Abstract
Predation is a major ecological and evolutionary driver of natural populations, greatly influencing fitness and behaviour of prey species. Small, long-lived petrels are vulnerable to predation at the breeding colonies and are expected to evolve behavioural strategies to minimize predation risks. Using an automatic nest monitoring system and nightly aerial counts, we examined the effect of vegetation cover and moonlight on colony attendance patterns and levels of burrow activity of breeding thin-billed prions, Pachyptila belcheri, on New Island, Falkland Islands. We further investigated how these parameters were related to predation by Falkland skuas. We monitored up to 32 nests in two habitats, one with Tussock grass and one with low vegetation cover. Individuals in both areas were more active at the nest before hatching, and those breeding in the low cover habitat were more active and arrived at the colony earlier, which might reflect an effect of reaction time over predation risk. Nocturnal activity peaks shifted in time as the season progressed, indicating behavioural adjustments to sunrise hours. Moon phase did not affect attendance and activity levels of breeders in either habitat or overall aerial activity, but influenced arrival time at the colony during chick-rearing, individuals arriving later in periods of full moon. Skua capture rates were positively correlated with aerial and nest activity but not with overall breeder attendance and were unaffected by moon phase. Thin-billed prions activity budgets are influenced by environmental parameters that affect their likelihood of being predated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is a major demographic and evolutionary driver of natural populations, greatly influencing survival, reproductive success and behaviour of prey species (Martin 1996; Lima 1998), which in turn are likely to adopt strategies that allow them to minimize survival costs (Harfenist and Ydenberg 1995). Especially when predation represents a major cause of mortality, anti-predator behaviour in prey is strongly selected for and may include habitat choice and temporal and/or spatial segregation of activity patterns that minimize encounters with predators (for a review, see Lima and Dill 1990).
The physical structure of the nesting habitat can provide shelter from predators, especially for ground-nesting birds. Vegetation cover improved reproductive success in gulls and provided significant shelter against avian predators in nocturnal auklets (Pierotti 1982; Miyazaki 1996). In the latter species, experimental removal of vegetation cover resulted in an increase in predator density and attacks towards breeding auklets which, in response, arrived later at the nests (Miyazaki 1996).
Most pelagic seabirds only come ashore during the breeding season and at this time they are vulnerable to native and introduced predators (e.g., Boersma et al. 1980; Watanuki 1986; Weidinger 1998; Nordstrom and Korpimaki 2004; Catry et al. 2007; Wanless et al. 2007). For example, gulls kill approximately 13% of the adult Leach’s storm-petrels (Oceanodroma leucorrhoa) annually on Daikoku Island (Watanuki 1986), which must represent a strong selective pressure. With very few exceptions, small petrels are nocturnal at the breeding colonies (Warham 1990), and nocturnality in this group has likely evolved in response to predation and/or prey availability (Imber 1975; Brooke and Prince 1991; Klomp and Furness 1992). Most studies examining the effects of predation pressure and ambient light on the behaviour of small petrels have found that aerial activity and vocalization levels of non-breeders are reduced during moonlit nights (e.g. Bretagnolle 1990; Mougeot and Bretagnolle 2000b; Boersma and Silva 2001). Conversely, the behaviour of breeders is poorly documented, but a few studies suggest that their activity is unaffected by light variation (Storey and Grimmer 1986; Bretagnolle 1990; but see Keitt et al. 2004). An exception to this trend is the small Leach′s Storm-Petrels, where both breeders and non-breeders, as determined by brood patch condition, decreased the frequency of colony visitation during moonlit nights (Watanuki 2002).
