Abstract
Depth-stratified vertical sampling was carried out during the New Zealand International Polar Year cruise to the Ross Sea on board the RV Tangaroa in February–March 2008. The distribution (horizontal and vertical), density and population biology of Salpa thompsoni were investigated. Salps were found at two of the four major sampling locations, e.g. near the continental slope of the Ross Sea and in the vicinity of seamounts to the north of the Ross Sea. Both abundance and biomass of S. thompsoni were highest near the seamounts in the Antarctic Circumpolar Current reaching ~2,500 ind 1,000 m−3 and 8.2 g dry wt 1,000 m−3 in the water column sampled. The data showed that S. thompsoni populations were able to utilize horizontal and vertical discontinuities in water column structure, in particular the warm Circumpolar Deep Water (CDW), to persist in the high Antarctic. Although salps appeared to continue migrating to the surface colder layers to feed, both aggregate chain and young embryo release seem to be restricted to the CDW. This study for the first time has provided evidence that low Antarctic salp species may successfully reproduce in the hostile high Antarctic realm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pelagic tunicate Salpa thompsoni is one of the most prominent metazoan filter feeders in the Southern Ocean (Voronina 1998; Pakhomov et al. 2002). In the core of its areal distribution (ca 45–55°S), S. thompsoni has the ability to undergo explosive population development during the austral summer, likely outcompeting other zooplankton species and dramatically altering the food web (Loeb et al. 1997; Walsh et al. 2001). This species is known as an efficient re-packager of small particles into fast sinking feces, playing a significant part, at least seasonally, in the Southern Ocean biological pump by channeling biogenic carbon from surface waters into the ocean’s interior and seafloor (Le Fèvre et al. 1998; Moline et al. 2000; Walsh et al. 2001; Phillips et al. 2009). Recently, it has been shown that S. thompsoni may also provide a direct and efficient link between surface production and benthic ecosystems (Gili et al. 2006).
In the classical work by Foxton (1966), S. thompsoni was described as an animal well adapted to oceanic, low Antarctic latitudes and seldom found in coastal seas surrounding the Antarctic continent. The southern boundary of the S. thompsoni distribution coincided with the northernmost extension of the winter sea ice (Foxton 1966). In recent decades, however, a southward shift in the distribution of S. thompsoni has been documented (Chiba et al. 1999; Pakhomov et al. 2002; Atkinson et al. 2004). Since S. thompsoni is a cold-temperate species, changes in its latitudinal distribution could be indicative of a large-scale environmental shift in the high Antarctic. Indeed, large-scale environmental changes have been observed over the past few decades (de la Mare 1997, 2009; Levitus et al. 2000), including a warming of the Antarctic midwaters (700–1,100 m) by 0.17°C between the 1950s and the 1980s (Gille 2002).
However, the mechanisms of the salp expansion into the high Antarctic are still not clear. Positive relationships between water column integrated temperature and salp distributions have been documented in various coastal seas around the Antarctic continent (e.g. Siegel et al. 1992; Pakhomov et al. 1994, 2002; Hosie et al. 1997; Nicol et al. 2000 and references therein), suggesting that salps may utilize the vertical structure of the Southern Ocean waters to penetrate southwards. Salps have been found to be restricted to the warmer water masses or layers (e.g. Siegel et al. 1992; Park and Wormuth 1993; Pakhomov et al. 1994; Pakhomov et al. 2006). Limited studies at fine vertical scales (tens of centimeters to tens of meters) in the epipelagic domain have shown that salps may indeed preferentially concentrate in the warmer water layers/lenses in the high Antarctic (Krakatitsa et al. 1993; Pakhomov 1993, 1994; Catalán et al. 2008). However, current evidence suggests that their expansion into areas previously considered as the realm of Antarctic krill is physiologically limited by their inability to reproduce at these low temperatures (Casareto and Nemoto 1986; Chiba et al. 1999; Pakhomov et al. 2002; Pakhomov et al., in review).
The generalized water column structure in the water adjacent to the Antarctic continent includes the cold Antarctic Surface Water (ASW) that occupies the top 200–300 m and may have a seasonally warmed near-surface layer. Beneath this layer is the warm Circumpolar Deep Water (CDW) that extends from ca 200 to ~3,000 m. This layer moves toward the Antarctic continent and is often recorded close to the continental slope (Naganobu et al. 2006). We hypothesize that the CDW may represent the mechanism for S. thompsoni transport into the cold water regions of the coastal Antarctic and a refuge enabling its persistence in this realm. Although the generalized vertical distribution and migrations of S. thompsoni have been studied in the Southern Ocean, to our knowledge there are no data sets on the vertically resolved distribution of S. thompsoni in the high Antarctic.
