Abstract
Measurements of the defecation rate of Salpa thompsoni were made at several stations during two cruises west of the Antarctic Peninsula in 2004 and 2006. Rates were quantified in terms of number of pellets, pigment, carbon and nitrogen for a wide size range of both aggregate and solitary salps. Measured defecation rates were constant over several hours when salps were held at near-surface conditions from which they had been collected. The defecation rate per salp increased with both salp size and the ambient level of particulate organic matter (POM) in the upper water column. The weight-specific defecation rate ranged between 0.5 and 6% day−1 of salp body carbon, depending on the concentration of available particulate matter in the water. Carbon defecation rates were applied to biomass estimates of S. thompsoni to calculate daily carbon defecation rates for the populations sampled during the two cruises. Dense salp populations of over 400 mg C m−2 were calculated to produce about 20 mg C m−2 day−1, comparable to other major sources of vertical flux of organic material in the Southern Ocean. Measured sinking rates for salp fecal pellets indicated that the majority of this organic material could reach deep sediments within a few days, providing a fast and direct pathway for carbon to the deep ocean.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salps (subphylum Urochordata, class Thaliacea) are pelagic tunicates found throughout the world’s oceans. Salpa thompsoni is the principal salp species in the Southern Ocean (Foxton 1966; Casareto and Nemoto 1986; Pakhomov et al. 1994). Salps are indiscriminate filter feeders, utilizing a pharyngeal mucus net to capture particles as water is pumped through the body (Madin and Diebel 1998). Therefore, they are assumed to ingest all particulate matter (both living and detrital) small enough to fit through their oral opening and large enough to be retained by the mucus net. This may include particles as small as 1–2 μm (Harbison and McAlister 1979; Kremer and Madin 1992; Bone et al. 2003). Measured ingestion rates of S. thompsoni, expressed as mg C ind.−1 day−1, have been estimated to be higher than any other Antarctic herbivore (Perissinotto and Pakhomov 1998).
Salps produce large, dense fecal pellets that are enriched in carbon and nitrogen (as percent dry weight) compared to salp body composition (Andersen 1998). These pellets sink rapidly, and when salps are abundant they may play a significant role in the transport of particulate matter to the deep sea (Wiebe et al. 1979; Bruland and Silver 1981; Fortier et al. 1994). The vertical flux is maximized if the pellets do not fragment or decompose before reaching the benthos (Caron et al. 1989; Dubischar and Bathmann 2002). This is particularly relevant in high latitude waters, where colder water temperatures (0–5°C) may decrease the rate of bacterial decomposition relative to other oceanic regions. S. thompsoni is known to vertically migrate up to 500 m day−1 (Nishikawa and Tsuda 2001), and therefore its overall role in transport of particulate organic matter (POM) could be affected by its depth distribution (Perissinotto and Pakhomov 1998).
The abundance and distribution of S. thompsoni has been measured in several studies (Foxton 1966; Huntley et al. 1989; Chiba et al. 1999; Pakhomov 2002; Atkinson et al. 2004) and in two cases population size and structure has been coupled with direct defecation measurements (Huntley et al. 1989; Pakhomov et al. 2006). However, these two studies measured defecation for only very few specimens that have been collected using plankton nets. The goal of this research was a more comprehensive quantification of the defecation rate of S. thompsoni, relating these rates to body size, temperature and food availability. The sinking rate of salp fecal matter was also investigated. From these measurements, estimates of the contribution to the vertical flux of POM by S. thompsoni were calculated for salp populations sampled during both the early summer (2004) and late summer (2006) in the region West of the Antarctic Peninsula.
