Abstract
Knowledge of diet is critical in interpreting the ecological roles of marine top predators and provides information towards their conservation and management. The Falkland Islands hold the largest number of breeding gentoo penguins. Yet knowledge of gentoo penguin diet at the Falklands is limited to either broad taxonomic divisions of prey items or dietary samples collected only on a single day. This study is the first to investigate gentoo penguin diet at Cow Bay, Falklands, to the species level, over repeated sampling intervals during the breeding period. Through stomach content analysis, we determined diet over a large temporal scale (2002/2003/2004–2011/2012/2013) and between the guard and crèche periods of chick rearing. The principle prey item by reconstituted mass was rock cod fish Patagonotothen spp., for all periods (47–78 %) except that of the 2012/2013 crèche period (19 %) when Falkland herring Sprattus fugensis made up the bulk of the diet (52 %). Of the cephalopods recovered, Patagonian squid Doryteuthis gahi was prominent (1–24 %), while crustaceans contributed negligibly to gentoo penguin diet. Our findings revealed that gentoo penguins breeding at the Falkland Islands were primarily demersal foragers with an ability for pelagic feeding. Diet choice appears to reflect prey availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding predator–prey relationships provides valuable information for elucidating ecosystem structure and function (Gon and Heemstra 1990; Cury et al. 2011). In seabirds, knowledge of diet facilitates an understanding of at-sea behaviour and reliance on marine habitats (Deagle et al. 2007; Iverson et al. 2007; Karnovsky et al. 2012). During the breeding period, seabirds are constrained in the total available habitat they may exploit by the need to return regularly to share incubating duties or to provision chicks (Alonzo et al. 2003; Ropert-Coudert et al. 2006; Masello et al. 2010). Thus, understanding seabird diet during chick rearing, a period of increased nutritional demand, can help determine factors influencing population numbers (Davoren and Montevecchi 2003) and identify particular prey or foraging areas that need protection (Hooker and Gerber 2004; Louzao et al. 2006; Karnovsky et al. 2012).
This study focuses on the diet of breeding gentoo penguins Pygoscelis papua at the Falkland Islands through analysis of stomach contents. The Falklands have the largest population of breeding gentoo penguins, accounting for 34 % of the world’s population (Baylis et al. 2013a). Previous studies assessed composition between broad taxonomic groups of prey items for gentoo penguins across the islands (Putz et al. 2001; Clausen and Putz 2002) or inferred diet from stable isotope analysis for a single breeding colony on the west, New Island (Weiss et al. 2009; Masello et al. 2010). A study conducted at five breeding colonies on the west of the Islands and a single colony in the south, found that gentoo penguins appeared to be selective in feeding during simultaneous diet observation and at-sea surveys of prey availability (Clausen et al. 2005). However, these studies did not explore temporal trends in diet at the taxonomic level of species, nor short-term variability in the diet within the guard and crèche periods of chick rearing (Putz et al. 2001; Clausen and Putz 2002; Clausen et al. 2005). Given variability in diet between breeding colonies, a detailed investigation of diet and dietary shifts during chick guard and crèche periods for gentoo penguins at the Falklands is timely. Such information is important for understanding the potential for short-term variability in diet and identifying potential threats towards the gentoo population, such as overlap of prey species with fisheries. Therefore, the aim of the study was to (1) describe in detail the diet of gentoo penguins breeding at Cow Bay in the Falklands, (2) for the first time investigate whether diet may differ during the chick guard and crèche periods at the Falklands, and (3) investigate whether inter-annual shifts in diet occur.
Materials and methods
Sample collection
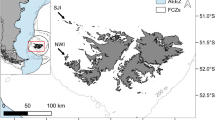
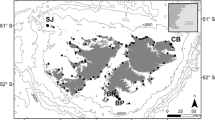
This study was conducted at Cow Bay (51°26′3.5″S, 57°52′39.2″W, Fig. 1), which lies in the north-east of the Falklands archipelago with approximately 1821 breeding pairs of gentoo penguins, calculated in 2012 during the annual surveys by the Falkland Islands Seabird Monitoring Program (Baylis et al. 2013a). Following the 5-yearly 2010 island-wide census, it was the 26th largest of 75 breeding colonies monitored that year (Baylis et al. 2013a). Diet samples were collected during the guard and crèche periods of the austral summers 2011/2012 (n Guard = 28, n Crèche = 10) and 2012/2013 (n Guard = 10, n Crèche = 11). The guard and crèche periods were defined as those when partners take turns to brood and guard chicks at the nest, and when chicks are left alone with other chicks in crèches, respectively (Polito and Trivelpiece 2008; Lescroël et al. 2009). Eight to ten birds were sampled on three occasions (22–24/11/2011; 5–9/12/2011; 19–21/12/2012) during the guard period of 2011/12, five birds each on two occasions (15/12/2012; 17/12/2012) in the guard period of 2012/13, and five to six birds daily over 2 days (17–18/01/2012 and 11–12/01/2013) during the crèche periods of these seasons. Data were available for ten diet samples collected at the same colony during the guard periods of the 2002/2003 and 2003/2004 seasons.
Birds were caught upon returning to the colony with a net attached to a 2-m pole (Otley et al. 2005; Masello et al. 2010). As gentoo penguins at Cow Bay and elsewhere are skittish around their nests (Reilly and Kerle 1981), this minimised disturbance in the colony. To ensure sampling of breeding individuals, only those birds with a vascularised brood patch were sampled (Volkman et al. 1980). Birds were suspended in a harness, and their mass recorded with a 10-kg Pesola spring balance, accurate to 100 g. Stomach lavage followed Wilson (1984) with modifications appropriate to the species. Specifically, two observers handled the bird with one gripping the feet while supporting the body on their legs, and the other holding the beak while massaging the neck. Stomach samples were drained of excess water over a 0.5-mm sieve, sealed in zip lock bags and frozen within 12 h (Putz et al. 2001).
Birds were marked with a green, temporary, waterproof wax marker (ROTO.STIK) on the breast feathers and then released in the direction of the colony. This mark lasts for approximately 10 days, minimising the chance of repeat sampling. It also allowed post-identification of sampled birds to facilitate detection of possible abnormal behaviour. There was a negligible chance of birds being repeatedly sampled between breeding periods or within years, owing to the size of the colony.