The oceanography of the Ross Sea and the Antarctic Circumpolar Current (ACC) to its north has been well described (e.g. Jacobs et al. 1970; Orsi et al. 1995; Picco et al. 2000; Naganobu et al. 2006; Orsi and Wiederwohl 2009; Rickard et al. 2010). It represents a perfect setting for the CDW refuge hypothesis. The ACC north of the Ross Sea shelf break (~72 to 73°S) to the Polar Front (PF) (~62°S) is characterized by a slow eastward flow of <2 cm s−1, accelerating to >10 cm s−1 at the southern boundary of the ACC (~65 to 66°S) and Antarctic Divergence region (AD) (~71.5 to 73°S). South of the PF to the Ross Sea shelf, surface waters are described as ASW, while cold (−1.9°C) and saline (>34.6‰) shelf waters (SW) are found over the Ross Sea shelf proper. It is well documented that in the vicinity of the Ross Sea, the CDW is clearly distinguishable between 150 and 2,000 m and penetrates all the way to the Ross Sea continental slope (Russo 2000; Naganobu et al. 2006).
Here, we describe a unique, albeit small, data set collected during the New Zealand (International Polar Year) IPY-CAML cruise to the Ross Sea that performed vertically stratified net tows in the region. The main aims of this paper are (a) to describe the horizontal and vertical distributions as well as stage development of the S. thompsoni population in the high Antarctic realm (the Ross Sea and its vicinity) in relation to environmental variables and (b) to look for evidence whether S. thompsoni may successfully reproduce in the high Antarctic.
Materials and methods
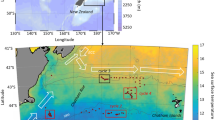
Plankton samples were collected during the New Zealand IPY-CAML cruise to the Ross Sea on board the RV Tangaroa (cruise 0802), between February 12 and March 11 of 2008 (Table 1; Fig. 1). In total, fourteen MOCNESS-1 (rectangular 1-m2 frame equipped with nine 200-μm mesh nets) tows were carried out, in three regions: the Ross Sea shelf, the Ross Sea slope and the ACC to the north of the Ross Sea (two locations). Generally, four (on two occasions, five) depth strata were sampled between the surface and near bottom (proximity to bottom ± 50–200 m) layers (Table 1). Most of the tows were conducted during the daytime. The exceptions were tows at stations 42, 57, 95 and at stations 170, 238 that were completed during nighttime and twilight, respectively. Tows at stations 168 and 232 started at twilight and completed in daylight (Table 1). The MOCNESS-1 was equipped with mechanical flow meters, net pitch and depth sensors. The towing speed was 1.5–2 knots, and volume filtered ranged between 235 and 1,953 m3, depending on the depth strata sampled. The towing procedure was to lower the net to the maximum sampling depth and subsequently tow it obliquely to the surface, closing and opening nets according to pre-identified sampling depth strata (Table 1). Immediately after the tow, the samples from each depth stratum were split using a Folsom splitter. A one-fourth or one-eighth subsample was preserved in a 4% buffered formaldehyde seawater solution and returned to the laboratory for subsequent numerical analyses. In addition to the stratified MOCNESS-1 tows, a single midwater trawl (10 mm mesh size) was conducted at station 193, at the depth interval 163–184 m. For comparative purposes, a random subsample of salps was preserved in a 4% buffered formaldehyde seawater solution.
Station positions occupied during the RV Tangaroa IPY-CAML survey in the Ross Sea and adjacent waters during February–March 2008. Open circles MOCNESS-1 net stations that were accompanied by the CTD cast; filled circles MOCNESS-1 net and midwater trawl (only station 193) stations that were not accompanied by the CTD cast; filled squares CTD casts
At seven of the MOCNESS-1 stations, CTD casts, generally from sea surface to sea floor, were completed prior to the net hauls to obtain vertical profiles of temperature, salinity, oxygen and chlorophyll-a concentrations (Fig. 1). Two additional CTD casts were completed at independent stations.
In the laboratory, all samples were examined for the presence of salps. When found, salps were identified, counted, separated into aggregate and solitary forms. The oral-atrial length (OAL) was measured to the nearest millimeter according to Foxton (1966), for specimens in either the entire preserved sample or a half to quarter subsample when salp numbers were very high. The maturity stages of S. thompsoni have previously been described in several papers (e.g. Foxton 1966; Casareto and Nemoto 1986; Chiba et al. 1999; Daponte et al. 2001). Following these descriptions, the maturity stages of aggregates were determined according to the morphological characteristics of the embryo inside an aggregate body. Five stages (from 0 to 4) were classified according to gradual growth of the embryo. At stage 0, the ovarian sac is spherical with no sign of embryo development. By stage 4, the embryo, often >4 mm in length, resembles in all features an early oozoid (Foxton 1966; Daponte et al. 2001). Stage 5 ‘spent’ was identified by the presence of a placenta scar, which indicated that the embryo had been released. Finally, we observed some aggregates containing what we assumed to be unfertilized eggs (the ovarian sac is clearly transparent) and aggregates with no visible embryos (possibly failed fertilization) or embryos that showed a remnant of some degree of development (possibly embryos with disrupted development). Following Chiba et al. (1999), we classified these specimens as stage ‘X1’ and stage ‘X2’, respectively. The developmental stages of S. thompsoni solitary forms were previously described by Foxton (1966), Casareto and Nemoto (1986) and Daponte et al. (2001) and are determined according to the morphology of the stolon.