The thin-billed prion, Pachyptila belcheri, is a medium-sized petrel that breeds in the sub-Antarctic (Marchant and Higgins 1990). Although some aspects of its biology have been studied well (e.g., breeding ecology: Strange 1980; Silva et al. 2007, foraging behaviour and chick provisioning: Chastel et al. 1995; Duriez 2000; Quillfeldt et al. 2006), their nocturnal activity budgets, as in most other small petrels, have not been documented in detail. On New Island, Falkland Islands, there is an estimated breeding population of 2 million pairs (Catry et al. 2003). They nest mainly in soft peat areas with reduced plant cover of short grass and in lower numbers in areas of Tussock (Poa flabellata). Higher densities in open areas do not reflect preference, but availability of nesting sites. In fact, prions nest at the base of tussock plants, and consequently, nest densities are limited by the density of the plants. One of their main predators at this colony is the Falkland skua, Catharacta a. antarctica, and those nesting close to the main concentrations of prion burrows seem to rely mainly on them as a food source. Predation pressure on adult prions during the breeding season, which lasts for up to 6 months, most likely constitutes a strong selective force for predator avoidance behaviour to evolve in this population. This study investigates the effect of vegetation cover and the phase of the moon on the behaviour of this small petrel and specifically addresses the following predictions: areas with high vegetation cover, such as the tussock habitat, will show (1) higher burrow activity and (2) earlier arrivals to the nest sites, since individuals are less exposed to predation relative to more open areas. Also, during periods of full moon, (3) individuals will decrease attendance and activity levels at the nest and (4) arrive later at the colony. To compare differences in the attendance profiles and activity levels of breeding thin-billed prions between habitats, we used an automatic nest logging system, which allowed to simultaneously, and unambiguously, monitor the two members of each pair.
Materials and methods
The study was carried out on New Island, Falkland Islands (51°73′S, 61°31′W) from mid-November 1999 until the end of February 2000. Thin-billed prions lay a single egg during the first half of November, and hatching begins at the end of December. Fledging occurs when nestlings are between 45 and 54 days old, typically during the second half of February (Strange 1980; Silva unpubl. data). At the beginning of the study, we randomly selected 16 nests in an exposed area of peat (mean 5% of plant cover, mostly Common Groundsel Senecio vulgaris and Yorkshire Fog Holcus lanatus, mean maximum plant height of 64.4 cm), hereafter designated as “low cover”. This area has approximately 50 prion burrow entrances per 50 m2 (transect 16 of Catry et al. 2003). We also randomly selected 16 nests in an area covered almost exclusively by Tussock (mean plant cover of 74%, mean maximum plant height of 160.0 cm), hereafter designated as “high cover”. This area has between 30 and 50 nest entrances per 50 m2 (transect 13 of Catry et al. 2003). In each habitat, the studied nests were within a 60-m-diameter area. A small hole was cut in the roof of each nest chamber to allow easy access to adults and chick. The hole was covered by a flat stone and securely sealed to protect the nest from rain and predators. In an area between the two habitats, we randomly selected 30 chicks, which we also measured and weighed (although the adults from these nests were not manipulated) and acted as a control group.
Burrows were equipped with an automatic monitoring system that allowed the simultaneous detection of activity of the two adults in up to 16 nests in each area over the duration of the study. This system records entrances and departures from the nest, being triggered by a small magnet (<1 g) attached to a tail feather (see Granadeiro et al. 1998 for details of the system). PVC tubes with two coils were placed at the entrance of the studied nests. The passage of the magnet sequentially activated these coils, determining the direction (nest entry or exit) of the movement. Opposite orientations of the magnet induced different signals that enabled discrimination between mates.
Adults were captured at the nest at the beginning of the incubating period, ringed or identified if recaptured, sexed by vent measurements (Boersma and Davies 1987), measured and weighed. The magnet was glued to a central tail feather and covered with cloth-backed tape for additional security. Males and females were also marked with a different colour spot on the forehead to allow identification at the nest without further handling. Data from the monitoring system were rarely checked against field observations at night during chick-rearing, but during incubation whenever eggs were left unattended. Adult mass was not significantly different between the two areas (t 66 = −0.31, P > 0.1), and chick growth rate was similar between habitats and no different from the control group (F 4,60 = 0.78, P > 0.5), suggesting no significant differences in quality of the breeders between the two habitats.
The system only required a one-time manipulation of each individual, and measuring and magnet attachment took up to 10 min per bird. Birds lost the magnet on two occasions because the tail feather broke, and these individuals were excluded from all the analyses until the magnet was replaced. Data from the monitoring system were collected between 24 November and the end of February, and up to 16 nests were simultaneously monitored in each area. Black rats Rattus rattus predated four chicks in the Tussock and one chick in the low cover areas when they were approximately 15 days old. These nests were included in most analyses until predation took place since it is unlikely that adult attendance or activity patterns were affected up to that moment.