Methods
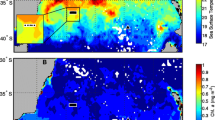
This study was conducted during two cruises of the ASRV Laurence M. Gould, November/December 2004 and February/March of 2006. Both cruises sampled the waters west of the Antarctic Peninsula (Fig. 1). Specimens of S. thompsoni were collected during both cruises (LMG 04-14 and LMG 06-02) using blue-water diving methods first described by Hamner (1975) and more recently by Haddock and Heine (2005). Salps were gently captured in quart jars and the time and depth were recorded by the diver. Specimens represented the range of available sizes of both aggregate and solitary stages of S. thompsoni. Selection of salps to use in this study was biased towards the aggregate stage since it dominated the biomass of the salp population.
Salps were gently transferred within ~1 h of collection into shipboard aquaria that had been filled at the collection station with subsurface raw seawater. Salps were segregated into different containers based on length and stage (5–10 aggregates or 1–2 solitaries per tank). In 2004, all defecation measurements were made using 19 L containers kept at ambient surface water temperature, 0°C. To regulate the temperature, incubation containers were immersed in large fiberglass aquaria filled with flowing surface seawater. In 2006, salps were kept in 132 L containers at 3°C, ambient surface water temperature during that cruise. Depending on the sea state, a lid was used to prevent water spillage.
There are several problems associated with holding salps. The size and shape of the container, availability of fresh seawater, and turbulent disturbance all affect a salp’s feeding and swimming behavior. Attempts to improve shipboard holding methods for salps have met with some success (Madin and Purcell 1992; Madin and Diebel 1998). Salpa spp. are particularly active swimmers and are therefore challenging to get to behave naturally when confined. Previous studies on Salpa spp. in shipboard experiments have used smaller containers than our 2006 aquaria with limited success (Huntley et al. 1989; Pakhomov 2004; Madin et al. 2006; Pakhomov et al. 2006). Since salps filter feed while propelling themselves through the water (Madin 1974), the feeding behavior, and consequently the defecation rate is directly affected by the salp’s ability to swim freely. We therefore assumed that defecation measurements would be most reliable if the salps were held in as large containers as possible and thus enlarged the incubation tanks for the 2006 cruise.
To determine the defecation rate of S. thompsoni, feces were periodically collected over several hours using an elongated pipette. Any fecal pellets released prior to transfer from the collection jar were included as part of the time series. After each quantitative recovery, pellets from each experimental tank were held in lightproof containers at ambient water temperature for the duration of the time series. Most of these incubations were 8–10 hours, but some longer time series quantified defecation rates for >24 h. Defecation rates were measured in terms of number of fecal pellets, chlorophyll-a plus phaeopigments, and elemental carbon and nitrogen. During 2006, simultaneous measurements of fecal pellet carbon, nitrogen and pigment were made for most defecation runs by visually dividing the total feces collected from each defecation run. For the 2004 cruise, only counts and pigment measurements were made for the defecation runs. Conversion to carbon and nitrogen was based on independently measured C:N:pigment ratios of S. thompsoni feces from salps collected at the same station.
Seawater was collected with Niskin bottles at one or two depths within 30 m of the surface and filtered through GFF filters (pre-combusted for elemental measurement) for analysis of pigment, carbon and nitrogen. Before each defecation time series, an aliquot of seawater from each container was also filtered for pigment, carbon and nitrogen for comparison with the CTD results. In addition, water from each incubation tank was filtered and extracted for Chl-a and phaeopigments following each run. The water temperature of each container was checked periodically to confirm the salps were held at the ambient surface water temperature.
Pigment analysis of both filters and feces was done on shipboard. Fecal matter was ground in 3 ml of 90% acetone in a glass tissue grinder using a motorized teflon pestle, and transferred into 13 mm diameter screw-top test tubes. Another 3 ml of acetone was used to rinse the tissue grinder and added to each test tube. Samples were held refrigerated in the dark for 24 h and centrifuged just prior to reading. Immediately after water samples were filtered, filter pads were transferred into 6 ml of 90% acetone and the pigment was extracted in a dark refrigerator for 24 h. Filter pads were removed and the test tubes centrifuged just prior to reading the fluorescence (Madin and Cetta 1984). Fluorescence was read with a Turner Designs 10 AU fluorometer, and dilutions using 90% acetone were made when necessary to prevent quenching. Following the initial fluorescence reading, samples were acidified with ~0.2 ml of HCl and reread. The fluorometer was calibrated using a spectrophotometer during each cruise using standard Chl-a derived from spinach. Chlorophyll-a and phaeopigments were calculated from standard equations (Strickland and Parsons 1972).