Salp densities were converted to individuals 1,000 m−3. The dry weight (mg 1,000 m−3) of salps was calculated for each station using their length frequency abundance composition and a relationship between the dry mass and OAL of fresh S. thompsoni from the Bellingshausen Sea (Dubischar et al. 2006). No correction for the salp length shrinking due to preservation was applied. The mean values of chlorophyll-a, temperature, salinity, oxygen and depth were calculated for each depth interval sampled by the MOCNESS-1 using the available CTD data. In the case of net stations without a CTD cast, the data from the nearest CTD cast to that station were applied. The relationship between these environmental data and log10(x + 1)-transformed salp density and biomass was then tested using Pearson correlation analysis. All analyses were performed using Statistica 6.0.
Results
The oceanographic environment
CTD stations reflected the known oceanographic environment for the region. The Ross Sea shelf region (stations 42, 57 and 95) was characterized by water temperatures <0°C throughout the water column (Fig. 2). The upper 50 m was comparatively warm at ~−0.8°C, while below this depth the temperature decreased to a uniform −1.8°C. The Ross Sea shelf break was characterized by cold (<−1°C) surface waters and an intrusion of warm (~0°C) Circumpolar Deep Water (CDW) (Fig. 3, stations 122, 136, 158 and 168). The CDW layer at shelf break stations was of variable thickness, extending from ~200 m to between 400 and 750 m, below which temperatures decreased to <−1°C. Station 170 although located close to the Ross Sea, shelf break was slightly further north than the other stations in this region (Figs. 1, 3). This station was oceanographically similar to the northern stations (Figs. 3, 4), indicating that there was a sharp separation at the shelf break between the continental shelf stations and the abyssal stations. The stations to the north of the Ross Sea had cold (−1.5°C) surface waters (Fig. 4). Below 200 m, these stations were characterized by CDW and temperatures between 0 and 1.5°C.
Vertical distribution of Salpa thompsoni abundance, water temperature and chlorophyll-a concentrations at stations near the Ross Sea continental slope during February–March 2008. When salp abundances were plotted with the CTD profiles, average sampling depth of the particular depth strata was used. It was assumed that the salp abundance was always zero at the surface
Chlorophyll-a concentrations were highest over the Ross Sea shelf, reaching 4.5 mg m−3 in the strongly stratified surface waters (Fig. 2). At the shelf break stations and further north, chlorophyll-a concentrations ranged between 0.8 and 2.5 mg m−3 in the top 100 m (Figs. 3, 4). At the slope stations, chlorophyll-a concentrations usually sharply decreased to almost zero below 300 m, while at the northern stations modest chlorophyll-a concentrations (ca 0.5–0.6 mg m−3) could be observed down to 250–300 m (Figs. 3, 4).
Horizontal and vertical salp distributions
Pelagic tunicates belonging to a single species, Salpa thompsoni, were present in nine of the fourteen MOCNESS stations sampled (Fig. 5). Salps were found at two of the four major sampling locations, the continental slope of the Ross Sea and in the vicinity of seamounts to the north of the Ross Sea (Fig. 5). S. thompsoni was completely absent from the stations on the Ross Sea shelf (stations 42, 57 and 95) and at the two stations in the ACC to the northwest of the Ross Sea (stations 261 and 283). Where salps did occur, their density distribution was patchy, varying by two to three orders of magnitude among stations (Table 2). Only median abundance and biomass of S. thompsoni were higher near the seamounts in the ACC compared with the north of the Ross Sea, while mean densities, although variable, were similar in both locations (Table 2).
The deepest depth stratum where salps were caught was 800–1,280 m, at station 168 (Table 1). At the stations with the highest salp densities (168, 170, 194 and 238), salps were primarily distributed in the upper 300 m of the water column. Stations 168 and 170 commenced at daytime 08:15 and 19:15 local time, respectively. Very high salp densities (up to 2,500 ind 1,000 m−3) were observed in the top 0–200(300) m coinciding with the highest chlorophyll-a concentrations (Table 1; Fig. 3). At station 238, commencing at 04:54 (twilight), salp densities reached >6,000 ind 1,000 m−3 (Table 1; Fig. 4). Salps were concentrated in the upper 100 m of the water column and, as with the previous two stations, coincided with the maximum chlorophyll-a concentrations. Sampling at station 194 commenced at midday, and the maximum density of salps was deeper than that in the previous three stations (occurring at ~300 m), in close proximity to the upper boundary of the CDW (Table 1; Fig. 4).
At slope stations 122, 156 and 158, sampled during daylight between 09:30 and 15:30, maximum salp densities (ca 40–400 ind 1,000 m−3) were found between 200 and 400 m and clearly situated within the warm CDW (Table 1; Fig. 3). Sampling at station 232, situated north of the Ross Sea, commenced at 07:20 and had low salp densities (<8 ind 1,000 m−3) that peaked at ~500 m in the CDW (Table 1; Fig. 4).
The Pearson correlation analysis found a significant positive relationship (r = 0.5, P < 0.05, df = 24) between salp abundance/biomass and chlorophyll-a concentrations and a significant negative correlation (r = −0.5, P < 0.05, df = 24) between salp abundance/biomass and depth. This was in part due to the significant inverse relationship between depth and chlorophyll-a concentrations (r = −0.67, P < 0.05, df = 24). There was no significant correlation between salp abundance/biomass and sampling time, depth strata averaged temperature, salinity and oxygen.