Stomach content analysis
Prior to sorting, samples were left to thaw overnight. Each sample was then placed in a 0.5-mm sieve allowing excess water to drain off. Total wet mass was recorded. Mass of stones, accounting for on average 1.6 % (range 0–13 %), was removed from the total wet mass and not included in the diet analysis (Clausen et al. 2005). When possible, the undigested part (e.g. whole squid, fish, and crustaceans) of the stomach content was separated from the digested part (Herling et al. 2005). These items were identified, weighed and measured accordingly: cephalopods, dorsal mantle length (DML); fish, total length (TL), measured to the fin tip (only rock cod fish Patagonotothen spp. were suitable owing to digestion extent); and crustaceans, total length (CTL), where CTL is the measure from the anterior edge of the eye to the distal end of the telson. From the remaining digested material, a 350-g subsample was used to inspect for saccular otoliths, cephalopod beaks, crustacean carapaces, or other hard part remains, as this is equivalent to roughly half the total wet mass (Polito et al. 2011). The remaining total composition of the sample was calculated from this proportion. Where others have used dark-bottomed pans (Miller et al. 2009; Polito et al. 2011), it was found that a large green container (≈350 mm × 200 mm) allowed easy inspection for both white otoliths and darker cephalopod beaks simultaneously. A small amount of the digested material (≈25 g) was placed into the container. Water was added to a depth of approximately 20 mm. The material was then lightly agitated apart with a fine spatula to search for hard part remains. These remains were identified to the lowest possible taxonomic level by comparing them with an extensive reference collection housed by the Falkland Islands Fisheries Department (FIFD) and published reference material (Clarke 1986; Xavier and Cherel 2009).
Length frequency and reconstituted mass
Intact lobster krill (Munida spp.) carapaces were measured using vernier callipers to the nearest 0.1 mm. Two species/morphs, Munida gregaria and Munida subrugosa, are commonly found within the continental shelf waters of South America and the Falklands (Matthews 1932; Tapella and Lovrich 2006). There are conflicting views on whether or not these are separate species (Tapella and Lovrich 2006), so reconstituted mass for Munida spp. was calculated only from regressions relating to Munida gregaria as this was the only identifiable morph during the study. Similarly, rock cod fish were largely digested and only occasionally were whole individuals identified as Patagonotothen ramsayii or Patagonotothen tessellata. These two species are most common in inshore waters of the Falklands, and distinguishing species by otoliths is only possible for those larger than observed in the study (otolith >7 mm, Paul Brickle pers. comm.; study: >7 mm, n = 2 of 1707). Therefore, average length and mass from the two species were used (Huin 2005).
Otolith length (OL) from fish, lower rostral length (LRL) of squid beaks, and lower hood length (LHL) of sepiolid squid and octopods were measured using an eye-piece graticule in a dissecting microscope, regularly recalibrated with a graticule scale. Both cephalopod beaks and otoliths undergo a significant ontogenetic morphological change (Smale et al. 1995). Otoliths of larval fish are virtually identical, and with increasing size, otoliths may be identified to a particular order, then family and ultimately a species (Gon and Heemstra 1990). Reconstituted mass and length were calculated from morphometric equations for each species following reference material or from regression equations developed during the study (Online Resource 1). When left and right otoliths were found from the same species and of similar size, the highest number of either side was used as a conservative estimate for total number of fish (Miller et al. 2009). Eroded fish otoliths and cephalopod beaks that were not attached to flesh were considered as accumulated items and not included in the final calculations of mass for each species (Van Heezik and Seddon 1989; Thompson 1994; Clausen et al. 2005). Accumulated squid beaks were, however, included when calculating species composition and size class distribution of the cephalopod portion of the diet (Thompson 1994). In cases where whole prey could be identified, but no reference equations existed or could be developed, the wet mass was used (Clausen and Putz 2003). Proportional size compared to a single, undigested, exemplar specimen was used in the case of the bobtail squid (Semirossia patagonica).
Composition of samples
Prey items were first described in terms of percentage number (%N), mass (%M), and frequency of occurrence (%FO) (Duffy and Jackson 1986), where:
-
%N is the proportion of the total number of individuals of one prey item compared to the total number of individuals of all prey items per sample.
-
%M is the proportion of the total mass of one prey item compared to the total mass of all prey items per sample.
-
%FO is the proportion of stomachs containing the prey type compared to all stomachs examined.
Samples were then described by an integrative value, the index of relative importance (IRI) (Pinkas et al. 1971):
This is a modification of the index where the original term of percentage by volume was replaced with %M (Koen Alonso et al. 1998). It incorporates from all samples, in a given sampling period, the summed value of %N and %M. Percentage FO has the advantage of being fast, with stomachs scored for the presence or absence data. It is, however, most appropriate when prey items are of similar size (Duffy and Jackson 1986). The IRI is most sensitive to %FO, which is a multiplicand, rather than %M and %N which are addends, but helps reduce the biases introduced by numerous small or a few very large items occurring in only one or a few stomachs (Duffy and Jackson 1986). It further reduces numerous tables making multiple comparisons which may lead to “data narcosis” (Duffy and Jackson 1986). As the index does not have a set scale, percentage index of relative importance was calculated (%IRI), where:
-
%IRI is the proportion of each species IRI relative to the total sum of IRI for a given sampling period.
This allowed for easier interpretation of the IRI and for a standard measure to compare different sampling periods (Cortes 1997; Huin 2005). Analyses could then be conducted on those prey items that were represented by >2 %IRI (Koen Alonso et al. 2000). As the IRI relies on the summed information for each sampling period, there is no variation in the result. Therefore, percentage mass (%M) was chosen to compare prey items at the level of the sampling unit (each penguin), again as this favours samples with varying prey size (Duffy and Jackson 1986; Ratcliffe and Trathan 2011).