Body length was measured for all salps as the distance between oral and aboral openings (omitting pointed tips for the aggregate form). The combined relationship for both years between body length and carbon weight followed a power relationship:
where Wcarbon is body carbon weight (μg) and L is salp length (mm).
Samples for carbon and nitrogen were dried at 60°C in a drying oven, then stored in a −20°C freezer and redried just prior to analysis. Elemental analysis was performed using a Carlo-Erba ES1500 elemental analyzer within 6 months of collection.
The sinking velocities of S. thompsoni fecal pellets were measured using a 2,000-ml graduated cylinder filled with seawater at ambient sea surface temperature (0°C in 2004, and 3°C in 2006). Pellets were collected opportunistically from defecation incubations and other shipboard experiments and represented the size range of both aggregate and solitary salps. The size and shape of each pellet was measured using a calibrated dissecting microscope. Measured pellets were gently released below the meniscus at the top of the cylinder and the time required to fall a standard distance (40 cm) was recorded. Measurements were made only when the ship motion was minimal and water in the cylinder was steady. A wide size range of salps were used and 5–10 pellets were measured to get average sinking rates for pellets from single chains of aggregates.
Net tows to determine the standing stock of salps were made with a 10 m2 Tucker trawl (2004) and a 1 m2 MOCNESS (2006). During 2004, trawls were made once a day (at minimum incident light) with an open net (3 mm mesh) to a depth of 100 m, unless limited by the depth of the bottom. During 2006 trawls sampling eight depth strata to 500 m were made multiple times a day at some stations as part of a study of salp diel vertical migration. Salps from each net were counted and measured at each station to determine the size frequency. For some hauls a representative aliquot was used to estimate the total. To calculate salp carbon biomass for each station, equations given above were applied to the size frequency data for each net haul and then divided by the estimated volume filtered for the tow. The depth interval for each net was then summed to calculate salp C m−2 for the station. At stations where several net tows were made, an average biomass and standard deviation was calculated. The defecation rate, mg C m−2 day−1, for the sampled population was calculated by applying empirically measured relationships (overall upper and lower estimates using data from both years) to the size frequency results from each net.
Results
A total of 40 defecation time series were measured at nine separate stations. Three of the sampling stations were occupied in 2004 and the remaining six in 2006 (Fig. 1). The stations in 2006 were further offshore from the Antarctic Peninsula than those in 2004, as salps were not abundant in the waters over the continental shelf during that year. In November–December 2004 the water temperature in the top 20 m at all sampling stations was ~0°C and in February–March 2006 it was ~3°C. Chlorophyll-a and particulate carbon and nitrogen varied from station to station (Table 1). Two stations in 2004 (2004–45 and 2004-Deception Island) were considerably higher in POM and Chl-a than the third, while only one station in 2006 had POM levels of >100 μg C l−1 (2006-1). Measured pigment in surface waters from both years was 75–95% Chl-a.
For each defecation run, pellet production rates by S. thompsoni aggregates were measured to be approximately constant over the measured time interval. Almost all salps exhibited an ‘acclimation period’ of 1–2 h immediately following their transfer from collection jars into aquaria (Fig. 2). After that acclimation, a relatively constant rate in pellet production was observed in all but two incubation tanks. This pattern is consistent with previous observations of cumulative pigment defecation over time (Madin and Kremer 1995; Pakhomov et al. 2006). The observed ‘acclimation period’ from this study differs from results of Pakhomov (2004), who observed a faster defecation rate in the first 4–8 h of incubation. Aggregate salps produced feces at a mean rate of 0.25 ± 0.12 pellets h−1 ind−1, or one pellet about every 4 h, with an overall range of 0.03–0.46 pellets ind.−1 h−1. Salps at high POM stations defecated more pellets ind.−1 day−1 (0.33 ± 0.12 pellets ind.−1 h−1, n = 9) than salps at low POM stations (0.19 ± 0.10 pellets ind.−1 h−1, n = 23; P < 0.01 t-test). Specimens of the solitary form of S. thompsoni (not shown) defecated 0.4–0.6 pellets h−1, or one pellet about every 2 h. The ‘acclimation period’ that was observed for aggregate defecation was not observed for solitaries.
