species in this calculation. We calculated the average reconstituted biomass of the main prey items as the sum of the reconstituted biomass of each item consumed by a penguin divided by the number of individuals that consumed each prey item.
Data analysis
To address the question on how diet varied by year and by breeding stage, we analyzed stomach load mass, Shannon-Weaver's diversity index, average reconstituted biomass and size of the main prey items consumed (total length for fish, carapace length for crustaceans and lower rostral length for cephalopods) by Magellanic penguins. We tested stomach load mass and the average reconstituted biomass as functions of year and breeding stage using two-way ANOVA and Tukey’s multiple comparisons. Shannon-Weaver's diversity index and sizes of the main prey items consumed were tested using Kruskall-Wallis separately for year and breeding stage. Tests to evaluate stomach load mass and size of the main prey items were repeated separately for male and female Magellanic penguins. We log10 transformed average reconstituted biomass and stomach load mass data to accomplish ANOVA assumptions of homogeneity of variances and normality. Late chick-rearing was excluded from the statistical analyses of crustacean size because of low sample size (<5).
To examine differences in diet between sexes we tested stomach load mass, Shannon-Weaver's diversity index and size of the main prey items consumed by male and female Magellanic penguins for each breeding stage and year. We used a t test or Mann-Whitney (W) test in case of lack of normality and pairwise multiple comparisons as post hoc-test. We assessed the effect of sex on average reconstituted biomass of main prey items consumed by penguins within each year with one-way ANOVA.
We performed all statistical analyses using Infostat software (Di Rienzo et al. 2009). We presented average values with their standard deviations (SD), considering P values <0.05 significant.
Results
Overall composition
We analyzed a total of 144 stomach contents heavier than 25 g. Over the 3 years we identified a total of 8,884 prey items from 13 taxa (species or species group), including one crustacean (Munida gregaria), five species of cephalopods (Enteroctopus megalocyathus, Loligo gahi, Moroteuthis ingens, Gonatus antarticus and Semirrosia sp,) and seven fish taxa (Sprattus fueguensis, Champsocephalus esox, Macruronus magellanicus, Cottoperca gobio, Agonopsis chiloensis and Notothenioidei, Paralichthyidae). Fish were the most represented taxa in all years. However, all main components varied in their percentage by mass between breeding stages and years (Fig. 2).
Variation among years and breeding stages
Stomach load mass
Stomach load mass is detailed in Table 1. We found no differences among years, stages or their interactions for females (2-way ANOVA, year: F 2, 65 = 1.97, P = 0.15, stage: F 2,65 = 2.85, P = 0.07; year * stage: F 3,65 = 1.21, P = 0.31) or males (2-way ANOVA year: F 2,75 = 0.97, P = 0.38; stage: F 2,75 = 0.19, P = 0.82; year * stage: F 4,75 = 1.77, P = 0.15).
Among-year variation in diversity and relative composition of the diet
Mean Shannon-Weaver's diversity indices differed among years (2006: 0.21, SD = 0.3; 2007: 0.44, SD = 0.42 and 2008: 0.45, SD = 0.55; Kruskal–Wallis H 2 = 7.09. P = 0.0205). Diversity in 2006 was lower than in 2007 and 2008 (multiple comparisons P = 0.01 and P = 0.05, respectively). The higher index values found in 2007 and 2008 seem to be related to the increase in the number of prey items and the decrease in the numerical dominance of fuegian sprat.
Fish occurred in 97, 98 and 93 % of the samples during 2006, 2007 and 2008, respectively. Cephalopods were next in occurrence (FO%: 2006: 45 %, 2007: 54 % and 2008: 46 %), while crustaceans occurred in 24, 32 and 39 % of the samples during 2006, 2007 and 2008, respectively. Fuegian sprat (Sprattus fuegensis) was the most frequent fish species in all years (FO%: 2006: 97 %, 2007: 98 % and 2008: 85 %) and the most important in terms of percentage by mass (%M: 2006: 86 %; 2007: 77 %; 2008: 51 %). The other fish species were rare during the first year (2 %). However, during the last 2 years the occurrence of Notothenioidei fish, Agonopsis chiloensis and icefish (Champsocephalus esox), increased in frequency of occurrence (2007: 10–37 % and 2008: 15–27 %), but their contribution in terms of biomass did not exceed 1.5 % in any 2 years, even when pooling all these items (Fig. 2).
Cephalopod occurrence was similar between consecutive breeding years, with the Patagonian squid (Loligo gahi) being the most represented (FO%: 2006: 37 %; 2007: 39 % and 2008: 44 %) and having the highest percentage by mass (2006: 10 %; 2007: 4 %; 2008:19 %). The octopus Enteroctopus megalocyathus was the second most represented (10 % in 2006 and 12 % in 2007 and 2008), and in terms of percentage by mass only in 2008 did it reach 1.5 %. All together, the other squid species were represented in less than 12 % of the samples in the 3 years, but their contribution in terms of percentage by mass was lower than 5 % (2006: <0.1 %, 2007: 1.2 %, 2008: 4 %). The squat lobster Munida gregaria was the only crustacean in the diet of the Magellanic penguins, and its importance increased in the consecutive years (%M: 2006: 4 %, 2007: 17 %, 2008: 23 %; Fig. 1).