Data analysis
All data analyses were performed using R version 3.0.1 (R Core Team 2013). As historical data (2002/2003 and 2003/2004) were represented by relatively small sample sizes, extensive sampling during the chick guard period of 2011/2012 allowed us to determine whether these samples were sufficient to represent the major prey items for each period. Specifically, prey species accumulation curves were plotted for each sampling period (package: vegan; function: specaccum) and the Chao estimator (Chao 1987) determined (package: vegan; function: specpool). Comparisons were made for intra-annual variation between chick guard and crèche periods and long-term variation through analysis of all chick guard data. Univariate and multivariate normality was assessed with Shapiro–Wilks and multivariate Shapiro–Wilks tests (package: mvnormtest; function: m.shapirotest), respectively. Bartlett’s test and multivariate Levene’s test (package: vegan; function: betadisper) were used to assess for univariate and multivariate homogeneity of variance, respectively. Means with standard deviations are given, and significance was assumed at p < 0.05 unless otherwise stated.
Differences in species composition were first visually assessed with ordination via nonmetric multidimensional scaling (nMDS) (package: vegan; function: metaMDS with autotransform = “F”), using the arc-sin-transformed percentage by mass data. The function ordispider (package: vegan) was applied to the ordination which plots the centroid (weighted mean) for each treatment. Centroids further apart indicate greater dissimilarity. Statistical differences in species assemblages were assessed using an ADONIS test (package: vegan; function: adonis with distance = “bray”). This function partitions sums of squares for multivariate data and is analogous to nonparametric MANOVA (Anderson 2001; Birk et al. 2012). It is similar to ANOSIM but is believed to be statistically more robust (Peay et al. 2010; Oksanen et al. 2015). Significance was tested against 999 null permutations. Where appropriate, SIMPER tests (package: vegan; function: simper) were performed post-ADONIS to assess which species had the most influential cumulative contribution to sample similarity. These tests are limited as they only compare two groups of samples at a time (Clarke and Warwick 2001). Univariate tests among individual prey species percentage mass, and size, included Kruskal–Wallis tests, followed by post hoc Mann–Whitney U tests with Bonferroni correction.
Results
A total of 3314 prey items were identified from 69 gentoo penguin stomach contents examined over four seasons, comprising 2249 individual fish, 412 individual cephalopods, 648 individual crustaceans, and five other individual items, which included two gastropods, and a single mytilid, nacellid, and venerid. A total of 17 of the 31 prey items were identified to species level over the study period (Table 1).
Combined across each individual sampling period, a total of eight prey items contributed >2 %IRI. These included: fish, rock cod Patagonotothen spp., Falkland herring Sprattus fugensis, Bull blenny Cottoperca gobio, and juvenile fish too small for identification; cephalopods, Patagonian squid Doryteuthis gahi, Gonatus antarcticus, and juvenile cephalopods; and one crustacean, Themisto gaudichaudii. The three most important items for all sampling periods were rock cod, Patagonian squid, and then Falkland herring.
Reliability of sample size
From the samples collected, prey species accumulation curves (Fig. 2) indicated that sampling was extensive enough to represent the major prey items as indicated by all accumulation curves reaching a plateau. The highest number of expected major prey items (>2 % IRI) in the diet occurred during the 2011/2012 crèche period with the Chao estimator indicating six prey items. For all sampling periods, there was zero deviation about the Chao estimator. As major prey items were found in at least two stomachs for each period, this was to be expected as once a species is found more than twice (doubletons), the estimator does not predict further increase in number of additional species.
Prey species accumulation curves for sampling periods. Each plot is labelled by the year and chick-rearing phase and includes the: [Chao estimator (Chao standard error)] (Chao 1987). Bold line represents the accumulation curve, with grey shading representing the 95 % confidence interval associated with the curve. Species used in each plot are those having >2 %IRI, for each sampling period
General diet composition
The relative contributions of major prey taxa, by percentage mass, during all sampling occasions are shown in Fig. 3. The lowest contribution of fish (66 %) was seen during the 2011/2012 crèche period, compared to all other periods where fish contributed to 80 % of the diet. During the 2011/2012 crèche period, there was an increase in the contribution of squid to 32 %; otherwise, it accounted for a maximum of 14 %. Crustaceans did not appear in large quantities during any of the sampling periods (max. = 4 %). ADONIS indicated no significant differences between sampling occasions when comparing major prey taxa (F 5,63 = 1.94, R 2 = 0.13, p = 0.075).
Temporal variation: breeding period
Variation in diet was evident both within and between the 2011/2012 and 2012/2013 breeding seasons. ADONIS indicated significant differences for the interaction of breeding season (inter-annual) and breeding period (intra-annual) (F 1,55 = 7.55, R 2 = 0.097, p = 0.002), where breeding period contributed most significantly and explained 12 % of the variation (F 1,55 = 9.51, R 2 = 0.12, p = 0.001) as opposed to breeding season which explained 7 % of the variation (F 1,55 = 6.07, R 2 = 0.07, p = 0.003). Evidence of these results is reflected in the nMDS ordination (Fig. 4a), where the centroids of both crèche periods are separated from their respective guard periods and separated from each other, indicating an effect between breeding seasons for the crèche periods and within each breeding season. No seasonal effect was present between the guard periods.
Two-dimensional nMDS ordination, showing the grouping of diet samples for gentoo penguins at Cow Bay. a Breeding period comparison for guard and crèche periods of 2011/2012 and 2012/2013. b Inter-annual comparison for guard periods only. Prey items included are those that had >2 %IRI. Function ordispider: plots arms from samples which join at the centroid (weight mean) for each group of samples. Centroids further apart indicate greater dissimilarity
This change in penguin diet within each breeding season was largely driven by the high proportion of Falkland herring, 53 %, in the 2012/2013 crèche period and the relatively low intake of rock cod in the 2011/2012 crèche period, 47 % (Fig. 5). As Falkland herring had zero contribution by %M during the 2011/2012 crèche and 2012/2013 guard period, comparisons were made only among rock cod and Patagonian squid for all four sampling occasions. Failing assumptions of normality, Kruskal–Wallis tests indicated significant differences for %M of consumed rock cod (H 3 = 14.524, p = 0.002). Post hoc testing indicated a significant difference for only the 2012/2013 crèche period where rock cod accounted for only 20 % of the diet compared to all other occasions where it accounted for on average 47–73 % (p < 0.05, all occasions). No difference by %M was observed for Patagonian squid (H 3 = 1.313, p = 0.726).
