Abstract
Predation risk on birds is often an important source of natural selection that shapes parental care and may promote behavioral changes. Parents can often estimate certain risks and adjust their behavior to reduce the likelihood of nest predation. The fragmentation of habitats is one of the main consequences of loss of habitats, and in general, for birds breeding in smaller patches, their daily nest-survival rate is lower due to increased nest predation. Since nest survival is an estimate of predation risk in the environment, we evaluated the daily survival rate (DSR) for nests of spectacled tyrants (Hymenops perspicillatus) and parental care behavior on fragmented and unfragmented grasslands. We conducted nest searching and monitoring during the 2012–2013 breeding season in small patches and in a continuous patch of grassland. In addition, parental activity was recorded using video monitoring. We found a lower DSR for the spectacled tyrant in fragmented grasslands, associated with increased nest predation risk; females showed a variation in parental care. This variation was evidenced by larger incubation bouts and lower visitation rate during the incubation period, and by a lower food delivery rate to nestlings, compensated by larger prey sizes. The results show that fragmentation not only reduces the fitness of individuals and impacts adversely on population, but individuals are also subjected to a strong selection pressure, and their reproductive success may depend to some extent on the ability of parents to estimate at least certain predation risk and adjust their behavior in this regard.
Zusammenfassung
Unterschiede in der Brutpflege beim Brillentyrann steht in Verbindung mit einer erhöhten Nest-Prädationsrate in fragmentiertem Grasland
Das Prädationsrisiko ist für Vögel eine wichtige Quelle natürlicher Selektion, die die Brutpflege formt und zu Verhaltensänderungen führen könnte. Oft können Eltern bestimmte Risiken abschätzen und ihr Verhalten anpassen, um die Wahrscheinlichkeit für Nest-Prädation zu reduzieren. Die Fragmentierung von Habitaten ist die wesentliche Folge von Habitatverlust, und generell ist für Vögel, die in kleineren Habitatflecken brüten, die auf den Tag umgerechnete Überlebensrate eines Nests (daily survival rate, DSR) aufgrund von höherer Nest-Prädation geringer. Weil Nest-Überlebensraten ein Schätzer für das Prädationsrisiko in der Umgebung sind, erhoben wir die DSR für Nester des Brillentyrann (Hymenops perspicillatus) zusammen mit Brutpflegeverhalten in fragmentiertem und unfragmentiertem Grasland. Wir führten Nestersuche und Nestmonitoring in der Brutsaison 2012–2013 für kleine Flecken und in einem zusammenhängenden Stück Grasland durch. Außerdem wurde das Brutpflegeverhalten mit Videomonitoring aufgenommen. Wir fanden, dass der Brillentyrann in fragmentiertem Grasland eine geringere DSR hatte, und die Weibchen zeigten in Verbindung mit dem erhöhten Prädationsrisiko eine Änderung im Brutpflegeverhalten. Diese Änderung zeigte sich in längeren Bebrütungsphasen und verringerter Anzahl dieser Phasen, und in selteneren Fütterungen, die aber durch größere verfütterte Beute kompensiert wurden. Die Ergebnisse zeigen, dass Habitat-Fragmentierung nicht nur die individuelle Fitness herabsetzt und sich negativ auf die Population auswirkt, sondern auch einen Selektionsdruck auf Individuen darstellt, indem ihr Reproduktionserfolg teilweise davon abhängen könnte, wie gut die Elterntiere zumindest bestimmte Prädationsrisiken abschätzen und mit Verhaltensänderungen darauf reagieren können.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nest predation is the primary cause of reproductive failure for most birds, and thus, represents an important source of natural selection (Ricklefs 1969; Martin 1995). Due to this, birds have developed different strategies to protect the nest and thus minimize predation risk. These strategies comprise diverse aspects such as crypsis, nest-site selection (i.e., nesting in concealed places in order to avoid predator’s detection) (Martin and Roper 1988; Weidinger 2002; Kearns and Rodewald 2013), passive defense (i.e., by adjusting parental care behavior) (Weidinger 2002; Eggers et al. 2005; Lima 2009), and ultimately, active nest defense to deter predators (Martin 1992; Pietz and Granfors 2005; Ellison and Ribic 2012).
Parental care is a behavior used by many taxa, including fish, reptiles, birds, and mammals. Over 90 % of bird species provide some kind of parental care (Kendeigh 1952), which essentially includes all those behaviors that increase the survivorship of eggs or chicks (Wesołowski 1994). Temporal and spatial variation in predation risk is thought to be one of the main selective forces to explain the adaptive adjustment of parental care. Thus, parents may recognize at least some predation risk cues in the environment, and accordingly adjust their reproductive investment (e.g., egg size, clutch size) (Fontaine and Martin 2006; Zanette et al. 2011), accompanying this investment with changes in nest attendance behavior, in order to avoid or reduce predation risk (Eggers et al. 2005; Fontaine and Martin 2006; Zanette et al. 2011). Birds use multiple sources of environmental information associated with predation in order to improve nest survival. Two types of information sources have been identified: private information, which is known to the individual only (e.g., their own nesting history), and public information, which is knowable to all (e.g., abundance of predators at a site; Wagner and Danchin 2010); both of them can be incorporated, for example, in order to adopt changes in parental care (e.g., Eggers et al. 2005; Fontaine and Martin 2006; Peluc et al. 2008; Chalfoun and Martin 2010).