Salpa thompsoni: length frequency distribution and development
Aggregate forms dominated the populations of S. thompsoni in both the Ross Sea slope and northern ACC stations (Table 2). The aggregate/solitary ratio was particularly high (>1,000) in the Ross Sea slope region. Solitaries were present in very low numbers (0.7–7.5 ind 1,000 m−3) in the upper depth strata (0–200 or 0–300 m) and were represented by large (58–80 mm OAL) specimens at advanced (3–5A) stages of development. At the stations to the north of the Ross Sea, the aggregate/solitary ratio was substantially lower at around 55 (Table 2). The solitaries were only found in the subsurface (>100 m) depth strata, with densities ranging from 3.5 to 59.7 ind 1,000 m−3. Unlike stations over the Ross Sea slope, almost all solitaries at the northern stations were composed possibly of “newly released” embryos (stage 0) with OAL varying between 3 and 5.5 mm. However, the possibility that small embryos might have been the net sampling artifact and could have been released by aggregates in the net cannot be discounted. Only a single large and mature (OAL = 60 mm, Stage 4B) solitary specimen was caught at station 238 in the 400–300 m depth strata.
The length frequency data averaged over the sampled water column were multimodal at both slope and northern sites, ranging between 3 and 25 mm in OAL (Fig. 6a, b). In both regions, a prominent small aggregate (OAL 6–7 mm) cohort was observed. Inter-comparison between MOCNESS and midwater trawls (stations 194 and 193) clearly pointed to a severe undersampling by the midwater trawl of aggregates smaller than 12 mm OAL (Fig. 6b). Generally, aggregates at early developmental stages (0–2) predominated across all stations (Fig. 6c). It should be noted that at stations where stage 0 was encountered, a substantial proportion (up to 25%) of aggregates had unfertilized eggs (stage X1). The contribution of either matured (stage 4) or spent (stage 5) aggregates was small (<5%) over the continental slope and modest (up to 20%) at the ACC stations. Occasionally, aggregates with no visible or malfunctioned embryos (stage X2) were found, at both slope and ACC stations, but their contribution never exceeded 5% of total aggregate numbers (Fig. 6c).
Depth-stratified length frequency distribution of developmental stages showed several similar patterns across all stations where salps were present (see Figs. 7, 8, 9, 10, 11). Highest relative contributions of large aggregates (OAL >15 mm) at advanced stages (3–5) of development were generally observed at depths >200–300 m (e.g. Figs. 7b, 8, 9b, 10b and 11b). The smallest aggregates, at early stages of development (1–3), were mainly found between 100 and 500 m (Figs. 8, 9a, b, 10a, b and 11a, b). It should be noted that stage X1 was only associated with the small-sized (probably just released) aggregates. Their contribution was the highest (up to 80–100% of total aggregates of the size class) within the subsurface midstrata (e.g. Figs. 9b, 10b and 11a), and they entirely vanished by the OAL of 10 mm. Conversely, stage X2 aggregates were typically between 10 and 20 mm OAL.
Discussion
Vertical distribution of Salpa thompsoni and persistence in the region
During austral summer, S. thompsoni has been shown to migrate strongly between the surface and midwater layers, down to 500–1,000 m (Hardy and Gunther 1935; Foxton 1966; Gili et al. 2006), although the core of the population appears to remain within the upper 300 m (Piatkowski 1985; Casareto and Nemoto 1986; Pakhomov 1994; Perissinotto and Pakhomov 1998a, b; Nishikawa and Tsuda 2001). Detailed studies in the Bransfield Strait revealed that although S. thompsoni migrated across a temperature range of −1.5° to +2.0°C, they tended to concentrate in the warmer (>0°C) water layers above and below the main thermocline (Pakhomov 1993, 1994). In addition, the gradient in the thermocline appeared to affect S. thompsoni migration. For example, within the main thermocline salps freely migrated across temperature gradients of 0.022–0.048 T°C m−1. However, at gradients >0.242 T°C m−1 salps tended to migrate only within the uppermost 100 m, concentrating below 30 m either within the upper thermocline or just above the cold layer during the daytime, and migrating closer to the surface (3–50 m) during the nighttime (Pakhomov 1994).
In the East Indian sector of the Southern Ocean during detailed sampling, Nishikawa and Tsuda (2001) described the vertical migrations of S. thompsoni in the region of relatively low (max ~0.6 mg m−3) subsurface chlorophyll-a concentrations. It was hypothesized that salps adapted their migration patterns to utilize limited food resources in the oligotrophic region. For example, salps started ascending toward the surface at midday but concentrated in the chlorophyll maximum layer avoiding the near-surface (0–30 m) strata, possibly avoiding visual predators (Nishikawa and Tsuda 2001). The temperature range salps experienced at their daytime maximum generally ranged between −0.5 and −1.0°C.