Remains of prions were collected every other day in nineteen skua breeding territories, from the beginning of December to the end of February. All skua territories were located between the two prion study areas and were chosen based on the presence of a pair and an initiated nest structure. All territories were cleaned of existing remains before monitoring started. As in other sub-Antarctic colonies, skuas catch prions on the ground as they arrive at the colony (Mougeot and Bretagnolle 2000b), and prion remains, consisting usually of the two wings connected by the sternum, are found scattered on skua breeding territories. During the study period, we collected only fresh remains and we only counted the right wings to account for the few occasions when only one wing was found. Since prions may have been consumed by skuas away from the breeding territories, these predation rates are a conservative estimate of how many prions were taken during the period of the study.
Immature prions visit the breeding colonies, prospecting for mates and nests, and are thus also exposed to predation risks, possibly higher than those faced by breeders (Bretagnolle et al. 1998). Aerial activity of both age groups was quantified by performing ten consecutive 1-min counts of all flying birds crossing the beam of a headlight pointing vertically, every night throughout the whole period of the study. The counts were carried out in an area adjacent to the low cover habitat, approximately 2 h after sunset.
The effect of moonlight was examined for 7-day window periods around the peaks of full and new moon. During incubation, there were up to 10 days of full moon and 7 days of new moon. During chick-rearing, we considered up to 14 days of new moon (depending on the date of hatching) and 14 days of full moon when considering time of arrival, and 7 days of full moon and new moon when looking at differences in attendance and nest activity to account for the seasonal decline of these parameters. Arrival time data are reported relative to civil twilight time (as calculated in www.esrl.noaa.gov/gmd/grad/solcal) of the mid-point date for the incubation and chick-rearing periods.
Data from the automatic monitoring system were analysed in terms of colony attendance, estimated by the proportion of breeders with magnet present at the colony on a given night, levels of activity at the nest, defined as the number of single movements, either an entry or exit from the nest, performed by breeders present at the colony on a given night, and arrival times. Aerial activity data, estimated by the counts, refer to both breeders and non-breeders. We used generalized linear mixed models (GLMM) to account for repeated measurements on the same individuals and also to account for a possible correlational structure in the behaviour of birds in a pair. We used the package nlme (Pinheiro et al. 2009) to fit the models, using individuals nested within nest as a random factor. The significance of the fixed terms was tested using log-likelihood tests on nested models, with and without the variables of interest. The statistics are presented as averages for individual birds or nests, as appropriate. Results are presented as mean ± S.D. unless otherwise stated.
Results
Colony attendance and nest activity levels throughout the breeding season
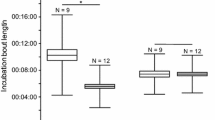
Attendance oscillated around 50% in both habitats during incubation and the beginning of chick-rearing, indicating that on average, one of the parents was present at the nest, but decreased significantly thereafter (low cover habitat: r = −0.73, n = 36 days, P < 0.0001; high cover habitat: r = −0.34, n = 37 days, P = 0.037) (Fig. 1).
Proportion of breeders visiting the colony during incubation and chick-rearing, as assessed by the automatic logging system. Full moon (open circles) and new moon (filled circles) periods are indicated. Hatched vertical line represents mean hatching date. Interrupted lines represent days of system failure
There was a difference in the pattern of activity during incubation and chick-rearing between the two habitats (Fig. 2). During incubation, adults in the low cover area were on average significantly more active at the nest than adults in the high cover area (low cover area: 2.84 ± 3.53 movements per night (n = 32 individuals), high cover area: 1.13 ± 1.05 movements per night (n = 28 individuals), log-likelihood test = 4.13, P = 0.042, but not during chick-rearing (low cover area: 2.49 ± 0.84 movements per night (n = 29 individuals), high cover area: 2.35 ± 1.17 movements per night (n = 31 individuals), log-likelihood test = 1.93, P = 0.164).
There was an initial period of increased activity after hatching, coinciding with the brooding period. In both habitats, the number of movements per bird eventually converged to an average of two movements per night (Fig. 2), indicating that during this period, adults entered the nest, fed the chick and left.