Food limitation is considered the other key factor that may shape not only avian life history traits (e.g., clutch size; Lack 1947; Martin 1987; Sofaer et al. 2013), but also parental care behavior, since, for example, by decreasing the amount of food in the environment, parents spend less time in the nest, while the feeding rate of chicks decreases (Lack 1947; Martin 1987, 1996; Conway and Martin 2000). Conversely, an increase in food availability reverses these behaviors, and in turn, nest predation rate decreases (Ward and Kennedy 1996; Duncan Rastogi et al. 2006; Zanette et al. 2006), by increasing nest defense activities (Martin 1987; Lima 1998; Nagy and Holmes 2005), such as nest guarding, which can potentially deter predators (Arcese and Smith 1988; Martin 1992; Ward and Kennedy 1996).
In addition, parental care is under high selective pressure, which involves a balance between preventing starvation and ensuring the proper development of nestlings (Naef-Daenzer and Keller 1999; Tremblay et al. 2003), and avoiding attracting predators to the nest due to the conspicuousness of the parents during nest attendance (Skutch 1949; Martin et al. 2000a). Consequently, selection should have favored the evolution of behaviors to reduce nest detectability by visual predators. During the incubation period, the main activities for birds are the incubation of eggs, provision of food to incubating females from their mates, or females leaving the nest to forage for themselves. In this sense, for species in which only females incubate, the most efficient behaviors in terms of reducing detectability by predators are that the females increase the incubation bout lengths to reduce the activity at the nest (e.g., Conway and Martin 2000; Ferretti et al. 2005; Fontaine and Martin 2006; but see Zanette et al. 2011), and that males decrease provision of food to incubating females (Ghalambor and Martin 2002; Fontaine and Martin 2006). During the nestling period, the main activity of the parents is to provide food, remove fecal sacs and keep the nest in good clean condition. In this case, the possibility of avoiding predator detectability is related to the reduction of foraging trips (Eggers et al. 2005; Zanette et al. 2011; Ghalambor et al. 2013), but this would result in a lower supply of food to the nestlings, limiting energy toward chick growth, at last producing lower weight brood (Zanette et al. 2011). One way to compensate for this dilemma, at least in part, is to provide the nestlings with larger and/or higher quality food, which would reduce the rate of visits to the nest without being detrimental to final chick growth (Martin 1996; Martin et al. 2000b).
One of the main disturbances associated with natural habitat loss is fragmentation, which involves the generation of patches immersed in landscape matrices of some land use (Andrén 1994). Fragmentation may lead to a higher rate of population decrease than that predicted from habitat loss alone (Wilcove 1985; Andrén 1994). One of the main detrimental drivers due to fragmentation is the “edge effect”, which in the case of birds may result in an increased nest predation risk near edges (Donovan et al. 1997; Winter et al. 2000; Batáry and Báldi 2004, but see Lahti 2001). Increased nest predation at habitat edges may result from increased density, activity or species richness of predators at edges (Chalfoun et al. 2002), combined with increased detectability of nests at edges (Winter et al. 2000; Bollinger and Gavin 2004).
The aims of this study were to assess the daily nest-survival rate of the spectacled tyrant (Hymenops perspicillatus), which provides a robust estimate of the nest predation risk in the environment (e.g., Ghalambor and Martin 2001; Chalfoun and Martin 2010; Ghalambor et al. 2013), and to assess whether there is a variation in parental care in the spectacled tyrant associated with grassland fragmentation. Specifically, we focused on the effect of nest predation because during two breeding seasons prior to this study, we recorded a high nest predation pressure in small patches of grassland, which significantly manifested with a smaller nesting success of spectacled tyrants in small patches of grassland than in unfragmented grasslands (Pretelli 2015). In addition, we knew that the spectacled tyrant has high nest-site fidelity (see “Methods”). In this context, and assuming that parental care may be modulated by the predation risk, and that predation would be higher in fragmented habitats than in non-fragmented ones, we predict that, in fragmented grasslands, parental care behaviors will be related to decrease nest detection by predators.
Methods
Study area and species
The study was conducted in the southeast Pampas region, Buenos Aires Province, Argentina (Cabrera 1976). This region has suffered a huge landscape transformation due to the suitability of soils for agricultural development (Viglizzo et al. 2001; Paruelo et al. 2005). However, in the east of this province, native grasslands are still well represented, because wet conditions and saline soils discourage agricultural development (León et al. 1984; Viglizzo et al. 2001). Cortaderia selloana grasslands are one of the most abundant native tall grasslands. They are extensively distributed within nature reserves (Bilenca and Miñarro 2004), and also occur in the form of small grassland patches immersed in a landscape matrix of different land uses (Pretelli et al. 2013). The landscape that dominates the study area is an agricultural matrix addressed mainly to cattle grazing (80 %), while cropping and cultivation occupy <10 % of the area (León et al. 1984; Baldi et al. 2006).
The spectacled tyrant (~20 g) is a member of the Tyrannidae family that inhabits open lands, grassy areas near water bodies, marshes, and fields and pastures (Fitzpatrick 2004). The spectacled tyrant is distributed from southern Argentina to Paraguay, central Bolivia, Uruguay and southern Brazil (Fitzpatrick 2004). In our study area, this species is present in spring and summer (Pretelli et al. 2013), and uses almost exclusively C. selloana grassland to nest from mid-October to late January (Pretelli and Isacch 2013). Spectacled tyrants show high breeding-site fidelity, and normally after nesting attempts (successful or not), females re-nest in the same area both within the breeding season and in consecutive breeding seasons (Mattos et al. 2011). They build open-cup nests, modal clutch size is two eggs, and nestlings hatch after 16 days of incubation and fledge 14 days after hatching (Pretelli and Isacch 2013). The spectacled tyrant shows a marked sexual dichromatism, given that male has black plumage contrasted with white primary feathers, while the female is less conspicuous being a dark brown and rufous color (Fitzpatrick 2004).