Among stages variation in diversity and relative composition of diet
Throughout 2006, mean diversity indices differed between breeding stages (Kruskal-Wallis H 2 = 13.27, P = 0.0005). During the incubation stage diversity (0.41) was higher than in the early and late chick-rearing stages (0.16 and 0.07, multiple comparisons P = 0.0031 and P = 0.00076, respectively). In 2007 and 2008 there were no differences in mean diversity indices among breeding stages (2007: incubation: 0.42, early chick-rearing: 0.46 and late chick-rearing: 0.39, Kruskal-Wallis H 2 = 1.1, P = 0.57, 2008: incubation: 0.48, early chick-rearing: 0.56 and late chick-rearing: 0.28, Kruskal-Wallis H 2 = 1.1, P = 0.27). However, the values of these indices were consistently high and similar to those recorded during the incubation stage of 2006, suggesting a greater diversity at all breeding stages in the last 2 years.
Fuegian sprat was the main prey item in terms of percentage by mass during all breeding stages and years, except during incubation and early chick-rearing of 2008 (Fig. 2). It was almost the only prey consumed during the late chick-rearing stage for the 3 years (Fig. 2). The importance of squat lobster was higher during incubation of the last 2 years than in 2006 and decreased during chick-rearing stages in all years, mainly during the late chick-rearing stage when it was almost absent (Fig. 2). Cephalopods were most important during the incubation stage of all years, except in 2008 when the highest percentage by mass was recorded during the early chick-rearing stage. Penguins consumed the lowest amount of cephalopods during the late chick-rearing stages of all years (Fig. 2).
Average reconstituted biomass variation of the main prey items
The average reconstituted biomass of fuegian sprat consumed by Magellanic penguins differed among years, but not among stages (2-way ANOVA F 2,131 = 3.6, P = 0.03; F 2,131 = 0.2, P = 0.82, respectively; Fig. 3). In 2006 the contribution of fuegian sprat to the diet was higher than in subsequent years, mainly with respect to 2008 (Tukey’s test, P < 0.05).
Average reconstituted biomass of the three main prey items (S. fuegensis, M. gregaria and L. gahi) of Magellanic penguin from Martillo Island, during each breeding stage and year. The boxes show the 0.25 and 0.75 percentiles, vertical bars represent 1 SD, black squares indicate the mean, the horizontal line the median and dots outside the boxes correspond to values outside of the percentiles. Numbers above bars or boxes indicate the number of stomachs in which this prey item was present
The average reconstituted biomass of squat lobster consumed by penguins was only evaluated for the incubation and early chick-rearing stages because of the null or low presence of this prey item in the samples from the late chick-rearing stages (see Fig. 3). We found differences in average reconstituted biomass of squat lobster among years but not between breeding stages (2-way ANOVA F 2,35 = 9.4, P = 0.0005; F 1,35 = 3.7, P = 0.06, respectively; Fig. 3). Contrary to what was observed for the fuegian sprat, a larger biomass of squat lobster was consumed in 2007 and 2008 than in 2006 (Tukey’s test, P < 0.05 both comparisons).
We found no differences in average biomass of Patagonian squid among different breeding stages or years (2-way ANOVA F 2,54 = 0.89, P = 0.42; F 2,54 = 2.0, P = 0.14, respectively, Fig. 3).
Size of the main prey consumed by Magellanic penguin
The size of fuegian sprat consumed by Magellanic penguins differed among years (Kruskal-Wallis H 2 = 233.8, P < 0.0001). In 2006 average size was smaller (mean = 92 mm, SD = 17 mm; Fig. 4a), whereas in 2007 we registered the largest sizes of sprat (mean = 110 mm, SD = 14 mm; 2008: mean = 100 mm, SD = 25 mm; P < 0.0001 all comparisons). Sprat size differed among breeding stages within each year for each sex, except for males in 2007 (Table 2).
Length frequency distribution of a fuegian sprat (Sprattus fuegensis) (total length, TL), b squat lobster (Munida gregaria) (carapace length, CL) and c Patagonian squid (Loligo gahi) (lower rostral length, LRL: mantle length, ML) taken by Magellanic penguin in each year (2006, 2007 and 2008). The arrows indicate the mature size (L50 %). Number (n) indicate sample size
Magellanic penguins consumed different squat lobster sizes (length) during the different years (Kruskal-Wallis H 2 = 85.78, P < 0.0001), with average size being smaller in 2006 (mean = 17.1 mm, SD = 2.9 mm) than in the last 2 years (2007: mean = 20.3 mm, SD = 2.1 mm; 2008: mean = 19.9, SD = 3.3 mm; P = 0.005 both comparisons; Fig. 4b). During the incubation stage, squat lobster size was larger than in the early chick-rearing in 2006 and 2008 (Mann-Whitney 2006: W = 1,666, P = 0.0103; 2008: W = 26,587.5, P = 0.007; 2007: W = 894.5, P = 0.28).
The sizes of Patagonian squid consumed by penguins were similar among years (2006: LRL = 0.77, SD = 0.4 mm; 2007: 0.81, SD = 0.4 mm and 2008: 0.95, SD = 0.7 mm; Kruskal-Wallis H 2 = 1.01, P = 0.6; Fig. 4c), consuming individuals with a mantle length (ML) of 52.4 mm (SD = 45.2). In Fig. 4c we show the size distribution of Patagonian squid for each year. The reconstituted mean mass of Enteroctopus megalocyathus, the second most important item among cephalopods in terms of numbers, was 1.05 g (SD = 0.7), and there were no differences in the size consumed by penguins during the 3 years (H 2 = 5.1, P = 0.08).