Levels of prion aerial activity, as indicated by night-time counts of flying birds, varied widely throughout the breeding season, with no apparent underlying pattern. This counting method was highly repeatable during the 10-min periods, as the first 5-min counts were significantly correlated with the second 5-min counts (r = 0.92, n = 100, P = 0.0001). The numbers of prions crossing the beam of light ranged between 0.20 ± 0.63 and 25.7 ± 5.14 prions per minute (n = 10 counts). Most peaks of aerial activity occurred during incubation, a period during which there was no relation between aerial activity and nest attendance of breeders (Fig. 3). During chick-rearing, aerial activity and attendance of breeders were positively correlated in the low cover (Spearman r = 0.52, n = 53, P < 0.0001) and high cover habitats (Spearman r = 0.44, n = 54, P < 0.0008) (Fig. 3). We also found a positive correlation between aerial activity and activity at the nest in the low cover habitat (r = 0.26, n = 82, P < 0.02) but not in the high cover habitat (P > 0.05).
Activity levels throughout the night
Nocturnal activity patterns were similar among habitats (Fig. 4a, b). During incubation, adults in both areas increased burrow activity levels, on average, ca. 2.30 h after sunset. The number of movements peaked around 02:00, ca. 2.30 h before sunrise, after which nest activity dropped sharply, as birds either returned to sea or to their burrows. Approximately 1 h before sunrise, birds stopped moving (Fig. 4a). Throughout the night, the total number of movements per unit of time in the Tussock was lower than in the low cover habitat. During chick-rearing, nocturnal activity of breeders in both areas reflected the pattern of attendance of the adults as they visited the colony to feed the chick (Fig. 4b). Numbers progressively build up as birds arrived at the colony approximately two and half hours after sunset, decreased slightly after this period and peaked again before dawn, at around 02:45, as birds left the colony. Although time of arrival at the colony during incubation and chick-rearing did not change significantly with the change of sunset hours, departure times were adjusted to sunrise hours, the last adults leaving the colony later during chick-rearing (Fig. 4a, b).
a Levels of nocturnal burrow activity during incubation in the two habitats. Vertical hatched lines indicate the average hour of sunset and sunrise for this period. The number of movements per bird was grouped into 15-min time intervals. Values presented are mean ± s.e. b Levels of nocturnal burrow activity during chick-rearing in the two habitats. Vertical hatched lines indicate the average times of sunset and sunrise for this period. The number of movements per bird was grouped into 15-min time intervals. Values presented are mean ± s.e
Effect of moonlight
There was no difference in the attendance levels of breeders between full and new moon periods during chick-rearing in either habitat, although the effect of habitat just failed to reach statistical significance (repeated measures ANOVA (on arcsin-root transformed attendance), with habitat as a main factor: habitat: F 1,44 = 3.89, P = 0.055; moon phase: F 1,44 = 0.32, P > 0.5; interaction F 1,44 = 3.19, P = 0.08). There was a tendency for chick-rearing adults to visit more frequently in the low cover area, irrespective of moon phase.
The phase of the moon did not also have an effect on the activity levels of breeders (number of single movements per bird) in both habitats during either incubation or chick-rearing (all log-likelihood tests were non-significant) (Table 1; Fig. 2). In contrast, both habitat and the phase of the moon had a significant effect on the breeders’ time of arrival to the colony (Table 1). During incubation, individuals in the high cover area arrived significantly later relative to birds breeding in the low cover area, irrespective of moon phase (log-likelihood tests: effect of moon = 1.31, P = 0.25, effect of area = 7.9, P = 0.005) (Table 1). During chick-rearing, breeders arrived significantly later in periods of full moon irrespective of the breeding habitat ((log-likelihood tests; effect of moon = 11.2, P < 0.001, effect of habitat = 1.96, P = 0.161) (Table 1).
Moon phase did not have an effect on the total aerial activity registered during either incubation or chick-rearing (all log-likelihood tests, P > 0.05)
Predation by skuas
A total of 764 prion remains was collected from 19 skua territories, corresponding to an average of 0.53 prions per day per skua territory. No other avian remains were found, except for a skua chick leg bone found in a pellet. During the skuas’ chick-rearing period, fish (whole or remains) were found consistently in only two territories.
Prion intake by skuas decreased significantly over the breeding season (r = −0.69, n = 34 days, P < 0.001), reflecting the decrease in activity, both of breeding and non-breeding prions, as the season progressed. In fact, we found a positive correlation between the number of prions collected on the skua territories and the number of flying prions counted on the previous night (r = 0.62, n = 33 days, P < 0.0002), although the same was not true for breeder attendance (r = 0.30, n = 34 days, P = 0.08). Capture rates of skuas were also positively correlated with the number of single movements per bird (reflecting prion nest activity) visiting the colony on the previous night (r = 0.43, n = 34 days, P = 0.01).