Sampling design
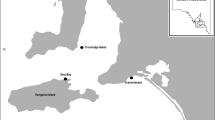
To assess the effect of grassland fragmentation on parental care behavior in the spectacled tyrant, we selected a large unfragmented patch of C. selloana (~900 ha) within the Mar Chiquita Biosphere Reserve (37°40′S, 57°23′W) (hereafter “reserve”). Moreover, two small patches of C. selloana growing in field margins along secondary unpaved roads (hereafter “patches”) were selected. During the sampling period, patches were surrounded by fields dedicated mainly to livestock production. Patches and reserve grasslands were not grazed by cattle or subjected to any other use. Since patch shape could modulate the access of predators to the nests, and modify edge effects (Lahti 2001), we selected rectangular shape patches with similar area (1.2 and 1.8 ha) and perimeter-to-area ratios (4.5 and 5.5 m−1). The area and perimeter of each patch was determined by using an on-line tool (http://www.freemaptools.com/area-calculator.htm). The study site was covered by a high resolution image from Google Earth (date: 1 July 2012) in which previously geopositioned patches were easily recognized.
Nest location and monitoring
We systematically searched for spectacled tyrant nests in patches and the reserve from the beginning of October 2012 to the beginning of January 2013. Nests were located using behavioral cues of adults and by systematic search (Martin and Geupel 1993). In the reserve, we searched for nests in a 100-ha permanent plot located >1.5 km from the reserve’s edge. Once found, we recorded GPS nest locations and marked the nest area with plastic tape to facilitate subsequent monitoring. Nests were visited at intervals of 3–5 days following standard procedures to avoid attracting predators to nests (Martin and Geupel 1993). Nests were checked until they were abandoned, depredated, or produced fledglings. At each visit, we recorded egg or chick loss and the presence of adults near the nest. The continued presence of eggs following the estimated date of hatching and/or the absence of parents was the criteria used to consider a nest abandoned. We considered a nest to have been depredated when the complete clutch disappeared between two subsequent visits, or when the chicks disappeared from their nests before they were old enough to fledge. We considered a nest successful if one or more chicks fledged. C. selloana grassland is typically host to a diversity of potential nest predators, including raptors (e.g., Milvago chimango, Circus buffoni) and passerines (e.g., Pitangus sulphuratus, Embernagra platensis, Phacellodomus striaticollis), mammals like opossums (Monodelphis dimidiata and Didelphis albiventris), skunks (Conepatus chinga), foxes (Lycalopex gymnocercus), feral cats (Oncifelis geoffroyi), lesser grisons (Galictis cuja), and small mammals (e.g., Oxymycterus rufus) and reptiles (Canepuccia et al. 2008; Baladrón et al. 2012; Cardoni et al. 2012; Pretelli et al. 2013; M. Pretelli, personal observation).
Video monitoring
Parental activity during incubation and nestling periods was recorded using small digital cameras (Mini-DV 200) at the nest. This type of camera (8 × 3 × 1 cm in length, width and height, respectively) gives the possibility to film hidden nests within tussocks of grass without the need to substantially modify the conditions and structure of the plant. We installed cameras between 0800 and 0900 h (local time), and recorded all the activity in the nest for 4 h, always during days without rain or strong winds. This approach standardized for time of day, duration of measurements, and weather conditions. Furthermore, for statistical analysis, we only used those nests where the female (female-only care, see “Results”) showed a confident behavior in front of the camera, which consisted of a relaxed and correct incubation posture, accompanied by preening. Some nests were filmed in more than one stage (i.e., incubation, nestling); however, only once within each stage. For nests found after hatching of nestlings, they were aged through body weight and using digital balances (accurate to ±0.1 g) (M. Pretelli, unpublished data). Since the age estimate from weight only may not be very precise, we assigned the age of the chicks into two age ranges (see below). Videotapes were scored in the laboratory for length of incubation bouts and inter-bout intervals, and parental visitation rates during incubation and nestling periods. Nests were filmed between 7 and 12 days of incubation after clutch completion to control for any potential age effects. Parental activity during the nestling stage was measured in two age groups: when chicks were 2–4 days of age (hereafter “young nestlings”) and when chicks were 8–11 days of age (hereafter “old nestlings”). In this period videotapes were scored for the following behaviors: the rate at which females fed the nestling (visits/h per nestling) and the rate at which females removed fecal sacs (number of fecal sacs/h per nestling). Moreover, we recorded the prey size when old nestlings were fed. The prey size [as total length (TL)] was estimated relative to the bill-length of the spectacled tyrant (mean bill-length: 15 mm; M. Pretelli, unpublished data) in the following size-classes: (1) TL ≤ 8 mm; (2) 8 mm < TL ≥ 15 mm; and (3) TL > 15 mm. In order to prevent the effects of seasonal variation on the contribution of prey in only one site (Cavalli et al. 2014; Pretelli et al. 2014), nests were simultaneously filmed throughout the breeding season at both sites.