A significant difference in size of consumed rock cod (H 3 = 124.130, p < 0.001) and Patagonian squid (H 3 = 14.936, p = 0.002) was evident among the differing sampling occasions. For rock cod (Fig. 6a), gentoo penguins consumed larger fish during the 2011/2012 crèche period (median 101, range 62–173 mm) and post hoc testing indicated only this period to have significant differences from all others (2011/2012 guard, median 90, range 62–295 mm; 2012/2013 guard, median 87, range 51–206 mm; 2012/2013 crèche, median 87, range 72–119 mm). For Patagonian squid, post hoc tests indicated differences to lie between the periods of 2011/2012 (median 97, range 41–131 mm) and 2012/2013 (median 83, range 62–103 mm, p < 0.001) crèche periods, and the 2012/2013 guard (median 97, range 62–138 mm) and 2012/2013 crèche (p = 0.011) periods, although no observable pattern in size of Patagonian squid consumed was apparent (Fig. 6b).
These results indicate a clear difference in diet over recently investigated seasons, where larger rock cod were taken during the crèche period of the 2011/2012 season and species composition changed between the chick guard and crèche periods in the 2012/2013 season and also between years for crèche periods. Inter-annual variation in diet during the guard phase was not evident.
Temporal variation: inter-annual
Only data from the chick guard period were used for the extended inter-annual comparison (due to above-mentioned differences between breeding periods and only guard data being available for the earlier period). The ADONIS test indicated no significant differences between chick guard periods for all years (F 3,44 = 1.39, R 2 = 0.086, p = 0.177), with this result being reflected in the nMDS ordination where centroids overlap or are situated in close proximity to each other (Fig. 4b).
SIMPER analysis identified rock cod and Patagonian squid as the most influential prey items by mass for all pairwise comparisons, except those involving the 2003/2004 season where rock cod and bull blenny were most influential. This is reflected in the species contribution by %M for each sampling occasion (Fig. 5), and no difference being found for %M of consumed rock cod over all periods (H 3 = 0.969, p = 0.808). However, when comparing the size of rock cod consumed, there were significant differences between seasons (H 3 = 108.963, p < 0.001). Similar size items were taken in the recent years (2011/2012 and 2012/2013, see above; p = 0.400), whereas prey items were smaller in 2011/2012 as compared to the 2002/2003 (median 83, range 19–292 mm, p = 0.007) season and in all cases smaller than those taken during the 2003/2004 season (median 116, range 44–177 mm, p < 0.001).
Discussion
General diet composition
This study provides the first detailed investigation of species consumed by gentoo penguins at Cow Bay, Falkland Islands. For the broad diet classification, fish were the main taxonomic group consumed by gentoo penguins, accounting for over 80 % of the diet by mass. This finding is consistent with a previous study at Cow Bay during the 1998/1999 guard period, where fish accounted for 98.6 % (Putz et al. 2001). Furthermore, our findings are consistent with a dietary trend across the Falklands, with fish dominant in gentoo penguin diet at breeding colonies in the north, while crustaceans are dominant at western and southern breeding colonies (Putz et al. 2001). At finer detail, the major prey items in this study were notothenioid fish rock cod, the Clupeidae, Falkland herring, and the loliginid squid Patagonian squid. All three of these items have been reported previously in the diet of gentoo penguins at the Falklands (Putz et al. 2001; Clausen and Putz 2002, 2003; Clausen et al. 2005), and other dietary studies over broad spatial ranges also report demersal fish, including notothenioids (Croxall et al. 1988; Bost et al. 1994; Coria et al. 2000).
The fact that rock cod was the most dominant prey item in the study can be attributed to its high relative abundance which does not vary much throughout a year (Arkhipkin et al. 2001; Laptikhovsky et al. 2013). It spawns on the shelf break during the austral winter (Arkhipkin et al. 2013a) and then during the spring, juvenile, resting, and maturing adults move onto the shelf (Brickle et al. 2006), with a majority found towards the highest abundance of macrozooplankton in the north of the Falklands (Agnew 2002). The size of rock cod consumed in this study indicates a benthic feeding behaviour for gentoo penguins as the juvenile stage of rock cod is characterised by individuals <150 mm which are primarily benthic themselves (Laptikhovsky and Arkhipkin 2003).
Similarly, Patagonian squid that spawn in the autumn cohort take advantage of this summer macrozooplankton bloom (Arkhipkin et al. 2013b). Spawning occurs in shallow waters <50 m (Arkhipkin et al. 2013b), and the highest densities of squid eggs have been found on the north-east coast (Arkhipkin et al. 2000). At the Falklands, adults are typically found in the south, south-east, and north-east waters (Arkhipkin et al. 2013b). Due to ontogenetic migration, maturing adults move beyond the shelf and a bi-modal size distribution in water <150 m deep is typically observed (Arkhipkin et al. 2013b). During the daytime, when gentoo penguins most often feed, Patagonian squid concentrate near the bottom. These concentrations include squid with DML of <80 mm in waters of <100 m depth and larger squid, DML 90–100 mm, being found in deeper water of 100–200 m depth (Arkhipkin et al. 2013b). This size range of squid fits well with the size consumed by gentoo penguins at Cow Bay and again points towards gentoo penguins being demersal foragers.
The third major prey item, Falkland herring, indicates gentoo penguins at Cow Bay can successfully utilise a pelagic foraging technique as these fish are associated with coastal, pelagic waters, typically schooling in the bottom layers of the water column around the Falklands (Laptikhovsky et al. 2001) in waters of 0–70 m depth (Whitehead et al. 1985). Around the Falklands, these fish spawn in September and October with adults then moving inshore to feed after spawning (Agnew 2002). Large shoals of adults have even been reported to strand in coves around the Falklands (Agnew 2002). At-sea surveys indicate the largest populations are towards the north-west of the Falklands (Laptikhovsky et al. 2001; Agnew 2002; Baylis et al. 2013b). These fish were also observed as the highest component in the diet of the South American Fur Seal Arctocephalus australis, via faecal samples collected in October in this region (Baylis et al. 2013b). However, as it coincided with spawning, the authors suggest that the diet may reflect opportunistic foraging on a seasonally abundant prey item. Similar conclusions were drawn when investigating the diet of dogfish Squalus acanthias and catsharks Schroederichtys bivius (Laptikhovsky et al. 2001) and Patagonian toothfish Dissostichus eleginoides (Arkhipkin et al. 2003) whose diet had the highest proportion of Falkland herring during September–November and November–January, respectively, around the Falklands.