Sexual variation of diet
Stomach load mass
Stomach load mass of males and females varied according to breeding stage and year (Table 1). Male’s stomach load mass was greater than that of females during the incubation stage of 2006 and in the late chick-rearing stage of 2008. However, during the late chick-rearing stage of 2006 females brought back heavier meals than males (Table 1).
Diversity and relative composition of diet
Mean Shannon-Weaver's index did not differ between sexes (Mann-Whitney, all tests P > 0.05), probably because both sexes consumed a similar proportion of the different prey items (Fig. 2) during each breeding stage and year.
Average reconstituted biomass variation of the main prey items
The average reconstituted biomass of fuegian sprat consumed by Magellanic penguins did not differ between males and females in each year (ANOVA: 2006: F 1,59 = 0.1, P = 0.76; 2007: F 1,36 = 0.6, P = 0.44; 2008: F 1,34 = 2.8, P = 0.11). Neither were there differences in the biomass of squat lobster (ANOVA: 2006: F 1,14 = 0.8, P = 0.38; 2007: F 1,10 = 0.2, P = 0.69; 2008: F 1,12 = 9.8 × 10−5, P = 0.99) nor in the average biomass of Patagonian squid consumed by males and females in each year (ANOVA: 2006: F 1,21 = 0.3, P = 0.61; 2007: F 1,14 = 0.4, P = 0.56; 2008: F 1,17 = 0.04, P = 0.84).
Size of the main prey consumed by Magellanic penguin
Males and females consumed different sprat sizes depending on the breeding stage and year (Table 2). Males consumed larger sprats than females during the incubation stage of 2006 and in the early chick-rearing stage of 2007, whereas females consumed larger sprats than males during the late chick-rearing stage of 2006 and in incubation stage of 2008 (Table 2). The remaining fish were juveniles and contributed little to the diet of Magellanic penguins.
Males and females did not consume different sizes of squat lobster (Mann-Whitney W = 60,262.50, P = 0.58).
Discussion
This study is the first to describe the diet composition of Magellanic penguins breeding at the southern limit of their geographical distribution. Magellanic penguins at Martillo Island fed mainly on fuegian sprat, which represents 75 % of the biomass consumed by birds during the studied period. They also fed on other prey items, whose biomass varies in importance from year to year as well as among stages within each breeding season. This result is consistent with the described latitudinal variation in the diet composition of the species (Wilson et al. 1995; Frere et al. 1996; Scolaro et al. 1999; Wilson et al. 2005).
Fuegian sprat is the dominant prey in the southern colonies of Argentine Continental Patagonia (above 50°S latitude, Fig. 1, Frere et al. 1996; Wilson et al. 2005) and is almost the only prey consumed in two colonies of the Magellan Strait (51°–52°S latitude, Fig. 1; Wilson et al. 1995; Radl and Culik 1999). Additionally, penguins of Martillo Island fed on squat lobster (12 %M pooling all years). This is consistent with the diet reported at the Malvinas (Falkland) Islands, where squat lobster represents 20 % of the overall diet composition (Pütz et al. 2001; Clausen and Pütz 2002). However, the squat lobster was not recorded for penguins breeding in Chile and Continental Patagonia (Wilson et al. 1995; Frere et al. 1996; Radl and Culik 1999; Scolaro et al. 1999; Wilson et al. 2005), except for one isolated record at Magdalena Island, Magellan Strait (Venegas and Sielfeld 1981). In the northern colonies along the Argentine coast (above 45°S latitude, see details Fig. 1), Magellanic penguins feed mainly on anchovy, Engraulis anchovita (Scolaro and Badano 1986; Frere et al. 1996; Scolaro et al. 1999; Wilson et al. 2005), and at two colonies (Clara Point, 43°58'S, and Dos Bahías Cape 44°54'S, Fig. 1) they have been reported to feed on a lower rate on hake, Merluccius hubbsi (Scolaro and Badano 1986; Frere et al. 1996). In northern Chile (Algarrobo, 33° 39'S, Fig. 1), Magellanic penguins feed almost exclusively on the anchovy Engraulis ringens (Wilson et al. 1995).
Unlike southern and northern colonies, where the diet is almost exclusively monospecific, in colonies located at the center of the penguin's distributional range (between 44° and 50°S latitude, Fig. 1) the diet is more diverse, with squid (mainly Loligo sp.) and other fish being more important, with silversides (Austroatherina sp. and Odontesthes smitti) and Syngnathus sp. being the most representative taxa. Fuegian sprat was also taken to a lesser extent at some central colonies (Frere et al. 1996; Scolaro et al. 1999; Wilson et al. 2005). Penguins of central Chile (Pinghuil Island., Chiloé 41°43′S, Fig. 1) feed primarily on E. ringens though they also consume significant amounts of squid Todarodes fillippovae (Wilson et al. 1995). Squids are also important in the diet of Magellanic Penguins at the Malvinas (Falkland) Islands, Gonatus antarcticus and Loligo gahi being the dominant species (Thompson 1993; Pütz et al. 2001; Clausen and Pütz 2002). At Martillo Island L. gahi was the third most important prey item in the diet, whereas the other cephalopods such as juvenile Enteroctopus megalocyathus and Gonatus sp. were less important. At the Malvinas (Falkland) Islands, a larger variety of fish species were taken, including several nototheniid species, as well as Merluccius sp. and Micromesistius australis. S.fuegensis was only present in the diet of western and southern colonies (Thompson 1993; Pütz et al. 2001; Clausen and Pütz 2002). At Martillo Island, juvenile nototheniid and other fish were present but their contribution was insignificant.