Statistical analysis
We estimated the nest predation risk in the environment for each site using the daily survival rate (DSR) estimator available in program MARK (White and Burnham 1999). Since the frequency of successful nests did not differ between small patches (Chi squared test: χ 2 1 = 1.29, p = 0.255), we pooled patches for comparison with the reserve. In addition, because we did not record a significant change in nest survival with age (Pretelli 2015), we estimated the DSR of a nest for the whole nesting cycle. Post hoc comparison of DSR between the patches and the reserve was done using the program CONTRAST (Hines and Sauer 1989). This program uses a Chi square-approach that is analogous to ANOVA in order to control for experiment-wise error and adjust for Type I errors (Hines and Sauer 1989). Values of DSR are presented as mean ±1 SE in order to make them comparable with other studies.
We were able to determine clutch-initiation dates for nests found during construction and egg-laying (N = 14 nests). Clutch-initiation dates were assigned by backdating from hatching dates (N = 30 nests) for nests found during incubation, and, for nests found after hatching (N = 12 nests), by using nestling weights (M. Pretelli, unpublished data). For 17 nests that failed during incubation, we estimated clutch-initiation dates (±1–5 days) by assuming that the observed period was halfway between the end of laying (nest age 2) and hatching (nest age 17; i.e., if a nest was observed for 4 days, we considered that it was observed between nest ages 8 and 11; if it was observed for 6 days, we considered that it was observed between nest ages 7 and 12). We standardized the observation period for each nesting attempt by setting a maximum length of 31 days (17 days for the egg-laying and incubation stages, and 14 days for the nestling stage; Pretelli and Isacch 2013). Observation periods started either the day the first egg was laid (for nests found during construction) or the day a nest was found.
To assess differences between the patches and the reserve in duration of incubation bout and inter-bout interval, we used single generalized linear mixed models (GLMM) with a gamma error structure and power (−1) link function (Crawley 2007). Since only the female incubates the eggs, its identity was included as a random term to account for non-independence of data. Model fit was visually assessed by inspecting plots of standardized deviance residuals for each model. We assessed goodness-of-fit for each model and estimated the variance inflation factor (ĉ) as residual deviance divided by degrees of freedom (Crawley 2007). We fitted GLMMs using the glmmPQL function of the MASS package in R software 3.0.1 (R Development Core Team 2013).
We used a Student’s t test to evaluate the null hypothesis of no difference in the latency of female returning to the nest after placing the camera. This would be an indicator of female wariness by prior experience in front of predators. For nests that were filmed more than once, the values were averaged. The number of nests filmed more than once, and the number of nests per nest stage (i.e., egg, young or old nestlings) was evenly distributed between the patches and the reserve (see “Results”) in order to avoid potential biases (Knight and Temple 1986; Montgomerie and Weatherhead 1988). In addition, we used the same test to evaluate the null hypothesis of no difference in female visitation rates during incubation between the patches and the reserve (Zar 1999). Furthermore, Mann–Whitney U tests were used to evaluate the null hypothesis of no difference in parental care (i.e., rate at which females fed the nestlings and removed fecal sacs) between the patches and the reserve (Zar 1999). To test for the differences between frequency distribution among different prey sizes brought to the nest by females in the patches and in the reserve, we used a Chi square test (Zar 1999). Additionally, we compared the proportion of different prey sizes that females brought to the nest between the patches and the reserve using the Z test for proportions (Siegel 1985). All analyses were carried out using R software 3.0.1 (R Development Core Team 2013). The level of significance in all tests was set to p < 0.05. Values are reported as mean ±1 SD.
Results
Nest survival
A total of 56 spectacled tyrant nests were monitored during the breeding season, 30 in patches and 26 in the reserve. We monitored nests at patches over 84 days (12 October–3 January) for a total of 247 exposure days, during which 11 nests were successful and 19 were depredated. In the reserve, we monitored nests over 56 days (24 October–17 December) for a total of 353 exposure days, with 14 successful nests and 12 depredated. No abandoned nests were recorded. The DSR at patches was 0.933 (SE 0.015; N = 30 nests) and at the reserve was 0.968 (SE 0.009; N = 26 nests). Post hoc comparisons of site-specific DSR show differences between the patches and the reserve (Chi square test: χ 2 1 = 4.14, p < 0.041).
Parental care
A total of 31 nests were filmed (13 in patches and 18 at the reserve). In the patches, nine nests were filmed during incubation and ten during nestling period. Of the 13 nests in patches, three nests were filmed three times, two nests twice and eight nests only once. In the reserve, 12 nests were filmed during incubation and 14 during nestling period. Of the 18 nests at the reserve, three nests were filmed three times, two nests twice and 13 nests only once. During incubation, the following were filmed: five nests in October (two in patches and three at the reserve), ten in November (four in patches and six at the reserve) and six in December (three in patches and three at the reserve). During the nestling period, the following were filmed: 11 nests in November (six in patches and five at the reserve), 14 in December (six in patches and eight at the reserve), and six in January (four in patches and two at the reserve).
On average, females became accustomed to the camera significantly sooner in the reserve (09:01 m:s, SD 04:41 m:s, N = 12 nests) than in the patches (19:04 m:s, SD 15:10 m:s, N = 13 nests) (t = 2.21, df = 23, p = 0.037). In addition, four females showed rejection to the camera in the patches. Parental care was the exclusive concern of females, which were in charge of building the nest, incubating the eggs and broods, and feeding and cleaning the chicks. Males were never seen at the nest.