Cumulative number of defecated pellets by S. thompsoni aggregates over time for a representative selection of defecation runs (L = body length in mm, n = number of salps in each run). Each series is an independent group of aggregate salps in a separate container. Note the depressed initial slope of each series, indicating a short acclimation period
Longer time series measurements did not show a clear diel cycle in pellet production (Fig. 3). It is important to note, however, that pellet counts for only one of these defecation runs were made with sufficient time resolution to effectively measure a diel pattern. There was enough inconsistency in pellet production rates from hour to hour (Fig. 3) to mask any diel variability of less than a factor of 2–3. Furthermore, the salps were not exposed to diel food variability during these incubations as in the field where they vertically migrate (Nishikawa and Tsuda 2001).
Two of the three stations sampled in 2004 (2004-45, 2004-Deception Island) measured more than five times higher in surface Chl-a than all other stations in this study (Table 1). When the results for both the aggregates and solitaries from these two stations are compared with results from stations with low Chl-a, there is an obvious effect of high ambient chlorophyll as well as salp size on the pigment-based defecation rates (Fig. 4). As stated above, salps from the high Chl-a/high POM stations produced fecal pellets at a rate about 70% faster than salps from the low Chl-a stations. In addition, the carbon:pigment ratio for feces produced by salps at these two stations were consistently low (20 ± 8 n = 20) compared with the results from 2006 when the ratios were more variable and typically >100.
In 2004 carbon and nitrogen were not measured directly as part of the defecation time series, but estimated using pigment:C:N ratios for S. thompsoni feces collected at the same stations but not from defecation rate measurements. Using these independently derived ratios along with direct measurements from 2006, aggregate salps were calculated to defecate between 8.4–503.2 μg carbon ind−1 day−1 (Fig. 5) and 0.8–79.1 μg nitrogen ind−1 day−1 (not shown). Lower and upper estimates of the allometric relationship between salp size and carbon defecation rate were derived from Fig. 5 and are expressed as:
Carbon defecation rates as a function of body mass for S. thompsoni aggregates. ‘High’ POM stations are defined as >150 μg C l−1 and >27 μg N l−1, ‘Low’ POM stations are <85 μg C l−1 and <21 μg N l−1. Trendlines represent ‘upper’ and ‘lower’ estimates of the relationship between carbon defecation and salp length
where w is body mass, expressed as mg C per individual. The fecal C:N ratio (by weight) for all stations averaged 6.2 ± 1.3. Larger salps generally defecated more carbon and nitrogen ind.−1 day−1, with increased variability for the largest aggregates. These larger specimens might be disturbed more easily by the relative size of the container to their bodies, or they may actually slow their ingestion and defecation as they near the end of their reproductive life. In general, the carbon defecation rates reflected the ambient particulate carbon of the surface waters (Table 1). In two instances, salps feeding in low-POM environments defecated at rates comparable to salps in high POM environments and in one case salps from high-POM waters defecated at a lower rate. The ‘lower’ and ‘upper’ trendlines of carbon defecation as a function of length (Fig. 5) encompass the range of results measured in this study.