Skua predation rates were not affected by the phase of the moon, as we found no significant differences in capture rates between periods of full and new moon (paired t-test15, P > 0.05).
Discussion
Despite the large number of studies focusing on petrel breeding biology (e.g, Warham 1990), only a handful have examined in detail patterns of colony attendance of small petrels, and specifically those of breeders. The monitoring system used in this study provided an efficient and non-intrusive way of discriminating the effects of vegetation cover and moon phase on attendance and activity patterns of breeding thin-billed prions (see also Granadeiro et al. 1998). Contrary to our predictions, breeding adults were more active at the nest in the habitat with the lowest level of vegetation cover, although only during incubation. This is surprising since skuas were often observed hunting on the ground in low cover areas, but were very seldom seen hunting in tussock areas at night. This result may reflect a trade-off between cover from predators and ability to escape rapidly. Although in the low cover habitat adults presumably face a higher risk of predation by skuas, the absence of dense vegetation allows the birds to move and take-off rapidly, as petrels do not move well in tall vegetation (Warham 1990, 1996). Also, non-vegetated areas are more exposed to the generally moderate to strong winds, and consequently, birds in the low cover area might afford to be more active if they can detect incoming predators, manoeuvre and get airborne more rapidly. This might also explain earlier arrivals to the colony in this habitat, a pattern especially seen during incubation. As the low cover habitat has a higher density of nests and a larger proportion of nests sharing the same entrance, a higher number of movements in this area may also be the result of more social interactions, involving both breeders and non-breeders (Granadeiro et al. 1998). In the year of the study, there was lower breeding success in the high cover habitat, which might have been the result of rat predation (Catry et al. 2007). However, we have no evidence suggesting that rats influence adult behaviour.
We also found an effect of ambient light on the patterns of attendance of the breeders. Nocturnal activity in both habitats shifted in time as the season progressed suggesting that birds adjusted their behaviour particularly to sunrise hours, a pattern also seen in other small petrel species (Boersma and Silva 2001). This dynamic strategy is likely to reduce predation risk, by concentrating phases of higher activity during the darker periods of the night.
Several reports of decreased colony attendance during periods of full moon in petrels, particularly by smaller species, have been interpreted as a strategy to reduce the risk of predation (Boersma et al. 1980; Watanuki 1986; Bretagnolle 1990; Mougeot and Bretagnolle 2000a, b). Most studies found that the intensity of moonlight influences mainly non-breeding individuals, which reduced both the levels of colony attendance as well as vocal activity (Bretagnolle 1990; Mougeot and Bretagnolle 2000b). Unlike other studies, we found no effect of moon phase on the patterns of colony attendance of prions, including breeders and non-breeders, as estimated by our daily counts (but see Harris 1969; Watanuki 1986; Mougeot and Bretagnolle 2000a). Furthermore, by looking specifically at the activity budgets of breeding individuals, we also found that the phase of the moon did not affect the patterns of colony attendance and levels of activity of incubating and chick-rearing adults (see also Storey and Grimmer 1986). Interestingly, adults feeding chicks in both habitats arrived significantly later at the colony during periods of full moon. A similar pattern found in Leach’s Storm-Petrel was interpreted as a predator avoidance behaviour, as their main predators (Larus gulls) were found to be more active on moonlit nights (Watanuki 1986; Huntington et al. 1996). Although we did not find a relationship between skua capture rates and moon phase, it is possible that skuas and other predators are more active during bright nights and prions respond accordingly, by arriving later in periods of full moon. Alternatively, prions might increase and/or extend their foraging activity in brighter nights as suggested in other petrels (Klomp and Furness 1992; Phalan et al. 2007; Yamamoto et al. 2008).
On sub-Antarctic islands, skuas show high levels of prey specificity and their diets reflect local species abundance (Mougeot et al. 1998). Although skuas breeding on or near prion nesting areas seem to rely essentially on this species as prey, capture rates were low, ca. 0.5 prion/day/territory, and significantly lower than those of skuas on other sub-Antarctic islands (Young 1976; Mougeot et al. 1998). This suggests that skua diet must include other prey species such as fish, crustaceans and occasionally penguin eggs and chicks, which are under-represented in prey remains left on their territory. Another plausible explanation is that skuas are hunting and consuming prey away from their nests.