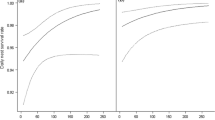
Females in the patches had larger incubation bouts than at the reserve (GLMM: t = −3.14, df = 19, p = 0.005; see Fig. 1a). However, inter-bout intervals were similar between patches and reserve (GLMM: t = −0.02, df = 19, p = 0.983; see Fig. 1b). Consequently, female visitation rate during incubation was greater in the reserve than in the patches (t = 2.69, df = 19, p = 0.014; see Fig. 2). In the patches, females spent on average 61 ± 13 % (N = 9 nests) of their time in the nest, and in the reserve they spent 45 ± 12 % (N = 12 nests).
Duration of incubation bouts (i.e., length of time, in minutes, that a female sits on the nest in a given bout) (a), and duration of inter-bout intervals (i.e., length of time, in minutes, a female is off the nest to forage during a break) (b). The boxes represent the standard error, whiskers represent the SD, and the lines inside the boxes represent the average. The asterisk above the bold horizontal line plot indicates significant differences between sites. The numbers above the plots represent the number of nests sampled
Female activity during incubation (number of visits per hour). The boxes represent the standard error, the whiskers represent the SD, and the lines inside the boxes represent the average. The asterisk above the bold horizontal line plot indicates significant differences between sites. The numbers above the plots represent the number of nests sampled
In the patches, during the nesting period with young nestlings, females spent on average 33 ± 21 % (N = 7 nests) of their time in the nest, and in the reserve they spent 43.2 ± 12 % (N = 6 nests). The rate at which females fed young nestlings was similar at both sites (Mann–Whitney U test: U = 17.00, p = 0.628, N = 13, see Fig. 3). In the patches, during the nesting period with old nestlings, females spent relatively less time in the nest, and it was on average 7.6 ± 7 % (N = 8 nests) of their time, while it was 7.9 ± 3 % (N = 8 nests) in the reserve. However, old nestlings were fed significantly less frequently in patches than in the reserve (U = 12.00, p = 0.038, N = 16, see Fig. 3). The rates at which females removed fecal sacs were similar both for young nestlings (U = 10.00, p = 0.909, N = 10) and for old nestlings (U = 21.00, p = 0.247, N = 16) in both sites (Fig. 4).
Number of visits per hour by females to feed their young on a per-nestling basis. The boxes represent the 25 and 75 % quartiles, the whiskers represent the minima and the maxima, and the median is the line within the box. The asterisk above the bold horizontal line plots indicates significant differences between sites. The numbers above the plots represent the number of nests sampled
Number of visits per hour by the females to remove fecal sacs from their young on a per-nestling basis. The boxes represent the 25 and 75 % quartiles, the whiskers represent the minima and the maxima, and the median is the line within the box. The numbers above the plots represent the number of nests sampled
We identified 479 prey items by size, 206 of which were in patches (N = 8 nests) and 273 of which were in the reserve (N = 8 nests). The distribution of different prey sizes that the female brought to the nest in the patches and in the reserve were significantly different at both sites (χ 2 2 = 20.72; p < 0.001; χ 2 2 = 32.18; p < 0.001; respectively) (Fig. 5). In the reserve, prey of size 1 was consumed more than in the patches (Z size1 = 2.72, p = 0.006); on the contrary, prey of size 2 was consumed more in patches than in the reserve (Z size2 = 0.16, p = 0.002). In both sites, prey of size 3 was equally consumed (Z size3 = 3.08, p = 0.871) (Fig. 5).
Size distribution of prey items that females brought to the nest at small grassland patches (N nests = 8) and at the reserve (N nests = 8). The horizontal lines above the bars indicate the statistical comparisons performed, and the asterisks denote significant differences between sites for each prey size
Discussion
Our results indicated that the DSR of the spectacled tyrant was lower in grassland fragments than in continuous grasslands, and the only detected driver of nest loss was predation. Then, we assumed that predation would be an important driver of the variation in parental care recorded throughout its nesting cycle between grasslands. This change was evidenced by larger incubation bouts and a lower visitation rate during the incubation period, and by a lower food delivery to nestlings compensated by larger prey sizes, all of them in patches as compared with continuous grasslands. The variation in parental care agrees with our prediction, and reflects the behavioral changes that can manifest in a species under different predation risk scenarios, and would respond to the need to adopt an elusive behavior to decrease the probability of being predated.
We found that the spectacled tyrant showed a lower DSR in small agricultural patches than in continuous grasslands, thus suggesting that nesting in fragmented habitats implies a higher predation risk for this species (Ghalambor and Martin 2001; Ghalambor et al. 2013). This pattern coincides with previous reports, which have shown that individuals that nest in small grassland patches are exposed to high nest predation risk (Johnson and Temple 1990; Herkert et al. 2003; Pretelli et al. 2015; but see Walk et al. 2010), and consider this factor as one of the main effects of habitat fragmentation (Pretelli et al. 2015).
Based on the observed results (i.e., difference in the DSR and variation in parental care between fragmented and continuous grasslands), and considering the nest-site fidelity of the spectacled tyrant (Mattos et al. 2011), we assume that this species would largely use its own nesting experience (i.e., private information) acquired in the same or previous seasons. An evidence of behavior modulated by private information may come from the level of tolerance to video cameras, as an indicator of prevention against predators. We observed that individuals nesting in the patches showed a higher avoidance for cameras than in the reserve. In addition, we also know that rodents are responsible for most nest predation events in the same study area (Cardoni et al. 2012; M. Pretelli, unpublished data), and while nest-predator assemblages may be little diverse, we do not know how predictable the abundance of rodents in the environment can be. In sites where the environmental nest-predation risk is unpredictable, public information loses its relevance over the use of private information (Chalfoun and Martin 2010).