In the Lazarev Sea, S. thompsoni was concentrating at 0–30 m during the nighttime where the maximum chlorophyll-a levels (0.8–1.2 mg m−3) were observed, but during the daytime salps were moving to the depth strata 30–90 and 90–200 m (Perissinotto and Pakhomov 1998a, b). During this migration, the population appeared to cross the cold water (~−0.5 to −1.5°C) layer between 30 and 100 m (Perissinotto and Pakhomov 1998b). The temperature gradients within thermoclines were modest, generally ranging from 0.03 to 0.10 T°C m−1 (Pakhomov et al. 2002).
Observations from this study appear to be most comparable to the Lazarev Sea data set. At the five daytime stations where salps were present (09:36–12:11, Table 1), salp vertical distribution was closely correlated with vertical water temperature patterns, with densities always being highest within the warm CDW. On three occasions, when stations were sampled during either the early morning (e.g. 07:20–08:15, stations 168 and 232) or twilight (04:54, station 238 and 19:14, station 170), salps were found in the upper water layers. It is worth noting that in all but one station, the upper stratum sampled was either 0–200 or 0–300 m, precluding precise location of salps within the epipelagic zone. The thermocline gradients at all stations with salps were modest, never exceeding 0.147 T°C m−1, and mostly ranged from 0.018 to 0.068 T°C m−1 (Table 1). The correlation analyses, however, did not reveal any relationship between log-transformed salp abundance and biomass and sampling time, seawater temperature, salinity or oxygen concentrations (P > 0.05). Our findings appeared to differ from results recently obtained in the Bransfield Strait where the salp distribution was largely related to oxygen concentrations (Catalán et al. 2008). In the Bransfield Strait data set, however, the sampling intervals within the upper most layer were narrow (ca 25 m), while during our study the minimum sampling resolution was at least 100 or 200 m.
Although no 24-h stations were carried out during this survey, comparison of the different net tows indicated that the vertical distribution of S. thompsoni had a consistent pattern that was strongly related to environmental variables. During the daytime, the salp population was concentrated at a depth associated with the upper part of warm CDW. Here, water temperatures were within the range typical of the low latitude Antarctic habitat of S. thompsoni documented by Foxton (1966). During the nighttime, salps migrated into the cold surface waters, with minimum temperatures of −1.5 to −1.6°C, presumably to take advantage of enhanced chlorophyll-a concentrations. This was evident as elevated epipelagic salp densities in the early evening to early morning hours. The above assessment of salp distribution patterns is supported by the Pearson correlation analysis. No significant relationship was observed between S. thompsoni densities/biomass and temperature, while they had a significant negative correlation with depth and positive correlation with chlorophyll-a (these two parameters having an obvious inverse relationship). Although being strongly associated with warm CDW, the occurrence of phytoplankton within the cold epipelagic zone necessitated salp migration out of CDW waters in order to feed. Although the sampling resolution was too coarse to identify exactly where salps were located within the epipelagic layer, the observed temperature gradients did not preclude their widespread occurrence. In the Bransfield Strait, Catalán et al. (2008) recently suggested that S. thompsoni populations have to utilize the vertical structure of the water column in a similar way in order to sustain their fitness. Below, we further hypothesize that warm water intrusions such as those observed up to the Ross Sea slope may be important not only for the advection of salps into the high Antarctic but also for their persistence in high Antarctic regions.
Development and life cycle of Salpa thompsoni in the high Antarctic
Surprisingly, little attention has been paid to the life cycle of S. thompsoni in recent years (e.g. Casareto and Nemoto 1986; Chiba et al. 1999; Pakhomov et al. 2006, submitted). It has only been described in detail by Foxton (1966) within the typical S. thompsoni habitat between 45° and 55°S. According to Foxton (1966), S. thompsoni aggregates dominate (aggregate/solitary ratio ≫ 50) in the surface layers between November and March. The aggregate/solitary ratio drops to <15 during the winter months (July–September). Near the surface, small aggregate forms (10–20 mm OAL) are found all year around. Foxton has noted that early spring (September) and late summer (March) were periods of sharp increase in S. thompsoni densities, particularly aggregates; these increases are linked to the active release of bud chains (aggregates) by solitary forms (Foxton 1966). There is a tendency for large and maturing aggregates to migrate to deep waters, particularly during March and April. Since solitaries, represented by small (<10 mm OAL) specimens, were almost entirely absent in the upper layers by fall, Foxton (1966) suggested March–April as an active embryo release period occurring in the subsurface layers. Small solitaries overwinter in the deep layers and probably grow to 10–20 mm (modal OAL) by September, when they appear near the surface, then to 30–40 mm by November and to 40–50 mm by February (Foxton 1966).
In the high Antarctic, it has been noticed that eggs in the aggregates could be either absent or not fertilized (up to 100% on some occasions), e.g. assigned as aggregates with the stage X (Casareto and Nemoto 1986; Chiba et al. 1999). In both the Bellingshausen and Lazarev seas, a substantial (up to 25%) proportion of stage X aggregates have also been encountered (Pakhomov et al. 2006, submitted). Based on these findings, it has been hypothesized that S. thompsoni may not be able to complete its life cycle at high latitudes (Casareto and Nemoto 1986; Chiba et al. 1999). It is likely that they occur as expatriate populations that are advected into the region with CDW waters (Pakhomov et al. 2006) and that these populations probably do not survive through the winter (e.g. in the Lazarev Sea, Pakhomov et al. submitted or west of the Ross Sea near Adelie Land, Chiba et al. 1999). This hypothesis has not yet been proven and physiological studies are required.