Variation among years and breeding stages
In addition to the variability in diet composition of Magellanic penguins among breeding sites, changes in prey items consumed among different breeding seasons and stages have also been registered (Scolaro and Badano 1986; Thompson 1993; Pütz et al. 2001; Clausen and Pütz 2002). In colonies at the Malvinas (Falkland) Islands, a considerable interannual variation in the diet was observed, alternating among fish, cephalopods and crustaceans. During certain years some taxa were not present, while in other years they acquired greater importance in the diet (Thompson 1993; Pütz et al. 2001; Clausen and Pütz 2002). Moreover, at Magdalena and Otway colonies, Magellan Strait, prey composition has been constant in all studies to date (Venegas and Sielfeld 1981; Scolaro and Badano 1986; Wilson et al. 1995; Radl and Culik 1999). In this study, regardless of fuegian sprat being the overall dominant prey item, diet composition varied among years and within each breeding season. The biomass of fuegian sprat consumed by penguins decreased throughout the studied years, and at the same time squat lobster consumption increased. In turn, the Patagonian squid contribution was low and relatively constant. Furthermore, the large diet diversity of the last 2 years reflects an increasing consumption of alternative prey, possibly linked to a decrease in the availability of fuegian sprat.
Variations in the energetic requirements of seabirds throughout the breeding season may be reflected in changes in diet composition. During the chick-rearing stage, seabirds have larger food demands and consume higher quality species (Culik 1994; Litzow et al. 2002) or choose more digestible prey given their parental duties (Thompson 1993). Thus, the amount and quality of prey consumed by seabirds may have important implications on their fitness (Pierotti and Annett 1990; Thayer and Sydeman 2007; Nicol et al. 2008; Tierney et al. 2009). In our study, as the chick-rearing period progressed, diet diversity decreased, and, although average sprat biomass ingested per bird that consumed this prey item did not increase, all penguins almost exclusively ate fuegian sprat. Instead, during the incubation stage penguins fed on a greater proportion of lobster squid and Patagonian squid.
Considering the energetic values of the three most important prey items consumed by Magellanic penguins, Patagonian squid has the lowest energy value (4.95 kJ/g wet weight (ww); Ciancio et al. 2007) compared 7.15 kJ/g (ww) in the fuegian sprat (Ciancio et al. 2007) and 7.5 kJ/g in the squat lobster (Romero unpublished data, recalculated from 18.86 kJ/g dry weight; Romero et al. 2006). Although squat lobster has an energy value similar to that of fuegian sprat, the first may have lower digestibility, and it has been suggested that chicks grow less on a diet based on squat lobster (Thompson 1993). The shift in diet from both low energy and less digestible prey such as squids and squat lobsters (Wilson et al. 1985) to fish with higher lipid content such as sprat (Clarke and Prince 1980) could be adaptive (Thompson 1993). A similar shift in diet was observed in colonies of Magellanic penguins located in the Malvinas (Falkland) Islands, where squid decreased and were replaced by fish (Thompson 1993; Clausen 2001; Pütz et al. 2001; Clausen and Pütz 2002; Tierney et al. 2009). These changes in diet composition within a breeding season reported by us could also be related to population dynamics of the prey, which results in variations in prey availability near the colony. Unfortunately, the prey population dynamics in the Beagle Channel is unknown.
Sexual variation
Sex differences in diet could be attributed to sexual segregation mechanisms produced in response to a decline of the main prey (González-Solís et al. 2000; Forero et al. 2002). Only one study evaluated changes in diet composition between sexes of Magellanic penguins (Forero et al. 2002). In northern Patagonian colonies, isotopic values indicated that males consume a greater proportion of anchovy than females do (Forero et al. 2002). At Martillo Island, the average biomass of fuegian sprat, squat lobster and Patagonian squid consumed by both sexes was similar during each breeding season. Males and females brought ashore the same food loads during most of the study period, but there were differences between sexes depending on the breeding stage and year. On one hand, differences found during the incubation stage of 2006 could be due to the degree of digestion of food in stomach contents depending on the distance to the feeding sites. During incubation males return to the colony with larger food loads than females do, which may indicate that female feeding sites are further away from the colony; thus, they have longer trips. This is supported by the finding that females of Martillo Island make longer foraging trips than males during incubation of the same breeding seasons (Scioscia 2011), which is as was recorded in previous studies (Scioscia et al. 2010; Raya Rey et al. 2012). However, such differences in stomach load mass were not found in all years, and this could be related to the fact that the previously mentioned differences in the duration of foraging trips also were not found in all years. On the other hand, during the late chick-rearing stage of 2006 females brought larger food loads to the colony than males, opposite to what was observed during the same stage in 2008. The differences between sexes within each year and breeding stage were inconsistent or irregular, indicating a great variability, maybe related to changes in specific conditions during each breeding season and stage. In conclusion, the few variations found between sexes of Magellanic penguins of Martillo Island in stomach load mass do not show a clear pattern to suggest an evident sexual segregation of prey selection.