Numerous solitary individuals were encountered during several net collections, accounting in some cases for >35% of the total carbon biomass. However, this study made only one direct measurement of carbon defecation by the solitary form, a 134 mm individual that defecated 3.42 mg C day−1. To supplement this result, C:pigment ratios from aggregate fecal samples were used to estimate carbon defecation rates on six other solitary individuals where pigment defecation was directly measured. From this very limited dataset, a solitary body mass-to-carbon defecation relationship was derived:
where w is body mass, expressed as mg C per individual. The (w – 5.17) term in Eq. 5 accounts for the observation that solitaries do not begin producing fecal pellets until they are ~40 mm in length, or 5.17 mg C body mass. This body size is typically reached within a few days after they are released from their aggregate ‘mothers’ (authors’ personal observations).
Daily defecation accounted for 0.5–6.6% of body carbon weight (Fig. 6) and 0.3–4.5% of body nitrogen weight (not shown). Defecation rates for solitary salps expressed in terms of body carbon were similar to the results for aggregates. Salps collected in high POM waters generally defecated more of their body mass (3.9 ± 1.5% body carbon weight day−1) than specimens collected in low POM regions (2.1 ± 1.8% body carbon weight day−1).
Despite a >3°C difference in ambient surface water temperature, no temperature effect was apparent in the defecation rates. Rates in both high and low POM environments yielded coherent results despite temperature differences between 2004 and 2006 (Fig. 5). The highest rates of defecation were measured during conditions where temperature was the lowest (high Chl-a and POM in 2004 at 0°C). Any temperature effect that depressed the rate of defecation at the colder temperature, was apparently more than offset by the high ambient levels of particulates. In 2004, the defecation measurements made at the single station with low levels of Chl-a and POM were consistent with defecation rates from low POM waters and higher temperature in 2006 (Figs. 5, 6).
A wide range of salp population densities and sizes were encountered during this study. The 2004 cruise was characterized by lower salp biomass, with no single net tow exceeding 33 mg C m−2 of salp biomass. Most of the salp biomass collected during 2004 was composed of aggregate salps 20–35 mm in length and very few solitaries. It is important to note that nighttime net tows in 2004 were relatively shallow (<100 m), as well as in shelf waters. The 3 mm mesh size for these Tucker trawl samples was also appreciably larger than for samples taken in 2006. Therefore, it is likely both the numbers and biomass of small aggregates are underestimated for the 2004 cruise. In contrast, the 2006 sampling was farther offshore in deeper water (see Fig. 1) and some MOCNESS tows were made to depths >500 m. The second cruise was also later in the austral summer and higher salp densities were generally encountered, as well as a greater proportion of solitary individuals. An overview of these data is presented in Table 2, along with estimates for low and high rates of carbon flux based on the relationships described by Eqs. 3 and 4 for aggregates and Eq. 5 for solitaries. Two stations (2006-4 and 2006-7) averaged a biomass ca. 400 mg C m−2 (seven and five tows, respectively) and a daily fecal pellet production rate of 4–20 mg C m−2 day−1. The highest salp biomass during this study was 863 mg C m−2 with an estimated daily fecal production rate of 8–42 mg C m−2 day−1.
Fecal pellet sinking velocities were measured for a wide size range of aggregate salps ranging from 10–55 mm in length. The pellet diameter ranged between 0.5–3.5 mm, with larger salps producing larger pellets. As there was some variability in pellet sinking rates from the same chain of aggregates, an average rate is reported for pellets (n = 5–10) measured from the same chain (Fig. 7). The relationship between measured pellet sizes and sinking velocity was approximately linear:
where V = sinking velocity, cm s−1, and D = pellet diameter, mm. Calculated sinking velocities ranged from 0.25–1.65 cm s−1 (200–1,400 m day−1), with an average rate of 0.81 cm s−1 (700 m day−1).
Fecal pellets produced by S. thompsoni solitaries were larger than their aggregate counterparts (3.0–5.5 mm in diameter), but were irregular in size and fragmented easily when handled. Sinking rates for solitaries were determined for only three body lengths (90, 105, 120 mm). A positive relationship was observed between solitary length and pellet diameter, and sinking rates for pellets from solitaries were consistent with rates for aggregate salps.