The current data were collected from the end of February to the beginning of March, coinciding with the second peak of aggregate release in the S. thompsoni life cycle described by Foxton (1966). Our findings are similar to those described by Casareto and Nemoto (1986) for the region to the east of the Ross Sea. The length frequency data and development stage composition of aggregates, and the presence of solitaries at the Ross Sea slope stations at an advanced stage of development conform well to the described life cycle timing. Although it has been suggested that the development rate of salp aggregates in high Antarctic regions may be slowed by low temperatures (Chiba et al. 1999; Pakhomov et al. 2006), it appears that the S. thompsoni population on the Ross Sea slope and to the north was not similarly affected. It is not unreasonable to suggest that effects of the low temperatures may have been minimized due to the presence of CDW underlying the cold surface waters, providing salps with a warm water refuge.
Depth-stratified length frequency and stage development analysis show several similar patterns. Firstly, the smallest aggregates were almost always observed in the 100–500 m depth strata. Secondly, the highest proportion of aggregates with unfertilized eggs was found in the same depth strata. Thirdly, the development of aggregates was positively related to the sampling depth, and the most advanced development stages always coincided with the deepest depth strata. Lastly, the ready-to-spawn solitaries were found in the upper (0–200 or 0–300 and 200–500 m) layers at the Ross Sea slope stations, while young, just released solitaries were only recorded within the subsurface (>100 m down to 400 m) depth strata at the northern stations. The above summary suggests that, similar to the life cycle described in Foxton (1966), S. thompsoni aggregates descend during their development. Both aggregate chains released by solitaries and embryos released by aggregates likely occur in the subsurface (100–400 m) layers.
Our data show that as many as 100% of newly released aggregates (OAL of <5 mm) in the subsurface depth strata may not yet be fertilized. This varied between stations, but remarkably, the proportion of unfertilized eggs had dropped sharply by the time aggregates reach 8–10 mm in OAL. Two processes may have been responsible for this phenomenon. Small, unfertilized aggregates may have a very high mortality, or they are fertilized soon after release. Aggregates with no visible or malfunctioned embryo are only found in small numbers (generally <5%) in the aggregates larger than 14 mm OAL. We may only speculate that large aggregates without visible embryos could in fact be remains of small aggregates with unfertilized eggs. Proper temporal studies in the high Antarctic region are urgently needed to verify the above suggestions.
The multimodal length frequency distributions and presence of small aggregates in high numbers in both slope and open ocean stations are indicative of multiple chain release events by solitaries. This confirms that reproduction is ongoing in the vicinity of the Ross Sea, as was suggested by Foxton (1966) for S. thompsoni populations further north. Although chain release was observed at both the Ross Sea slope and ACC stations in this study, free-living young embryos were only found at the latter. This despite spent aggregates being identified in the subsurface layers of the slope stations, albeit in low numbers. It is difficult to explain this finding. The newly released embryos judging from their size could be the sampling artifact and might have been released in the net at the northern stations. It is also possible that either young solitary densities were very low and/or natural mortality among them was very high at the Ross Sea slope stations. It appears that S. thompsoni aggregates might have already released embryos at the slope stations, while they were at the pre-releasing stage at the northern stations. Although S. thompsoni (=S. gerlachei, Casareto and Nemoto 1987) have been documented from the Ross Sea and its adjacent waters by Foxton (1964), our study has for the first time provided evidence that this species may successfully reproduce in the high Antarctic realm during the summer season.
Conclusions
The presented data set indicates that S. thompsoni populations are able to utilize horizontal and vertical discontinuities in water column structure, in particular the warm CDW, to persist in the high Antarctic. Although salps need to continue migrating to the surface layers to feed, both aggregate chain and young embryo release seem to be restricted to the CDW. This may allow S. thompsoni to complete the sexual reproduction within their life cycle in the hostile high Antarctic realm. It is therefore expected that the regions where the CDW penetrates close to the shelf regions (e.g. Bellingshausen, Lazarev, Cooperation and Ross Sea) may represent hot spots for the S. thompsoni southward expansion (Pakhomov et al. 1994; Nicol et al. 2000; Klinck et al. 2004; Smedsrud 2004; Naganobu et al. 2006). Annual studies are required to prove that S. thompsoni is capable surviving through the winter and completing its life cycle. Recent studies showed that the warming trend of the Southern Ocean could be largely explained by the poleward migration of the ACC although other mechanisms, for example increased heat flux from the atmosphere or increased poleward eddy heat flux, are also possible (Gille 2008). Such displacement could bring anomalously warm waters into contact with the ice shelves and increase their melting (e.g. Shepherd et al. 2001, 2004; Rignot and Jacobs 2002). Therefore, if the Southern Ocean warming continues to accelerate in the future due to climate change, such regions may become the first areas where S. thompsoni populations may become properly established.