Characteristics of prey taxa
Breeding penguins are central place foragers (Orians and Pearson 1979), performing foraging trips to the area adjacent to the breeding colony (Raya Rey et al. 2010) as they must return in a short time to carry out their parental duties. As a consequence, this work not only adds novel information on the trophic niche of the Magellanic penguin, but it also contributes to the understanding of the food web in this zone, complementing previous information on presence and diversity of marine resources (López et al. 1996; Comoglio and Amin 1999; Raya Rey and Schiavini 2000, 2001; Romero et al. 2004; Vanella et al. 2007; Diez et al. 2009; Riccialdelli et al. 2010).
Fuegian sprat is the most abundant pelagic resource and potentially the most important in the region (Cousseau 1982). It is distributed along the Patagonian coast of Santa Cruz and Tierra del Fuego (43° 30′–55° S) and around the Malvinas (Falkland) Islands (Cousseau and Perrotta 1998). It reproduces during the austral spring-summer, probably in the coastal waters of Santa Cruz and Tierra del Fuego (Cousseau and Perrotta 1998). The mean size of fuegian sprat consumed by Magellanic penguins is smaller than its size at maturity (L50 % = 119.94 mm) (Madirolas et al. 2000). However, during the last two seasons penguins consumed a greater proportion of larger sized fuegian sprats. Consumption of large-size sprat temporally coincides with longer foraging trips, whereas in periods during which penguins take shorter trips the sizes of sprat are smaller (Scioscia 2011). Thus, considering that the largest sizes of sprat were recorded at the eastern tip of the Beagle Channel (Madirolas et al. 2000) and that there is a positive relationship between foraging trip duration and distance to feeding sites (Boersma et al. 2009; Sala et al. 2012), it could be that during the chick-rearing stages of 2007, penguins that chose feeding sites further away from the colony consumed larger sprat. One hypothesis for this behavior would be a lower availability of fuegian sprat closer to the colony during these stages. This would be supported by the lower amount of sprat in the penguins’ diet in the last 2 years during these stages and longer foraging trips during the chick-rearing stages of 2007 than in the other years (Scioscia 2011). Although the knowledge on system dynamics of the Beagle Channel is poor, especially for pelagic organisms such as fuegian sprat (Hansen 1999), we can hypothesize that differences observed in size and abundance of fuegian sprat in penguin’ diet can be explained by natural fluctuations in the population of this species related to their life cycle or to environmental changes, similar to what happens in other pelagic species (Bertrand et al. 2004; Gutierrez et al. 2007). Thus, it is possible that penguins may feed on the same sprat cohort, which moves to the east of the channel mouth as it grows in size through the years.
The next important prey in the diet of Magellanic penguin is Munida gregaria (= M. subrugosa, Pérez-Barros et al. 2008), which represents 50 % of the biomass of the benthic community of the Beagle Channel (Arntz et al. 1999). This species presents two morphs, “subrugosa” with benthic habits and “gregaria” with benthic and pelagic habits (Peréz-Barros 2001; Tapella and Lovrich 2006; Diez et al. 2012). Penguins consumed mature squat lobster (size at maturity for males and females: 8 and 9.9 mm CL, respectively; Tapella et al. 2002) during incubation and the early chick-rearing stages, a time when most squat lobsters are at post-moult stage (Tapella 2002), with a soft and therefore more digestible exoskeleton.
Patagonian squid spawn and die in shallow waters and have two spawning peaks in austral spring and autumn, the coasts along the Beagle Channel being one of the spawning areas (Brunnetti et al. 1999). The sizes of Patagonian squid consumed by penguins did not differ between breeding stages and were smaller than those consumed at the Malvinas (Falkland) Islands (incubation: ML = 10.1, SD = 4.8 cm, chick-rearing ML = 6.9, SD = 2.9 cm; Clausen 2001). Most of the Patagonian squids consumed at Martillo Island were immature individuals (maturity size: ML = 50–60 mm; Pineda et al. 1996). Enteroctopus megalocyathus were very small in size (ca. ML = 5 mm) indicating juvenile cephalopods, recently hatched (ML = 7–9.5, Ortiz et al. 2006), which share planktonic and benthic characteristics.
In summary, at Martillo Island Magellanic penguins’ diet is composed mainly of fuegian sprat, followed by squat lobster and Patagonian squid. Although fuegian sprat is the dominant prey, interannual variations in the relative composition of the diet, as well as among breeding stages, are evident. Magellanic penguins show certain flexibility in the use of resources probably as a response to changes in prey populations. In years where the fuegian sprat occurred in a low proportion in penguins' diet, squat lobster and Patagonian squid increased their contribution. Variability in the diet among different reproductive stages could be related to changes in the distribution and abundance of their main prey near the colony during the breeding season. These changes could take place together with changes in the energy requirements of seabirds.