Spectacled tyrants breeding in C. selloana grasslands showed differences in parental care between patches and the reserve throughout the nesting cycle. During the incubation stage, this behavioral difference was evidenced by an increase in nest attentiveness in sites where the predation risk was higher (i.e., patches), where females invested in larger incubation bouts in comparison to those of continuous grasslands. However, we did not find differences in the duration of inter-bout intervals between sites, which resulted in a lower nest visitation rate in fragmented grasslands, and, ultimately, reduced conspicuousness of the female. This variation in parental care coincides with patterns found in previous studies, which found that females may increase the duration of incubation bouts in order to reduce nest activity in response to high levels of nest predation risk (e.g., Ferretti et al. 2005; Fontaine and Martin 2006; Kleindorfer 2007; Massaro et al. 2008). It is interesting to note that this pattern is not restricted to particular species or habitats, since it has been observed in different species, locations and geographic areas (Conway and Martin 2000).
Differences in parental care behavior between habitats also occurred during the nestling period, and were evidenced by a decrease of parental visitation rates to feed old nestlings in fragmented grasslands. This response was consistent with previous studies that showed how parent birds assess nest predation risk in the environment and adjust their reproductive strategies by decreasing the feeding rate of nestlings under high nest predation risk (Martin et al. 2000a; Eggers et al. 2005; Fontaine and Martin 2006; Zanette et al. 2011). Reduced rates of visiting the nest to feed offspring might constrain energy for growth and adversely affect physiological processes (Kempster et al. 2007) together with brain development (MacDonald et al. 2006), with negative impact on the survival of juveniles. A strategy to compensate for reduced feeding visitation rates would be increasing the size of food loads brought to the nest on each visit (Skutch 1949; Martin 1996; Martin et al. 2000b). Our study system was in agreement with that strategy, since reduced feeding visitation rate of the spectacled tyrant in patches was accompanied by larger prey items brought to nestlings, thus compensating for the lower contribution of prey to the nest by an increase in the prey sizes (Skutch 1949). An alternative explanation to the observed pattern may be that larger prey items are more abundant in the patches than in the reserve. Nevertheless, Cavalli et al. (2014), studying abundance of insects in similar habitats within the study area and at the same time of year, found that orthopterans (prey size 3; Pretelli et al. 2014) were relatively more abundant in agricultural landscapes than in native grasslands, being equally consumed by spectacled tyrants in our study, while lepidopterans (mostly prey size 2; Pretelli et al. 2014), were more abundant in continuous grasslands than in agricultural landscapes, but were more consumed in patches than in the reserve. These results would not support the possibility that the difference in the availability of insects between habitats is the cause of the behavioral difference of parental care.
The grassland fragmentation by agriculture generates patches of relatively lower quality in terms of the higher risk of nest predation as compared with continuous grasslands. This causes adverse demographic effects at the population level by decreasing the reproductive performance of spectacled tyrants. As a consequence of that, a strong selection pressure on individuals nesting in agro patches would be expected. The use of private information by spectacled tyrants would be a strategy to counterbalance nest-predation risk and ultimately increase nest survival through fragmented grasslands.
References
Andrén H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71:355–366
Arcese P, Smith JNM (1988) Effects of population density and supplemental food on reproduction in song sparrows. J Anim Ecol 57:119–136
Baladrón AV, Malizia AI, Bó MS, Liébana MS, Bechard MJ (2012) Population dynamics of the southern short-tailed opossum (Monodelphis dimidiata) in the Pampas of Argentina. Aust J Zool 60:238–245
Baldi G, Guerschman JP, Paruelo JM (2006) Characterizing fragmentation in temperate South America grasslands. Agric Ecosyst Environ 116:197–208
Batáry P, Báldi A (2004) Evidence of an edge effect on avian nest success. Conserv Biol 18:389–400
Bilenca D, Miñarro F (2004) Identificación de áreas valiosas de pastizal (AVPs) en las pampas y campos de Argentina, Uruguay y sur de Brasil. Fundación Vida Silvestre, Buenos Aires
Bollinger EK, Gavin TA (2004) Responses of nesting Bobolinks (Dolichonyx oryzivorus) to habitat edges. Auk 121:767–776
Cabrera AL (1976) Regiones fitogeográficas argentinas. In: Kugler WF (ed) Enciclopedia argentina de agricultura y jardinería. Editorial ACME S.A.C.I., Buenos Aires
Canepuccia AD, Farias AA, Escalante AH, Iribarne OO, Novaro A, Isacch JP (2008) Differential responses of marsh predators to rainfall-induced habitat loss and subsequent variations in prey availability. Can J Zool 86:407–418
Cardoni DA, Isacch JP, Iribarne O (2012) Effects of cattle grazing and fire on the abundance, habitat selection, and nesting success of the bay-capped wren-spinetail (Spartonoica maluroides) in coastal saltmarshes of the Pampas region. Condor 114:803–811