References
Atkinson A, Siegel V, Pakhomov EA, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Casareto BE, Nemoto T (1986) Salps of the Southern ocean (Australian sector) during the 1983–84 summer, with special reference to the species Salpa thompsoni. Mem Natl Inst Polar Res 40:221–239
Casareto BE, Nemoto T (1987) Latitudinal variation of the number of muscle fibres in Sapla thompsoni (Tunicata, Thaliacea) in the Southern Ocean, implications for the validity of the species Salpa gerlachei. Proc NIPR Symp Polar Biol 1:90–104
Catalán IA, Morales-Nin B, Company JB, Rotllant G, Palomera I, Emelianov M (2008) Environmental influences on zooplankton and micronekton distribution in the Bransfield Strait and adjacent waters. Polar Biol 31:691–707
Chiba S, Ishimaru T, Hosie GW, Wright SW (1999) Population structure change of Salpa thompsoni from austral mid-summer to autumn. Polar Biol 22:341–349
Daponte MC, Capitanio FL, Esnal GB (2001) A mechanism for swarming in the tunicate Salpa thompsoni (Foxton, 1961). Antarct Sci 13:240–245
de la Mare WL (1997) Abrupt mid-twentieth-century decline in Antarctic sea-ice extent from whaling records. Nature 389:57–60
de la Mare WK (2009) Changes in Antarctic sea-ice extent from direct historical observations and whaling records. Clim Change 92:461–493
Dubischar CD, Pakhomov EA, Bathmann U (2006) The tunicate Salpa thompsoni ecology in the Southern Ocean—II. Proximate and elemental composition. Mar Biol 149:629–632
Foxton P (1964) Salpa fusiformis Cuivier and related species. Discov Rep 32:1–32
Foxton P (1966) The distribution and life history of Salpa thompsoni Foxton with observations on a related species S. gerlachei Foxton. Discov Rep 34:1–116
Gili J-M, Rossi S, Pagès F, Orejas C, Teixidó N, López-González PJ, Arntz WE (2006) A new trophic link between the pelagic and benthic systems on the Antarctic shelf. Mar Ecol Prog Ser 322:43–49
Gille ST (2002) Warming of the Southern Ocean since the 1950s. Science 295:1275–1277
Gille ST (2008) Decadal-scale temperature trends in the Southern Hemisphere Ocean. J Climate 21:4749–4765
Hardy AC, Gunther ER (1935) The plankton of the South Georgia whaling ground and adjacent waters. Discov Rep 11:1–456
Hosie GW, Cochran TG, Pauly T, Beaumont KL, Wright SW, Kitchener JA (1997) Zooplankton community structure of Prydz Bay, Antarctica, January–February 1993. Proc NIPR Symp Polar Biol 10:90–133
Jacobs SS, Amos AF, Bruchhausen PM (1970) Ross Sea oceanography and Antarctic bottom water formation. Deep Sea Res 17:935–962
Klinck JM, Hofmann EE, Beardsley RC, Salihoglu B, Howard S (2004) Water-mass properties and circulation on the west Antarctic Peninsula continental shelf in austral fall and winter 2001. Deep Sea Res II 51:1925–1946
Krakatitsa VV, Karpenko GP, Pakhomov EA (1993) Distribution peculiarities of zooplankton depending on temperature stratification of the 1-metre surface water layer in the Cooperation Sea. In: Voronina NM (ed) Pelagic ecosystems of the Southern Ocean. Nauka Press, Moscow, pp 151–157
Le Fèvre J, Legendre L, Rivkin RB (1998) Fluxes of biogenic carbon in the Southern Ocean, roles of large microphagous zooplankton. J Mar Syst 17:325–345
Levitus A, Antonov JI, Boyer TP, Stephens C (2000) Warming of the World Ocean. Science 287:2225–2229
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Moline MA, Claustre H, Frazer TK, Grzymski J, Schofield O, Vernet M (2000) Changes in phytoplankton assemblages along the Antarctic Peninsula and potential implications for the Antarctic food web. In: Davison W, Howard-Williams C, Broady P (eds) Antarctic ecosystems, models for wider ecological understanding. The Caxon Press, Christchurch, pp 263–271
Naganobu M, Nishiwaki S, Yasuma H, Matsukura R, Takao Y, Taki K, Hayashi T, Watanabe Y, Yabuki T, Yoda Y, Noiri Y, Kuga M, Yoshikawa K, Kokubun N, Murase H, Matsuoka K, Ito K (2006) Interactions between oceanography, krill and baleen whales in the Ross Sea and adjacent waters: an overview of Kaiyo Maru-JARPA joint survey in 2004/05. Japanese Whale Research Program under Special Permit in the Antarctic (JARPA) Review Meeting, Paper SC/D06/J23. http://www.icrwhale.org/eng/SC-D06-J23.pdf
Nicol A, Pauly T, Bindoff NL, Wright S, Thlele D, Hosie GW, Strutton PG, Woehler E (2000) Ocean circulation off east Antarctica affects ecosystem structure and sea-ice extent. Nature 406:504–507
Nishikawa J, Tsuda A (2001) Diel vertical migration of the tunicate Salpa thompsoni in the Southern Ocean during summer. Polar Biol 24:299–302