Capture rates were found to be highest during prion incubation and hatching periods, similar to the pattern found with Brown skuas in Mayes Island (Mougeot et al. 1998). In their study, maximum capture rates were found to be related to periods of high colony attendance by non-breeding petrels (Mougeot and Bretagnolle 2000b). We found that nightly intake of prions was positively correlated with the number of prions flying on the previous night but not correlated with the number of visiting breeders, suggesting that also on New Island, skua diet might include a significant proportion of non-breeding individuals. This age group spends more time flying and moving around above ground while looking for burrows and advertising for potential mates (Bretagnolle et al. 1998), which increases their exposure to predation by skuas. This relation also shows that skua predation rates are a function of prion availability, suggesting that skuas need to rely on other prey species particularly on nights of low prion activity.
References
Boersma PD, Davies EM (1987) Sexing monomorphic birds by vent measurements. Auk 104:779–783
Boersma PD, Silva MC (2001) Fork-tailed storm-petrel (Oceanodroma furcata). In: Poole A, Gill F (eds) The Birds of North America. Birds of North America, Philadelphia, no. 569
Boersma PD, Wheelwright NT, Nerini MK, Wheelwright ES (1980) The breeding biology of the fork-tailed storm-petrel (Oceanodroma furcata). Auk 97:268–282
Bretagnolle V (1990) Effet de la lune sur l’activité des petrels (classe Aves) aux îles selvages (Portugal). Can J Zool 68:1404–1409
Bretagnolle V, Genevois F, Mougeot F (1998) Intra- and inter-sexual functions in the call of a non-passerine bird. Behaviour 135:1161–1184
Catry P, Campos A, Segurado P, Silva MC, Strange IJ (2003) Population census and nesting habitat selection if thin-billed prion Pachyptila belcheri on New Island, Falkland Islands. Polar Biol 26:202–207
Catry P, Silva MC, MacKay S, Campos A, Masello J, Quillfeldt P, Strange IJ (2007) Can thin-billed prions Pachyptila belcheri breed successfully on an island with introduced rats, mice and cats? The case of New Island, Falkland Islands. Polar Biol 30:391–394
Chastel O, Weimerskirch H, Jouventin P (1995) Body condition and seabird reproductive performance: a study of three petrel species. Ecology 76:2240–2246
de Brooke ML, and Prince PA (1991) Nocturnality in seabirds. Proc Int Orn Congres XX:1113–1121
Duriez O (2000) Regulation of chick provisioning in the thin-billed prion: an interannual comparison and manipulation of parents. Can J Zool 78:1275–1283
Granadeiro JP, Burns MD, Furness RW (1998) Patterns of activity and burrow attendance in Cory’s Shearwater Calonectris diomedea as revealed by a novel logging technique. Ibis 140:458–466
Harfenist A, Ydenberg RC (1995) Parental provisioning and predation risk in rhinoceros auklets (Cerorhinca monocerata): effects on nestling growth and fledging. Behav Ecol 6:82–86
Harris MP (1969) The biology of storm-petrels in the Galapagos Islands. Proc Calif Acad Sci 37:95–166
Huntington CE, Butler RG, Mauck RA (1996) Leach’s storm-petrel (Oceanodroma leucorrhoa). In: Poole A, Gill F (eds) The Birds of North America. Birds of North America, Philadelphia, no. 233
Imber MJ (1975) Behaviour of petrels in relation to the moon and artificial lights. Notornis 22:302–306
Keitt BS, Tershy BR, Croll DA (2004) Nocturnal behavior reduces predation pressure on black-vented shearwaters Puffinus opisthomelas. Mar Ornithol 32:173–178
Klomp NI, Furness RW (1992) Patterns of chick feeding in Cory’s Shearwater and the associations with ambient light. Colon Waterbirds 15:95–102
Lima SL (1998) Stress and decision-making under the risk of predation: recent developments from behavioral, reproductive and ecological perspectives. Adv Study Behav 27:215–290
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Marchant S, Higgins PJ (eds) (1990) The handbook of Australian, New Zealand and Antarctic Birds. Oxford University Press, Melbourne
Martin TE (1996) Nest predation and avian life-history evolution in Europe versus North America: a possible role for humans? Am Nat 147:1028–1046