References
Agnew D, Kerry K (1995) Sexual dimorphism in penguins. In: Dann P, Norman I, Reilly P (eds) The penguins: ecology and management. Surrey Beatty & Sons, Sydney, pp 299–318
Arntz W, Gili JM, Reise K (1999) Unjustifiably ignored: reflections on the role of benthos in marine ecosystems. In: Gray JS (ed) Biogeochemical cyucling and sediment ecology. Klawers Academic Publishers, Netherlands, pp 105–124
Bertrand A, Segura M, Gutiérrez M, Vásquez L (2004) From small-scale habitat loopholes to decadal cycles: a habitat-based hypothesis explaining fluctuation in pelagic fish populations off Peru. Fish Fish 5(4):296–316
Boersma DP (2008) Penguins as marine sentinels. Bioscience 58(7):597–607
Boersma PD, Stokes DL, Yorio PM (1990) Reproductive variability and historical change of Magellanic Penguins (Spheniscus magellanicus) at Punta Tombo, Argentina. In: Davis LS, Darby JT (eds) Penguin biology. Academic Press, San Diego, pp 15–43
Boersma D, Rebstock GA, Frere E, Moore SE (2009) Following the fish: penguins and productivity in the South Atlantic. Ecol Monogr 79(1):59–76
Brunnetti NE, Ivanovic ML, Sakai M (1999) Calamares de importancia comercial en la Argentina. Biología, distribución, pesquerías, muestreo biológico. Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP), Mar del Plata
Ciancio J, Pascual M, Beauchamp D (2007) Energy density of Patagonian aquatic organisms and empirical predictions based on water content. Trans Am Fish Soc 136:1415–1422
Clarke MR (1986) A handbook for the identification of cephalopods beak. Clarendon Press, Oxford
Clarke A, Prince PA (1980) Chemical composition and calorific value of food fed to Mollymauck chicks (Diomedea melanophris) and (D. chrysostoma) at Bird Island, South Georgia. Ibis 122:488–494
Clausen A (2001) Falkland island seabird monitoring programme. Annual Report 2000/2001. Falklands Conservation, Port Stanley
Clausen AP, Pütz K (2002) Recent trends in diet composition and productivity of Gentoo, Magellanic and Rockhopper Penguins in the Falkland Islands. Aquat Conserv 12:51–61
Collm NS (2002) Análisis morfométrico de los huesos del cráneo de la sardina fueguina Sprattus fuegensis. Degree thesis. Universidad de Buenos Aires, Argentina
Comoglio LI, Amin OA (1999) Feeding habits of the false southern king crab Paralomis granulosa (Lithodidae) in the Beagle Channel, Tierra del Fuego, Argentina. Sci Mar 63(1):361–366
Cousseau MB (1982) Revisión taxonómica y análisis de los caracteres morfométricos y merísticos de la sardina fueguina, Sprattus fuegensis (Jenyns, 1842) (Pisces, Clupeidae). Rev Invest Desarr Pesq INIDEP 3:77–94
Cousseau MB, Perrotta RG (1998) Peces marinos de Argentina. Biología, distribución, pesca 3rd edn. Instituto Nacional de Investigación y Desarrollo Pesquero, INIDEP, Mar del Plata
Croxall JP, Trathan PN, Murphy EJ (2002) Environmental change and Antarctic seabird populations. Science 297:1509–1514
Culik BM (1994) Energy requirements of pygoscelid penguins: a synopsis. Rep Polar Res 150:1–76
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2009) Infostat. FCA Universidad Nacional de Córdoba, Argentina
Diez MJ, Romero MC, Obenat S, Albano MJ, Tapella F (2009) Distribución of benthic invertebrates in the Beagle Channel, Argentina. An Inst Patagon 37(2):29–40
Diez MJ, Perez-Barros P, Romero MC, Scioscia G, Tapella F, Cabreira AG, Madirolas A, Raya Rey A, Lovrich G (2012) Pelagic swarms and beach strandings of the squat lobster Munida gregaria (Anomura: Munididae) in the Beagle Channel, Tierra del Fuego. Polar Biol 35:973–983
Forero MG, Hobson KA, Bortolotti GR, Donázar JA, Bertellotti M, Blanco G (2002) Food resource utilisation by the Magellanic penguin evaluated through stable-isotope analysis: segregation by sex and age and influence on offspring quality. Mar Ecol Prog Ser 234:289–299
Forero MG, González-Solis J, Hobson KA, Donázar JA, Bertellotti M, Blanco G, Bortolotti GR (2005) Stable isotopes reveal trophic segregation by sex and age in the southern giant petrel in two different food webs. Mar Ecol Prog Ser 296:107–113
Frere E, Gandini P, Lichtschein V (1996) Variación latitudinal en la dieta del Pingüino de Magallanes (Spheniscus magellanicus) en la costa patagónica, Argentina. Ornitol Neotrop 7:35–41
Gandini PA, Frere E, Helik TM (1992) Implicaciones de las diferencias en el tamaño corporal entre colonias para el uso de medidas morfométricas como método de sexados en Spheniscus magellanicus. Hornero 13:211–213
Gandini P, Frere E, Boersma PD (1996) Status and conservation of Magellanic penguins Spheniscus magellanicus in Patagonia, Argentina. Bird Conserv Int 6:307–316
González-Solís J, Croxall JP, Wood AG (2000) Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos 90:390–398
Gosztonyi AE, Kuba L (1996) Atlas de huesos craneales y de la cintura escapular de peces costeros patagónicos. Informes Técnicos del Plan de Manejo Integrado de la Zona Costera Patagónica 4:29 pp