Orsi AH, Wiederwohl CL (2009) A recount of Ross Sea waters. Deep Sea Res II 56:78–795
Orsi AH, Whitworth T III, Nowlin WD Jr (1995) On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Res I 42:641–673
Pakhomov EA (1993) Vertical distribution and diel migrations of Antarctic macroplankton. In: Voronina NM (ed) Pelagic ecosystems of the Southern Ocean. Nauka Press, Moscow, pp 146–150
Pakhomov EA (1994) Diel vertical migrations of Antarctic macroplankton. Salpidae, Ctenophora, Coelenterata, Chaetognatha, Polychaeta, Pteropoda. Oceanology 33:510–511
Pakhomov EA, Grachev DG, Trotsenko BG (1994) Distribution and composition of macroplankton communities in the Lazarev Sea (Antarctic). Oceanology 33:635–642
Pakhomov EA, Froneman PW, Perissinotto R (2002) Salp/krill interactions in the Southern Ocean, spatial segregation and implications for the carbon flux. Deep Sea Res II 49:1881–1907
Pakhomov EA, Dubischar CD, Strass V, Brichta M, Bathmann U (2006) The tunicate Salpa thompsoni ecology in the Southern Ocean—I. Distribution, biomass, demography and feeding ecophysiology. Mar Biol 149:609–623
Pakhomov EA, Dubischar CD, Hunt BPV, Strass V, Cisewski B, Siegel V, von Harbou L, Gurney L, Kitchener J, Bathmann U (in review) Pelagic tunicates in the Lazarev Sea, Southern Ocean. Deep Sea Res II
Park C, Wormuth JH (1993) Distribution of Antarctic zooplankton around Elephant Island during the austral summers of 1988, 1989, and 1990. Polar Biol 13:215–225
Perissinotto R, Pakhomov EA (1998a) Contribution of salps to carbon flux of marginal ice zone of the Lazarev Sea, Southern Ocean. Mar Biol 131:25–32
Perissinotto R, Pakhomov EA (1998b) The trophic role of the tunicate Salpa thompsoni in the Antarctic marine ecosystem. J Mar Syst 17:361–374
Phillips B, Kremer P, Madin LP (2009) Defecation by Salpa thompsoni and its contribution to vertical flux in the Southern Ocean. Mar Biol 156:455–467
Piatkowski U (1985) Distribution, abundance and diurnal migration of macrozooplankton in Antarctic waters. Meeresforsch 30:262–279
Picco P, Bergamasco A, Demicheli L, Manzella G, Meloni R, Paschini E (2000) Large-scale circulation features in the central and western Ross Sea (Antarctica). Ross Sea Ecology. Springer, New York, pp 95–105
Rickard GJ, Roberts MJ, Williams MJM, Dunn A, Smith MH (2010) Mean circulation and hydrography in the Ross Sea sector, Southern Ocean: representation in numerical models. Antarct Sci. doi: 10.1017/S0954102010000246
Rignot EJ, Jacobs SS (2002) Rapid bottom melting widespread near Antarctic ice sheet grounding lines. Science 296:2020–2023
Russo A (2000) Water mass characteristics during the ROSSMIZE cruise (Western sector of the Ross Sea, November–December 1994). Ross Sea Ecology. Springer, New York, pp 83–93
Shepherd A, Wingham DJ, Mansley AD, Corr HFJ (2001) Inland thinning of Pine Island Glacier, West Antarctica. Science 291:862–864
Shepherd A, Wingham DJ, Rignot E (2004) Warm Ocean is eroding West Antarctic ice sheet. Geophys Res Lett 31:L23402. doi:10.1029/2004GL021106
Siegel V, Skibowski A, Harm U (1992) Community structure of the epipelagic zooplankton community under the sea-ice of the northern Weddell Sea. Polar Biol 12:15–24
Smedsrud LH (2004) Warming of the deep water in the Weddell Sea along Greenwich meridian, 1977–2001. Deep Sea Res I 52:241–258
Voronina NM (1998) Comparative abundance and distribution of major filter-feeders in the Antarctic pelagic zone. J Mar Syst 17:375–390
Walsh JJ, Dieterle DA, Lenes J (2001) A numerical analysis of carbon dynamics of the Southern Ocean phytoplankton community, the roles of light and grazing in effecting both sequestration of atmospheric CO2 and food availability to larval krill. Deep Sea Res I 48:1–48
Acknowledgments
We would like to thank the captain and crew of the RV Tangaroa for invaluable help during the IPY-CAML sampling program and our colleagues for kind assistance in sample collections. The surveys were financially supported by the NIWA. Finally, NIWA (New Zealand), University of British Columbia (Canada), Alfred Wegener Institute for Polar and Marine Research and Alexander von Humboldt Foundation (Germany) are greatly acknowledged for providing funds and facilities allowing the completion of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pakhomov, E.A., Hall, J., Williams, M.J.M. et al. Biology of Salpa thompsoni in waters adjacent to the Ross Sea, Southern Ocean, during austral summer 2008. Polar Biol 34, 257–271 (2011). https://doi.org/10.1007/s00300-010-0878-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0878-9