Cavalli M, Baladrón AV, Isacch JP, Martínez G, Bó MS (2014) Prey selection and food habits of breeding burrowing owls (Athene cunicularia) in natural and modified habitats of Argentine pampas. Emu 114:184–188
Chalfoun AD, Martin TE (2010) Parental investment decisions in response to ambient nest-predation risk versus actual predation on the prior nest. Condor 112:701–710
Chalfoun AD, Thompson FR III, Ratnaswamy MJ (2002) Nest predators and fragmentation: a review and meta-analysis. Conserv Biol 16:306–318
Conway CJ, Martin TE (2000) Evolution of avian incubation behavior: influence of food, climate and nest predation. Evolution 54:670–685
Crawley MJ (2007) The R book. Wiley, West Sussex
Donovan TM, Jones PW, Annand EM, Thompson FR III (1997) Variation in local-scale edge effects: mechanisms and landscape context. Ecology 78:2064–2075
Duncan Rastogi A, Zanette LY, Clinchy M (2006) Food availability affects diurnal nest predation and adult antipredator behaviour in song sparrows, Melospiza melodia. Anim Behav 72:933–940
Eggers S, Griesser M, Ekman J (2005) Predator-induced plasticity in nest visitation rates in the Siberian jay (Perisoreus infaustus). Behav Ecol 16:309–315
Ellison KS, Ribic CA (2012) Nest defense: grassland bird responses to snakes. In: Ribic CA, Thompson FR III, Pietz PJ (eds) Video surveillance of nesting birds. Studies in avian biology (no. 43). University of California Press, Berkeley, pp 149–160
Ferretti V, Llambías PE, Martin TE (2005) Life-history variation of a neotropical thrush challenges food limitation theory. Proc R Soc B 272:769–773
Fitzpatrick JW (2004) Family Tyrannidae. In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the birds of the world, vol 9., Cotingas to pipits and wagtailsLynx Edicions, Barcelona, pp 170–462
Fontaine JJ, Martin TE (2006) Parent birds assess nest predation risk and adjust their reproductive strategies. Ecol Lett 9:428–434
Ghalambor CK, Martin TE (2001) Fecundity-survival trade-offs and parental risk-taking in birds. Science 292:494–497
Ghalambor CK, Martin TE (2002) Comparative manipulation of predation risk in incubating birds reveals variability in the plasticity of responses. Behav Ecol 13:101–108
Ghalambor CK, Peluc SI, Martin TE (2013) Plasticity of parental care under the risk of predation: how much should parents reduce care? Biol Lett 9:20130154
Herkert JR, Reinking DL, Wiedenfeld DA, Winter M, Zimmerman JL, Jensen WE, Finck EJ, Koford RR, Wolfe DH, Sherrod SK, Jenkins MA, Faaborg J, Robinson SK (2003) Effects of prairie fragmentation on the nest success of breeding birds in the midcontinental United States. Conserv Biol 17:587–594
Hines JH, Sauer JR (1989) Program CONTRAST: a general program for the analysis of several survival or recovery rate estimates. U.S. Department of the Interior, Fish and Wildlife Service, Washington
Johnson RG, Temple SA (1990) Nest predation and brood parasitism of tallgrass prairie birds. J Wildl Manag 54:106–111
Kearns LJ, Rodewald AD (2013) Within-season use of public and private information on predation risk in nest-site selection. J Ornithol 154:163–172
Kempster B, Zanette L, Longstaffe FJ, MacDougall-Shackleton SA, Wingfield JC, Clinchy M (2007) Do stable isotopes reflect nutritional stress? Results from a laboratory experiment on song sparrows. Oecologia 151:365–371
Kendeigh SC (1952) Parental care and its evolution in birds. Ill Biol Monogr 22:1–358
Kleindorfer S (2007) The ecology of clutch size variation in Darwin’s small ground finch Geospiza fuliginosa: comparison between lowland and highland habitats. Ibis 149:730–741
Knight RL, Temple SA (1986) Why does intensity of avian nest defense increase during the nesting cycle? Auk 103:318–327
Lack D (1947) The significance of clutch-size. Ibis 89:302–352
Lahti DC (2001) The “edge effect on nest predation” hypothesis after twenty years. Biol Conserv 99:363–374
León RJC, Rusch GM, Oesterheld M (1984) Pastizales pampeanos—impacto agropecuario. Phytocoenologia 12:201–218
Lima SL (1998) Nonlethal effects in the ecology of predator–prey interactions: what are the ecological effects of anti-predator decision making? Bioscience 48:25–34
Lima SL (2009) Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol Rev 84:485–513
MacDonald IF, Kempster B, Zanette L, MacDougall-Shackleton SA (2006) Nutritional stress impairs development of song-control brain regions in juvenile male and female song sparrows (Melospiza melodia). Proc R Soc B 273:2559–2564
Martin TE (1987) Food as a limit on breeding birds: a life-history perspective. Annu Rev Ecol Syst 18:453–487
Martin TE (1992) Interaction of nest predation and food limitation in reproductive strategies. Curr Ornithol 9:163–197
Martin TE (1995) Avian life history evolution in relation to nest sites, nest predation and food. Ecol Monogr 65:101–127
Martin TE (1996) Life history evolution in tropical and south temperate birds: what do we really know? J Avian Biol 27:263–272
Martin TE, Geupel GR (1993) Nest-monitoring plots: methods for locating nests and monitoring success. J Field Ornithol 64:507–519
Martin TE, Roper JJ (1988) Nest predation and nest site selection in a western population of the hermit thrush. Condor 90:51–57
Martin TE, Scott J, Menge C (2000a) Nest predation increases with parental activity: separating nest site and parental activity effects. Proc R Soc B 267:2287–2293