For the majority, these data all imply that gentoo penguins typically utilise a demersal feeding technique within the confines of the continental shelf, on readily available prey, as reported at other breeding locations (Trivelpiece et al. 1986, 1987; Wilson et al. 1998; Miller et al. 2009). We expect the location of Cow Bay would further promote a demersal feeding technique because the shelf slope, defined by the 200-m isobath, which is a depth towards the maximal ability of gentoo penguins, is approximately 100 km away. This is well beyond the 30-km average foraging range of gentoo penguins (Trivelpiece et al. 1987; Wilson et al. 1998; Miller et al. 2009). This argument is supported in the fact that demersal prey was also observed in the west of the islands at breeding colonies with similar bathymetry where there was a gently sloping seabed (Clausen et al. 2005).
In terms of pelagic feeding, this has been observed when gentoo penguins target krill (Bost et al. 1994; Hinke et al. 2007; Kokubun et al. 2010). The technique is more typical when the birds are foraging in deeper near-shore waters, as seen in Antarctica where gentoo penguins at Cape Sherriff had significantly more fish in their diet compared to those at Admiralty Bay, where the continental shelf edge (and hence, deep water) is closer to shore (Miller et al. 2010). Therefore, while pelagic appears more atypical than demersal foraging, clearly gentoo penguins at Cow Bay can apparently utilise both demersal and pelagic foraging techniques during the chick guard and crèche period. The preferred method is most likely influenced by variations in abundance and migration of major prey species in and out of feeding areas. Ultimately, a demersal foraging technique may be more beneficial as prey distribution is limited by the sea floor which increases spatial predictability of prey items (Kokubun et al. 2010).
Temporal variation: breeding period
This is the first study to explore differences in gentoo penguin diet between the guard and crèche periods at the Falklands. We revealed a shift in the primary prey species between the guard and crèche periods of the 2012/2013 chick-rearing season. Also notable was the significant difference in the size of rock cod consumed between the guard and crèche periods of the 2011/2012 season. Predictions based on optimal foraging theory may help to explain the dietary differences we observed between guard and crèche periods.
Optimal foraging theory predicts that animals should forage in a manner that maximises energy gain therefore promoting fitness (Emlen 1966; Macarthur and Pianka 1966). The theory may also be applied to the selection of different food items (Stephens and Krebs 1986), in particular larger items or those with higher energy content which would favour a reduced foraging duration. This might additionally be influenced by the breeding stage of an individual that has different energy requirements (Williams and Rothery 1990). As the capture of larger prey items is facilitated by larger body size (Williams 1991), one might expect gentoo penguins at the Falklands to consume the largest prey items at this location. However, those breeding at South Georgia had a diet consisting of 48 % fish, which were much larger (130–180 mm) (Reid et al. 2005), on average, than those consumed in this study (83–116 mm), despite these penguins exhibiting a smaller body size (see Lynch 2012).
The hypothesis with respect to capture of higher energy prey items is compelling, because it could help to explain the shift in prey items between the 2012/2013 guard and crèche periods. Of all three major prey items in the study, Falkland herring has the greatest energy density (7148 j g−1) compared to rock cod (P. tesellata = 4034 j g−1, P. ramasayi = 4798 j g−1) and Patagonian squid (4952 j g−1) (Ciancio et al. 2007). For the yellow-eyed penguin in New Zealand, European sprat Sprattus sprattus in the diet was associated with higher fledgling mass and greater reproductive success (van Heezik 1990), and the importance of this prey item as a higher energy target species was also recognised by Browne et al. (2011). Furthermore, in feeding trials on black-legged kittiwakes Rissa tridactyla and tufted puffins Fratercula cirrhata, those chicks that were fed higher energy density forage fish had higher growth increments (Romano et al. 2006). Therefore, in gentoo penguins at the Falklands, when higher energy prey items are available, these may be preferentially captured so as to provide even greater support for growing chicks compared to a diet of rock cod.
Temporal variation: inter-annual
The key difference in diet composition between years was in the size of rock cod consumed over the guard periods. Our findings are in contrast to Putz et al. (2001), who found marked differences in prey composition between years for gentoo penguins breeding at the Falklands. However, they investigated changes only between broad taxonomic divisions (fish, squid, crustacean) at the Falklands and in some years included different breeding colonies, which may bias results as diet is seen to vary greatly even at the scale of the archipelago (Lescroël et al. 2004; Clausen et al. 2005; Miller et al. 2010). There was, however, annual variation in the diet between the two crèche periods of the recent investigation.
This inter-annual difference in diet has been observed elsewhere for gentoo penguins in both size of fish and krill consumed and prey composition (Croxall et al. 1999; Coria et al. 2000; Bevan et al. 2002; Libertelli et al. 2004; Hinke et al. 2007). For example, Bost et al. (1994) found differences in the contribution of either Euphausiids or Themisto gaudichaudii over two seasons. Also, in a multispecies study conducted at South Georgia, changes in both predator diets and prey biomass estimated through acoustic surveys occurred on an annual basis. However, when assessing intra-annual variation, this same study found no marked differences present for either gentoo penguins or Antarctic fur seals Arctocephalus gazella (Croxall et al. 1999). This again points to temporal changes in species composition of gentoo penguin diet, in this study and others, being attributed largely to changes in prey availability within their foraging range (Volkman et al. 1980; Adams and Klages 1989; Coria et al. 2000; Libertelli et al. 2004). This argument is further supported by comparative studies at the Falklands (Clausen et al. 2005) and Kerguelen archipelago (Lescroël et al. 2004). These studies both found differences in diet during concurrent investigations at different colonies, which were reflective of known prey availability.