Discussion and conclusions
Salps are particularly difficult to use experimentally, and the measured rates presented here are the most detailed defecation results obtained for any salp species. Altogether, forty time series measurements were made during two cruises, at nine stations over two different years and seasons. This study includes measurements of defecation in terms of pellet number, pigment, and carbon/nitrogen for a broad size range of both solitary and aggregate forms of S. thompsoni. Ambient environmental conditions during this study covered a wide range of temperatures (<0 to >3°C), chlorophyll a (<0.1 to >3 μg l−1), and POC (<60 to >230 μg l−1). Prior to this study, there have been very few defecation rates published for S. thompsoni (Huntley et al. 1989; Pakhomov et al. 2006). For comparison, these rates are presented with results for similar sized aggregates from the present study (Table 3) including both lower and upper estimates for this study based on regressions from Fig. 5.
The rates of defecated pigment were generally comparable between our results and the results for two chains previously published (Pakhomov et al. 2006). Unfortunately, there are no data for the ambient Chl-a concentration at one of the two stations from that earlier study, and no defecation rates in terms of pigment from Huntley et al. (1989). Therefore, previous results cannot be used to support our findings about the strong effect of near-surface Chl-a on the defecation rate (as measured by pigment release).
Fecal production in terms of both carbon and nitrogen were higher in the earlier studies (Huntley et al. 1989; Pakhomov et al. 2006) than rates measured in this study for the same sizes of salps and similar ambient POM (Table 3). The small sample size from these earlier studies (total n = 3) precludes a detailed analysis of any systematic differences among the results, but the difference has a direct effect on defecation estimates for both individuals (Table 4) and populations (Table 6). Based on our results (Fig. 6, Table 4), S. thompsoni would be expected to defecate 4–6% of its body carbon per day when found in particle rich waters (>2 μg Chl-a l−1, >200 mg C l−1), and 0.5–2% in waters with low particulate concentrations.
Fecal pellet production was approximately constant for several hours, with the cumulative number produced increasing linearly over time. Only one other study has reported pellet production rates for S. thompsoni (Pakhomov et al. 2006). Their results for two chains of aggregates averaged 0.08 pellets ind.−1 h−1, near the low end of the rate of pellet production measured in this study.
Diel periodicity is a potentially important consideration when calculating daily defecation rates. This study measured defecation rates for freshly collected salps only at near-surface conditions of both Chl-a and POM. No experimental manipulations were carried out to simulate conditions once the salps migrate to depth. In the absence of results from such experimental manipulations, daily defecation rates for this study (Tables 2, 3, 4, 6) were extrapolated from measurements at near-surface conditions. It is likely, however, that these rates considerably overestimate actual in situ rates from an active vertically migrating population of salps. Defecation rate would be expected to decrease once the salps vertically migrate below the higher stock of particulate matter in the photic zone. Assuming the daytime defecation rates at depth are comparable to the rates measured at the low POM concentrations (Table 4), the upper estimates of our flux calculations (Table 2 high average and Table 6) would decrease by 40%.
Other studies have conservatively calculated salp contribution to vertical flux assuming fecal production for only 8 h/day (Madin et al. 2006) or 16 h/day (Wiebe et al. 1979; Madin et al. 1997), based on the reasoning that defecation occurs primarily when salps are actively feeding in warm surface water where there is more POM. Based on our unpublished observations of the time S. thompsoni spent near the surface (6–8 h), a conservative estimate of 24 h defecation rates would reduce measured rates for freshly collected salps by 70%.
The highly variable pellet production rates observed for the salp chains held >24 h in this study (Fig. 3) did not indicate any clear diel pattern. This is not surprising, as the conditions of the incubation were comparable to the near-surface waters, higher in both Chl-a and POM than ambient conditions typical of the daytime depths of S. thompsoni at >200 m, (Nishikawa and Tsuda 2001; Nishikawa et al. (personal communication)). We did not observe the “substantially lower” pellet production rates during daylight hours reported by Pakhomov (2004) and Pakhomov et al. (2006), which were based on a comparison of two salp chains.