Gutierrez M, Swartzman G, Bertrand A, Bertrand S (2007) Anchovy (Engraulis ringens) and sardine (Sardinops sagax) spatial dynamics and aggregation patterns in the Humboldt Current ecosystem, Peru, from 1983–2003. Fish Oceanogr 16(2):155–168
Hansen J (1999) Estimación de parámetros poblacionales del efectivo de sardina fueguina (Sprattus fuegensis) de la costa continental argentina. Inf Tec INIDEP 27:28
Jackson GD, Buxton NG, George MJA (1997) Beak lenght analysis of Moroteuthis ingens (Cephalopoda: Onychoteuthidae) from the Falkland Islands region of the Patagonia Shelf. J Mar Biol Assoc UK 77:1235–1238
Litzow M, Piatt J, Prichard A, Roby D (2002) Response of pigeon guillemots to variable abundance of high-lipid and low-lipid prey. Oecologia 132(2):286
López HL, García ML, San Román NA (1996) Lista comentada de la ictiofauna del Canal de Beagle, Tierra del Fuego, Argentina. CADIC, Contribución Científica, Publicación Especial 23:01–85
Madirolas A, Sánchez R, Hansen J, Álvarez Colombo G, Reta R (2000) Distribución, abundancia, biología y hábitat de la sardina fueguina (Sprattus fuegensis). Inf Tec INIDEP, p 46
Montevecchi WA (1993) Birds as indicators of change in marine prey stocks. In: Furness RW, Greenwood JJD (eds) Birds as monitors of environmental change. Chapman & Hall, Londres, pp 217–266
Montevecchi WA, Myers RA (1995) Prey harvests of seabirds reflect pelagic fish and squid abundance on multiple spatial and temporal scales. Mar Ecol Prog Ser 117(1):1–9
Montevecchi WA, Myers RA (1996) Dietary changes of seabirds indicate shifts in pelagic food webs. Sarsia 80(4):312–322
Nicol S, Clarke J, Romaine SJ, Kawaguchi S, Williams G, Hosie GW (2008) Krill (Euphausia superba) abundance and Adélie penguin (Pygoscelis adeliae) breeding performance in the waters off the Béchervaise Island colony, East Antarctica in 2 years with contrasting ecological conditions. Deep Sea Res Part II 55:540–557
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. Ohio State University Press, Columbus, pp 154–177
Ortiz N, Ré ME, Márquez F (2006) First description of eggs, hatchlings and hatchling behaviour of Enteroctopus megalocyathus (Cephalopoda: Octopodidae). J Plankton Res 28(10):881–890
Peréz-Barros P (2001) Crustáceos decápodos bentónicos asociados a la captura de Munida spp. (Decapoda: Anomura) en el Canal Beagle. Degree thesis, Universidad de Buenos Aires, Argentina
Pérez-Barros P, D’amato ME, Guzmán N, Lovrich GA (2008) Taxonomic status of two South American sympatric squat lobsters, Munida gregaria and Munida subrugosa (Crustacea: Decapoda: Galatheidae), challenged by DNA sequence information. Biol J Linn Soc 94:421–434
Pierotti R, Annett CA (1990) Diet and reproductive output in seabirds. Bioscience 40(8):568–574
Pineda SE, Aubone A, Brunetti NE (1996) Identificación y morfometría comparada de las mandíbulas de Loligo gahi y Loligo sanpaulensis (Cephalopoda, Loliginidae) del Atlántico Sudoccidental. Rev Invest Desarr Pesq INIDEP 10:85–99
Pütz K, Ingham RJ, Smith JG, Croxall JP (2001) Population trends, breeding success and diet composition of gentoo Pygoscelis papua, magellanic Spheniscus magellanicus and rockhopper Eudyptes chrysocome penguins in the Falkland Islands. Polar Biol 24:793–807
Radl A, Culik BM (1999) Foraging behaviour and reproductive success in Magellanic penguins (Spheniscus magellanicus): a comparative study of two colonies in southern Chile. Mar Biol 133:381–393
Raya Rey A, Schiavini A (2000) Distribution, abundance and associations of seabirds in the Beaggle Channel, Tierra del Fuego, Argentina. Polar Biol 23:338–345
Raya Rey A, Schiavini A (2001) Filling the groove: energy flow to seabirds in the Beagle Channel, Tierra del Fuego, Argentina. Ecol Austral 11(115):122
Raya Rey A, Bost CA, Schiavini A, Pütz K (2010) Foraging movements of Magellanic Penguins Spheniscus magellanicus in the Beagle Channel, Argentina, related to tide and tidal currents. J Ornithol 151:933–943
Raya Rey A, Pütz K, Scioscia G, Lüthi B, Schiavini A (2012) Sexual differences in the foraging behaviour of Magellanic Penguins related to stage of breeding. Emu 112(2):90–96
Raya Rey A, Rosciano N, Liljeström M, Saenz Samaniego R, Schiavini A (2014) Species-specific population trends detected for penguins, gulls and cormorants over 20 year in sub-Antartic Fuegian Archipelago. Polar Biol. doi:10.1007/s00300-014-1526-6
Riccialdelli L, Newsome SD, Fogel ML, Goodall RNP (2010) Isotopic assessment of prey and habitat preferences of a cetacean community in the southwestern South Atlantic Ocean. Mar Ecol Prog Ser 418:235–248
Romero MC, Lovrich GA, Tapella F, Thatje S (2004) Feeding ecology of the crab Munida subrugosa (Decapoda: Anomura: Galatheidae) in the Beagle Channel, Argentina. J Mar Biol Ass UK 84:359–365
Romero MC, Lovrich GA, Tapella F (2006) Seasonal changes in dry mass and energetic content of Munida subrugosa (Crustacea: Decapoda) in the Beagle Channel, Argentina. J Shellfish Res 25(1):101–106