Ecological implications
Monitoring predator diet and prey abundance in the environment is fundamental in understanding predator–prey interactions (Reid et al. 2005). The fact that rock cod is the primary prey item for gentoo penguins at Cow Bay during the breeding period, could indicate a possible interaction with fisheries. Prior to 2006, rock cod was viewed as a bycatch species but has since been commercialised and catch per unit effort (kg h−1) has increased 30-fold between 2002–2004 and 2009–2011. The ability for increased CPUE is thought to be possible because of increased rock cod population growth due to overfishing of southern blue whiting in the region which has reduced exploitative competition between these planktivorous fish (Laptikhovsky et al. 2013). Stocks of southern blue whiting declined from the 1990s, and in 2011–2012 total catches in Falklands and Argentinian waters were, conservatively, 25-fold less than they were between 1989 and 1995 (Laptikhovsky et al. 2013). For hake Merluccius hubbsi, M. australis, and kingklip Genypterus blacodes, whose diet also consisted of over 50 % rock cod, mean daily catches over the same period have increased five- and two fold, respectively, and are thought to be a consequence of increased rock cod abundance (Laptikhovsky et al. 2013). For gentoo penguins, breeding pair numbers have also increased from approximately 570 pairs in 2003/2004 (Huin 2005) to 1821 breeding pairs in 2012/2013 (Baylis et al. 2013a).
Population changes through shift in ecosystem structure, particularly change in prey availability, have been recognised elsewhere in seabirds. Furness and Barrett (1985) noted how the removal of some top predators and increased abundance in a forage fish, capelin Mallotus villosus, supported an increasing seabird population in Norway. Along with potential impacts of climate change, Lescroël and Bost (2006) suggest that reduced numbers in the gentoo penguin population at the Kerguelen archipelago may be related to depletion of fish stocks, in particular reduction in the mackerel icefish Champsocephalus gunnari which accounted for 40 % of the diet during the winter of 1987 (Lescroël et al. 2004), but now is no longer a viable commercial species in the region. Prey availability also played a role in population numbers of magellanic penguins Spheniscus magellanicus breeding along the Argentinian coastline in Patagonia. Those breeding colonies that were closest to required prey resources had the largest populations probably due to enhanced provisioning and associated increase in lifetime reproductive success (Wilson et al. 2005).
Conclusion
Gentoo penguins at Cow Bay, Falkland Islands, foraged primarily for demersal prey during the breeding period, which look to be readily available during this time. This was reflected in their two main prey items being rock cod and Patagonian squid, which, at the size consumed in this study, are found at or near the seabed. It would be valuable for future studies to assess how representative our findings are for other gentoo penguin breeding colonies at the Falkland Islands. In addition, given rock cod are one of the primary commercial resources at the Falkland Islands and the Falkland Islands holds the largest proportion of the global gentoo penguin population, it would be valuable for future research to investigate to what extent gentoo penguins and commercial fisheries interact in order to support the conservation and management of gentoo penguins.
References
Adams NJ, Klages NT (1989) Temporal variation in the diet of the gentoo penguin Pygoscelis papua at sub-Antarctic Marion Island. Colon Waterbird 12:30–36
Agnew DJ (2002) Critical aspects of the Falkland Islands pelagic ecosystem—distribution, spawning and migration of pelagic animals in relation to oil exploration. Aquat Conserv 50:39–50
Alonso MK, Crespo EA, Garcia NA et al (1998) Diet of dusky dolphins, Lagenorhynchus obscurus, in waters off Patagonia, Argentina. Fish Bull 96:366–374
Alonso MK, Crespo EA, Pedraza SN (2000) Food habits of the South American sea lion, Otaria flavescens, off Patagonia, Argentina. Fish Bull 98:250–263
Alonzo SH, Switzer PV, Mangel M (2003) An ecosystem-based approach to management: using individual behaviour to predict the indirect effects of Antarctic krill fisheries on penguin foraging. J Appl Ecol 40:692–702
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Arkhipkin A, Laptikhovsky V, Middleton DJ (2000) Adaptations for cold water spawning in loliginid squid: Loligo gahi in Falkland waters. J Molluscan Stud 66:551–564
Arkhipkin A, Brickle P, Laptikhovsky V et al (2001) Variation in the diet of the red cod with size and season around the Falkland Islands (south-west Atlantic). J Mar Biol Assoc UK 81:1035–1040
Arkhipkin A, Brickle P, Laptikhovsky V (2003) Variation in the diet of the Patagonian toothfish with size, depth and season around the Falkland Islands. J Fish Biol 63:428–441
Arkhipkin A, Jurgens E, Howes PN (2013a) Spawning, egg development and early ontogenesis in rock cod Patagonotothen ramsayi (Regan, 1913) caught on the Patagonian Shelf and maintained in captivity. Polar Biol 36:1195–1204
Arkhipkin A, Hatfield EMC, Rodhouse PG (2013b) Doryteuthis gahi, Patagonian long-finned squid. In: Rosa R, O’Dor R, Pierce G (eds) Advances in squid biology, ecology and fisheries, part 1. Nova Science, New York, pp 123–158
Baylis AMM, Crofts S, Wolfaardt AC (2013a) Population trends of gentoo penguins (Pygoscelis papua) breeding at the Falkland Islands. Mar Ornithol 41:1–5
Baylis AMM, Arnould JPY, Staniland IJ (2013b) Diet of South American fur seals at the Falkland Islands. Mar Mammal Sci 30:1210–1219
Bevan RM, Butler PJ, Woakes J, Boyd IL (2002) The energetics of gentoo penguins, Pygoscelis papua, during the breeding season. Funct Ecol 16:175–190
Birk HJB, Lotter AF, Juggins S, Smol JP (2012) Tracking environmental change using lake sediments: data handling and numerical techniques, 5th edn. Springer, New York