Miyazaki M (1996) Vegetation cover, kleptoparasitism by diurnal gulls, and timing of arrival of nocturnal Rhinoceros Auklets. Auk 113:698–702
Mougeot F, Bretagnolle V (2000a) Predation as a cost of sexual communication in nocturnal seabirds: an experimental approach using acoustic signals. Anim Behav 60:647–656
Mougeot F, Bretagnolle V (2000b) Predation risk and moonlight avoidance in nocturnal seabirds. J Avian Biol 31:376–386
Mougeot F, Genevois F, Bretagnolle V (1998) Predation on burrowing petrels by the brown skua (Catharacta skua lonnbergi) at Mayes Island, Kerguelen. J Zool 244:429–438
Nordstrom M, Korpimaki E (2004) Effects of island isolation and feral mink removal on bird communities on small islands in the Baltic Sea. J Anim Ecol 73:424–433
Phalan B, Phillips RA, Silk JRD, Afanasyev V, Fukuda A, Fox J, Catry P, Higuchi H, Croxall JP (2007) Foraging behaviour of four albatross species by night and day. MEPS 340:271–286
Pierotti R (1982) Habitat selection and its effect on reproductive output in the Herring gull in Newfoundland. Ecology 63:854–868
Pinheiro J, Bates D, DebRoy S, Sarkar D, R. Core team (2009) nlme: linear and nonlinear mixed effects models. R Package Version 3:1–92
Quillfeldt P, Maselalo J, Strange IJ, Buchanan KL (2006) Begging and provisioning of thin-billed prions, Pachyptila belcheri, are related to testorone and corticosterone. Anim Behav 71:1359–1369
Silva MC, Boersma PD, MacKay S, Strange IJ (2007) Egg size and parental quality in thin-billed prions, Pachyptila belcheri: effects on offspring fitness. Anim Behav 74:1403–1412
Storey AE, Grimmer BL (1986) Effect of illumination on the nocturnal activities of Manx Shearwaters: colony avoidance or inconspicuous behavior? Bird Behav 6:85–89
Strange IJ (1980) The thin-billed prion, Pachyptila belcheri, at New Island, Falkland Islands. Le Gerfaut 70:411–445
Wanless RM, Angel A, Cuthbert RJ, Hilton GM, Ryan PG (2007) Can predation by invasive mice drive seabird extinctions? Biol Lett 3:241–244
Warham J (1990) The petrels. Their ecology and breeding systems. Academic Press, London
Warham J (1996) The behaviour. Population biology and physiology of the petrels. Academic Press, London
Watanuki Y (1986) Moonlight avoidance behavior in leach’s storm-petrels as a defense against slaty-backed Gulls. Auk 103:14–22
Watanuki Y (2002) Moonlight and activity of breeders and non-breeders of Leach′s storm-petrels. J Yamashina Inst Ornithol 34:245–249
Weidinger K (1998) Effect of predation by skuas on breeding success of the Cape petrel Daption capense at Nelson Island, Antarctica. Polar Biol 20:170–177
Yamamoto T, Takahashi A, Yoda K, Katsumata N, Watanabe S, Sato K, Trathan PN (2008) The lunar cycle affects at-sea behaviour in a pelagic seabird, the streaked shearwater, Calonectris leucomelas. Anim Behav 76:1647–1652
Young EC (1976) Behavioural ecology of lonnbergi skuas in relation to environment on the Chatham Islands. NZ J Zool 5:401–416
Acknowledgments
The authors are grateful to S. MacKay for invaluable help with fieldwork, database organization and review of early versions of the manuscript. We are also grateful to the Physics Department, University of Washington, for access to equipment necessary to roll the coils, and to M. Burns, University of Glasgow for help with system maintenance. We thank the owners and crew of the “Professor Molchanov” for transportation to New Island. P Catry provided useful comments on the manuscript. Funding was provided by grants from Fundação para a Ciência e a Tecnologia (BD/9356/96 and BPD/22276/05) to MCS and also by the project Albatroz (PTDC/MAR/099366/2008). We also thank the New Island Conservation Trust, Falkland Islands Government and Wildlife Conservation Society for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, M.C., Granadeiro, J.P., Boersma, P.D. et al. Effects of predation risk on the nocturnal activity budgets of thin-billed prions Pachyptila belcheri on New Island, Falkland Islands. Polar Biol 34, 421–429 (2011). https://doi.org/10.1007/s00300-010-0897-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0897-6