Sala J, Wilson R, Frere E, Quintana F (2012) Foraging effort in Magellanic penguins in coastal Patagonia, Argentina. Mar Ecol Prog Ser 464:273–287
Schiavini A, Yorio P, Gandini P, Raya Rey A, Boersma PD (2005) Los pingüinos de las costas argentinas: estado poblacional y conservación. Hornero 20:5–23
Scioscia G (2011) Ecología trófica del pingüino de Magallanes (Spheniscus magellanicus) y sus implicancias en la ecología reproductiva en el Canal Beagle, Tierra del Fuego. Doctoral thesis, Universidad Nacional de Mar del Plata, Mar del Plata, Buenos Aires, Argentina
Scioscia G, Raya Rey A, Favero M, Schiavini A (2010) Factores que afectan el éxito reproductivo y la calidad de la nidada en el Pingüino de Magallanes (Spheniscus magellanicus) en el Canal Beagle, Tierra del Fuego. Argentina. Hornero 25(1):17–25
Scolaro JA, Badano LA (1986) Diet of the Magellanic penguin Spheniscus magellanicus during the chick-rearing period at Punta Clara, Argentina. Cormorant 13:91–97
Scolaro JA, Hall MA, Ximenez IM (1983) The Magellanic Penguin (Spheniscus magellanicus): Sexing adults by discriminant analysis of morphometric characters. Auk 100:221–224
Scolaro JA, Wilson RP, Laurenti S, Kierspel M, Gallelli H, Upton JA (1999) Feeding preferences of the Magellanic penguin over its breeding range in Argentina. Waterbirds 22(1):104–110
Tapella F (2002) Reproducción, crecimiento, distribución y abundancia de la langostilla Munida subrugosa (Anomura: Galatheidae) del canal Beagle, Tierra del Fuego, Argentina. Doctoral thesis, Universidad de Córdoba, Argentina
Tapella F, Lovrich GA (2006) Morphological diferences between ‘subrugosa’ and ‘gregaria’morphs of adult Munida (Decapoda: gnomura: Galatheidae) from the Beagle Channel, southern South America. J Mar Biol Ass UK 86:1149–1155
Tapella F, Lovrich GA, Romero MC, Thatje S (2002) Reproductive biology of the crab Munida subrugosa (Decapoda: Anomura: Galatheidae) in the Beagle Channel. Argentina J Mar Biol Ass UK 82:589–595
Thayer JA, Sydeman WJ (2007) Spatio-temporal variability in prey harvest and reproductive ecology of a piscivorous seabird, Cerorhinca monocerata, in an upwelling system. Mar Ecol Prog Ser 329:253–265
Thompson KR (1993) Variation in Magellanic penguin Spheniscus magellanicus diet in the Falkland Islands. Mar Ornithol 21:57–67
Tierney M, Emmerson L, Hindell M (2009) Temporal variation in Adélie penguin diet at Béchervaise Island, east Antarctica and its relationship to reproductive performance. Mar Biol 156(8):1633–1645
Vanella FA, Fernández DA, Carolina Romero M, Calvo J (2007) Changes in the fish fauna associated with a sub-Antarctic Macrocystis pyrifera kelp forest in response to canopy removal. Polar Biol 30(4):449–457
Venegas C (1998) Pingüinos crestados (Eudyptes chrysocome Forster 1781, E. chrysolophus Brandt 1837) y de Magallanes (Spheniscus magellanicus Forster 1781) en Isla Noir, Chile. An Inst Patagon 26:59–67
Venegas C, Sielfeld W (1981) Utilizacion de aves como indicadoras de presencia y potencialidad de recursos marinos eventualmente manejables. Universidad de Valparaíso, Jornadas Ciencias del Mar Chile, p 83
Williams TD (1995) The penguins. Oxford University Press, Oxford
Wilson (1984) An improved stomach pump for penguins and other seabirds. J Field Ornithol 55(1):110–112
Wilson R, La Cock G, Wilson M-P, Mollagee F (1985) Differential digestion of fish and squid in Jackass Penguins Spheniscus demersus. Ornis Scand 16(1):77–79
Wilson RP, Duffy DC, Wilson M-P, Araya B (1995) Aspects of the ecology of species replacement in Humboldt and Magellanic penguins in Chile. Le Gerfaut 85:49–61
Wilson RP, Scolaro JA, Grémillet D, Kierspel MAM, Laurenti S, Upton J, Gallelli H, Quintana F, Frere E, Müller G, Straten MT, Zimmer I (2005) How do Magellanic penguins cope with variability in their access to prey? Ecol Monogr 75(3):379–401
Acknowledgments
This research was made possible through the support of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Financial support was provided by the Wildlife Conservation Society. We thank Jorge, Andrés and Alejandro Greco from Piratur for logistic support and transportation to the island. We are also grateful to Museo Acatushun and Natalie Goodall. We thank Mariano Diez,Federico Tapella, Marcela Liljesthröm, Natalia Paso Viola, Noelia Volpe, Guillermo Panisse, M. Florencia Colavita, Alfredo Barreto, Constanza Alberio, Natalia Dellabianca, N. Rosciano and M. Paula Sotelano for field and/or laboratory assistance and Marcela Liljesthröm for advice on language. We are grateful to the editor and three anonymous reviewers for providing useful suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scioscia, G., Raya Rey, A., Saenz Samaniego, R.A. et al. Intra- and interannual variation in the diet of the Magellanic penguin (Spheniscus magellanicus) at Martillo Island, Beagle Channel. Polar Biol 37, 1421–1433 (2014). https://doi.org/10.1007/s00300-014-1532-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1532-8