Bost C, Koubbi P, Genevois F et al (1994) Gentoo penguin Pygoscelis papua diet as an indicator of planktonic availability in the Kerguelen Islands. Polar Biol 14:147–153
Brickle P, Arkhipkin A, Shcherbich Z (2006) Age and growth of a sub-Antarctic notothenioid, Patagonotothen ramsayi (Regan 1913), from the Falkland Islands. Polar Biol 29:633–639
Browne T, Lalas C, Mattern T, Van Heezik Y (2011) Chick starvation in yellow-eyed penguins: evidence for poor diet quality and selective provisioning of chicks from conventional diet analysis and stable isotopes. Austral Ecol 36:99–108
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791
Ciancio JE, Pascual M, Beauchamp D (2007) Energy density of Patagonian aquatic organisms and empirical predictions based on water content. Trans Am Fish Soc 136:1415–1422
Clarke MR (1986) A handbook for the identification of cephalopod beaks. Clarendon Press, Oxford
Clarke KR, Warwick RM (2001) Changes in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth
Clausen AP, Putz K (2002) Recent trends in diet composition and productivity of gentoo, magellanic and rockhopper penguins in the Falkland Islands. Aquat Conserv Mar Freshw Ecosyst 12:51–61
Clausen AP, Putz K (2003) Winter diet and foraging range of gentoo penguins (Pygoscelis papua) from Kidney Cove, Falkland Islands. Polar Biol 26:32–40
Clausen AP, Arkhipkin AI, Laptikhovsky V, Huin N (2005) What is out there: diversity in feeding of gentoo penguins (Pygoscelis papua) around the Falkland Islands (Southwest Atlantic). Polar Biol 28:653–662
Coria N, Libertelli M, Casaux R, Darrieu C (2000) Inter-annual variation in the autumn diet of the gentoo penguin at Laurie Island, Antarctica. Waterbirds 23:511–517
Cortes E (1997) A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Can J Fish Aquat Sci 54:726–738
Croxall AJP, Davis RW, Connell MJO (1988) Diving patterns in relation to diet of gentoo and macaroni penguins at South Georgia. Condor 90:157–167
Croxall J, Reid K, Prince P (1999) Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar Ecol Prog Ser 177:115–131
Cury PM, Boyd IL, Bonhommeau S et al (2011) Global seabird response to forage fish depletion. One-third for the birds. Science 334:1703–1706
Davoren GK, Montevecchi WA (2003) Consequences of foraging trip duration on provisioning behaviour and fledging condition of common murres Uria aalgae. J Avian Biol 34:44–53
Deagle BE, Gales NJ, Evans K et al (2007) Studying seabird diet through genetic analysis of faeces: a case study on macaroni penguins (Eudyptes chrysolophus). PLoS One 2:e831
Duffy DC, Jackson S (1986) Diet studies of seabirds: a review of methods. Colon Waterbirds 9:1–17
Emlen JM (1966) The role of time and energy in food preference. Am Nat 100:611–617
Furness RW, Barrett RT (1985) The food requirements and ecological relationships of a seabird community in North Norway. Ornis Scand 16:305–313
Gon O, Heemstra PC (1990) Fishes of the southern ocean. JLB Smith Institute of Ichthyology, Grahamstown
Herling C, Culik BM, Hennicke JC (2005) Diet of the Humboldt penguin (Spheniscus humboldti) in northern and southern Chile. Mar Biol 147:13–25
Hinke JT, Salwicka K, Trivelpiece SG et al (2007) Divergent responses of Pygoscelis penguins reveal a common environmental driver. Oecologia 153:845–855
Hooker SK, Gerber LR (2004) Marine reserves as a tool for ecosystem-based management: the potential importance of megafauna. Bioscience 54:27–39
Huin N (2005) Falkland Islands seabird monitoring programme annual report 2003/2004/2005. Falklands Conservation, Falkland Islands
Iverson S, Springer A, Kitaysky A (2007) Seabirds as indicators of food web structure and ecosystem variability: qualitative and quantitative diet analyses using fatty acids. Mar Ecol Prog Ser 352:235–244
Karnovsky N, Hobson K, Iverson S (2012) From lavage to lipids: estimating diets of seabirds. Mar Ecol Prog Ser 451:263–284
Kokubun N, Takahashi A, Mori Y et al (2010) Comparison of diving behavior and foraging habitat use between chinstrap and gentoo penguins breeding in the South Shetland Islands, Antarctica. Mar Biol 157:811–825
Laptikhovsky V, Arkhipkin A (2003) An impact of seasonal squid migrations and fishing on the feeding spectra of subantarctic notothenioids Patagonotothen ramsayi and Cottoperca gobio around the Falkland Islands. J Appl Ichthyol 19:35–39
Laptikhovsky V, Arkhipkin A, Henderson C (2001) Feeding habits and dietary overlap in spiny dogfish Squalus Acanthias (Squalidae) and narrowmouth catshark Schroederidhthys bivius (Scyliorhinidae). J Mar Biol Assoc UK 81:1015–1018
Laptikhovsky V, Arkhipkin A, Brickle P (2013) From small bycatch to main commercial species: explosion of stocks of rock cod Patagonotothen ramsayi (Regan 1931) in the Southwest Atlantic. Fish Res 147:399–403
Lescroël A, Bost CA (2006) Recent decrease in gentoo penguin populations at Iles Kerguelen. Antarct Sci 18:171–174
Lescroël A, Ridoux V, Bost C (2004) Spatial and temporal variation in the diet of the gentoo penguin (Pygoscelis papua) at Kerguelen Islands. Polar Biol 27:206–216
Lescroël A, Bazjak C, Bost C (2009) Breeding ecology of the gentoo penguin Pygoscelis papua at Kerguelen Archipelago. Polar Biol 32:1495–1505
Libertelli MM, Daneri GA, Piatkowski U et al (2004) Predation on cephalopods by Pygoscelis papua and Arctocephalus gazella at South Orkney Islands. Pol Polar Res 25:267–274
Louzao M, Hyrenbach KD, Arcos JM et al (2006) Oceanographic habitat of an endangered Mediterranean procellariiform: implications for marine protected areas. Ecol Appl 16:1683–1695
Lynch HJ (2012) Gentoo penguin (Pygoscelis papua). In: Borboroglu PG, Boersma PD (eds) Penguins: natural history and conservation. University of Washington Press, Washington, DC, pp 73–90
Macarthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Masello JF, Mundry R, Poisbleau M et al (2010) Diving seabirds share foraging space and time within and among species. Ecosphere 1:art19. doi:10.1890/ES10-00103.1
Matthews LH (1932) Lobster-krill. Discov Rep 5:467–484
Miller AK, Karnovsky NJ, Trivelpiece WZ (2009) Flexible foraging strategies of gentoo penguins Pygoscelis papua over 5 years in the South Shetland Islands, Antarctica. Mar Biol 156:2527–2537