Martin TE, Martin PR, Olson CR, Heidinger BJ, Fontaine JJ (2000b) Parental care and clutch sizes in North and South American birds. Science 287:1482–1485
Massaro M, Starling-Windhof A, Briskie JV, Martin TE (2008) Introduced mammalian predators induce adaptive shifts in parental behaviour in an endemic New Zealand bird. PLoS ONE 3:e2331
Mattos E, Cozzani N, Zalba S (2011) Selecciona el Pico de Plata (Hymenops perspicillatus) los mismossitios de cría cada temporada? In: Libro de resúmenes, XIV Reunión Argentina de Ornitología, Ciudad de Formosa, p 105
Montgomerie RD, Weatherhead PJ (1988) Risks and rewards of nest defence by parent birds. Q Rev Biol 63:167–187
Naef-Daenzer B, Keller LF (1999) The foraging performance of great and blue tits (Parus major and P. caeruleus) in relation to caterpillar development, and its consequences for nestling growth and fledging weight. J Anim Ecol 68:708–718
Nagy LR, Holmes RT (2005) Food limits annual fecundity of a migratory songbird: an experimental study. Ecology 86:675–681
Paruelo JM, Guerschman JP, Verón SR (2005) Expansión agrícola y cambios en el uso del suelo. Ciencia Hoy 15:14–23
Peluc SI, Sillett TS, Rotenberry JT, Ghalambor CK (2008) Adaptive phenotypic plasticity in an island songbird exposed to a novel predation risk. Behav Ecol 19:830–835
Pietz PJ, Granfors DA (2005) Parental nest defense on videotape: more reality than myth. Auk 122:701–705
Pretelli MG (2015) Efecto de la fragmentación del pastizal sobre las aves en pastizales costeros de la región Pampeana. Doctoral thesis, Universidad Nacional de Mar del Plata, Buenos Aires, Argentina
Pretelli MG, Isacch JP (2013) Breeding biology of spectacled tyrant (Hymenops perspicillatus) in the southeastern Pampas region, Argentina. Wilson J Ornithol 125:275–279
Pretelli MG, Isacch JP, Cardoni DA (2015) Effects of fragmentation and landscape matrix on the nesting success of grassland birds in the Pampas grasslands of Argentina. Ibis 157:688–699
Pretelli MG, Isacch JP, Cardoni DA (2013) Year-round abundance, richness and nesting of the bird assemblage of tall grasslands in the south-east Pampas region, Argentina. Ardeola 60:327–343
Pretelli MG, Cardoni DA, Isacch JP (2014) Diet of nestling spectacled tyrants (Hymenops perspicillatus) in the southeast Pampas region, Argentina. Wilson J Ornithol 126:754–759
R Development Core Team (2013) R: A language and environment for statistical computing. R foundation for statistical computing, Viena
Ricklefs RE (1969) An analysis of nesting mortality in birds. Smithson Contrib Zool 9:1–48
Siegel S (1985) Estadística no paramétrica aplicada a las ciencias de la conducta. Editorial Trillas, México
Skutch AF (1949) Do tropical birds rear as many young as they can nourish? Ibis 91:430–455
Sofaer HR, Sillett TS, Peluc SI, Morrison SA, Ghalambor CK (2013) Differential effects of food availability and nest predation risk on avian reproductive strategies. Behav Ecol 24:698–707
Tremblay I, Thomas D, Lambrechts MM, Blondel J, Perret P (2003) Variation in blue tit breeding performance. Ecology 84:3033–3043
Viglizzo EF, Lértora FA, Pordomingo AJ, Bernardos JN, Roberto ZE, Del Valle H (2001) Ecological lessons and applications from one century of low-external input farming in the pampas of Argentina. Agric Ecosyst Environ 81:65–81
Wagner RH, Danchin E (2010) A taxonomy of biological information. Oikos 119:203–209
Walk JW, Kershner EL, Benson TJ, Warner RE (2010) Nesting success of grassland birds in small patches in an agricultural landscape. Auk 127:328–334
Ward JM, Kennedy PL (1996) Effects of supplemental food on size and survival of juvenile northern goshawks. Auk 113:200–208
Weidinger K (2002) Interactive effects of concealment, parental behavior and predators on the survival of open passerine nests. J Anim Ecol 71:424–437
Wesołowski T (1994) The origin of parental care in birds: a reassessment. Behav Ecol 15:520–523
White GC, Burnham KP (1999) Program mark: survival estimation from populations of marked animals. Bird Study 46(Supplement):120–138
Wilcove DS (1985) Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66:1211–1214
Winter M, Johnson DH, Faaborg J (2000) Evidence for edge effects on multiple levels in tallgrass prairie. Condor 102:256–266
Zanette LY, Clinchy M, Smith JNM (2006) Combined food and predator effects on songbird nest survival and annual reproductive success: results from a bi-factorial experiment. Oecologia 147:632–640
Zanette LY, White AF, Allen MC, Clinchy M (2011) Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334:1398–1401
Zar JH (1999) Biostatistical analysis. Prentice-Hall, Englewood Cliffs
Acknowledgments
This paper benefitted from the comments of two anonymous reviewers. We thank Diego Metzadour for the language editing. The research received financial support from Neotropical Grassland Conservancy (NGC), Beca “Conservar la Argentina” (Aves Argentinas), Universidad Nacional de Mar del Plata, and Agencia de Promoción Científica y Tecnológica (PICT 12-461). MGP was supported by a doctoral scholarship from CONICET.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. G. Guglielmo.
Rights and permissions
About this article
Cite this article
Pretelli, M.G., Isacch, J.P. & Cardoni, D.A. Variation in parental care in the spectacled tyrant Hymenops perspicillatus is associated with increased nest predation in grassland fragments. J Ornithol 157, 451–460 (2016). https://doi.org/10.1007/s10336-015-1300-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1300-8