Miller AK, Kappes M, Trivelpiece SG, Trivelpiece WZ (2010) Foraging-niche separation of breeding gentoo and chinstrap penguins, South Shetland Islands, Antarctica. Condor 112:683–695
Oksanen J, Blanchet FG, Kindt R et al (2015) vegan: community ecology package. R package version 2.2-1. http://CRAN.R-project.org/package=vegan
Otley HM, Clausen AP, Christie DJ, Pütz K (2005) Aspects of the breeding biology of the gentoo penguin Pygoscelis papua at Volunteer Beach, Falkland Islands, 2001/2002. Mar Ornithol 33:167–171
Peay KG, Kennedy PG, Davies SJ et al (2010) Potential link between plant and fungal distributions in a dipterocarp rainforest: community and phylogenetic structure of tropical ectomycorrhizal fungi across a plant and soil ecotone. New Phytol 185:529–542
Pinkas L, Oliphant M, Iverson I (1971) Food habits of albacore, bluefin tuna and bonito in California waters. Fish Bull 152:1–105
Polito MJ, Trivelpiece WZ (2008) Transition to independence and evidence of extended parental care in the gentoo penguin (Pygoscelis papua). Mar Biol 154:231–240
Polito MJ, Trivelpiece WZ, Karnovsky NJ et al (2011) Integrating stomach content and stable isotope analyses to quantify the diets of pygoscelid penguins. PLoS One 6:e26642
Putz K, Ingham RJ, Smith JG, Croxall JP (2001) Population trends, breeding success and diet composition of gentoo Pygoscelis papua, magellanic Spheniscus magellanicus and rockhopper Eudyptes chrysocome penguins in the Falkland Islands. Polar Biol 24:793–807
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Ratcliffe N, Trathan PN (2011) A review of the diet and at-sea distribution of penguins breeding within the CCAMLR convention area. CCAMLR Sci 19:75–114
Reid K, Hill SL, Diniz TC, Collins MA (2005) Mackerel icefish Champsocephalus gunnari in the diet of upper trophic level predators at South Georgia: implications for fisheries management. Mar Ecol Prog Ser 305:153–161
Reilly PN, Kerle JA (1981) A study of the Gentoo penguin Pygoscelis papua. Notornis 28:189–202
Romano MD, Piatt JF, Roby DD (2006) Testing the junk-food hypothesis on marine birds: effects of prey type on growth and development. Waterbirds 29:407–414
Ropert-Coudert Y, Kato A, Wilson RP, Cannell B (2006) Foraging strategies and prey encounter rate of free-ranging little penguins. Mar Biol 149:139–148
Smale MJ, Watson G, Hecht T (1995) Otolith atlas of southern African marine fishes.Ichthyological monographs. J.L.B. Smith Institute of Ichthyology, Grahamstown
Stephens DW, Krebs J (1986) Foraging theory. Princeton University Press, Princeton
Tapella F, Lovrich GA (2006) Morphological differences between “subrugosa” and “gregaria” morphs of adult Munida (Decapoda: Anomura: Galatheidae) from the Beagle Channel, southern South America. J Mar Biol Assoc UK 86:1149–1155
Thompson KR (1994) Predation on Gonatus antarcticus by Falkland Islands seabirds. Antarct Sci 6:269–274
Trivelpiece WZ, Bengtson JL, Trivelpiece SG et al (1986) Foraging behavior of gentoo and chinstrap penguins as determined by new radiotelemetry techniques. Auk 103:777–781
Trivelpiece WZ, Trivelpiece SG, Volkman NJ (1987) Ecological segregation of Adélie, gentoo, and chinstrap penguins at King George Island, Antarctica. Ecology 68:351–361
Van Heezik Y (1990) Seasonal, geographical, and age-related variations in the diet of the yellow-eyed penguin (Megadyptes antipodes). N Z J Zool 17:201–212
Van Heezik Y, Seddon P (1989) Stomach sampling in the yellow-eyed penguin: erosion of otoliths and squid beaks [Erosión de otolitos y picos de calamares en el estómago de Pingüinos (Megadyptes antipodes)]. J Field Ornithol 60:451–458
Volkman NJ, Presler P, Trivelpiece W (1980) Diets of pygoscelid penguins at King George Island, Antarctica. Condor 82:373–378
Weiss F, Furness RW, McGill R et al (2009) Trophic segregation of Falkland Islands seabirds: insights from stable isotope analysis. Polar Biol 32:1753–1763
Whitehead PJP, Nelson GJ, Wongratana T (1985) Clupeoid fishes of the world (suborder Clupeoidei): Chirocentridae, Clupeidae, and Pristigasteridae. Food and Agriculture Organisation, London
Williams TD (1991) Foraging ecology and diet of gentoo penguins Pygoscelis papua at South Georgia during winter and an assessment of their winter prey consumption. Ibis 133:3–13
Williams TD, Rothery P (1990) Factors affecting variation in foraging and activity patterns of gentoo penguins (Pygoscelis papua) during the breeding season at Bird Island, South Georgia. J Appl Ecol 27:1042–1054
Wilson RP (1984) An improved stomach pump for penguins and other seabirds. J Field Ornithol 55:109–112
Wilson RP, Alvarrez B, Latorre L et al (1998) The movements of gentoo penguins Pygoscelis papua from Ardley Island, Antarctica. Polar Biol 19:407–413
Wilson RP, Scolaro JA, Grémillet D et al (2005) How do magellanic penguins cope with variability in their access to prey. Ecol Monogr 75:379–401
Xavier JC, Cherel Y (2009) Cephalopod beak guide for the Southern Ocean. British Antarctic Survey, Cambridge
Acknowledgments
This project was generously supported by the Rufford Small Grants Foundation, John Cheek Trust, Falkland Islands Environmental Planning Department, and Nelson Mandela Metropolitan University Research Capacity Department. Additional stipends were provided by the National Research Foundation of South Africa. Special thanks to Zhanna Shcherbich and staff of the Falkland Islands Fisheries Department, and Dr. Yves Cherel (CNRS, France) for assistance in sample identification. We are grateful to Jan Cheek and wardens of Johnsons Harbour for access to the study colony. We are extremely thankful to the volunteers who assisted with sample collection.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Handley, J.M., Baylis, A.M.M., Brickle, P. et al. Temporal variation in the diet of gentoo penguins at the Falkland Islands. Polar Biol 39, 283–296 (2016). https://doi.org/10.1007/s00300-015-1781-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1781-1