If daily rates are calculated from a simple extrapolation of measured hourly rates at near-surface conditions, salps in this study defecated on average the equivalent of 2.5% of their body C day−1. The few other measurements for S. thompsoni estimated daily defecation rates in the range of 6–20% body C day−1 (Table 4). As expected, salps from other (warmer) oceanic areas have been measured to defecate at higher rates, 9–31% when expressed as a percentage of their body C (Table 4). Increased ingestion associated with warmer seawater temperatures has been demonstrated for a related species, S. fusiformis (Andersen 1986), and presumably defecation and ingestion rates are tightly coupled.
We have combined our results for defecation rates with measurements of salp biomass and water particulates (pigment and carbon) to estimate the rate of removal by salps of particulate matter from the upper water column (Table 5). In making these calculations, we assume the following: the salps stay in the upper 50 m no more than 10 h per day (Nishikawa and Tsuda 2001; Nishikawa et al. 2008); there is no more than 50% degredation of chlorophyll and phaeopigments into non-fluorescing compounds (Madin and Purcell 1992; Madin et al. 2006); and the carbon assimilation efficiency (1-defecation/ingestion) is 75%, a reasonable estimate for omnivorous zooplankton. Not surprisingly, for 2004, when stocks of particulates were high and salps stocks low, the calculated removal rates in terms of both carbon and pigment were very low, <0.1% day−1. Calculated removal rates for 2006, when salp stocks were much higher, increased but were still low, <1% day−1 (Table 5). Based on these calculations, salps did not have an appreciable effect on phytoplankton stocks during the time of our observations.
The C:N ratio of feces from S. thompsoni measured in this and other studies was generally lower than the ratio (range 8–24) measured for other species of salps (Table 4). This difference may reflect an environment for salps in the Southern Ocean with seasonally higher food quality than the habitats of many salp species, oligotrophic environments where nitrogen is in very short supply. In this study, the average C:N ratio by weight of particulates in the near-surface seawater was 4.8 ± 0.8, slightly lower than the ratio of the feces (6.2 ± 1.3). This increase in the C:N ratio between food source and feces is not surprising, given the feeding mechanism of salps that uses carbon-rich mucus to capture food particles. This mucus matrix remains in the fecal pellet following digestion, producing feces that are relatively carbon rich compared with the ingested food particles. This is especially the case in habitats where food is scarce.
In addition, some of the ambient POM was comprised of material that salps could ingest but not digest. Pteropod shells and crustacean bits were abundant in both the gut contents and feces of salps at several stations in 2006, in particular stations 2006-3, 2006-4, and 2006-5. Feces collected from station 2006-1 contained crustacean fragments but no pteropod shells were observed. Pteropod shells are composed of calcium carbonate and are a prime example of ‘non-labile’ POM. High concentrations of these shells, along with undigested crustacean fragments, might explain some of the variability and inconsistency in our results.
Overall, salps feeding and defecating in higher-POM water were measured to defecate more carbon and nitrogen than salps found in low-POM water. This implies that salps feeding in waters with elevated levels of POM will contribute more per unit biomass to vertical flux than their counterparts feeding in waters with lower POM. Nevertheless, high densities of salps are usually found offshore, and overall salp biomass as well as specific defecation rate is critical in determining the overall contribution to vertical flux from salps. Offshore, salps may be relatively more important to the overall flux of organic matter as other major sources such as phytoplankton blooms, copepods and krill are relatively less abundant.
Salpa thompsoni fecal material has the potential to reach the benthos within one to several days, depending on water depth and the size of the salps producing the fecal pellets (Fig. 7). Water depths where salps were collected in this study ranged from 500–4,000 m; most stations in our 2006 survey were beyond the continental shelf and at the upper end of this depth range. The salp population sampled in 2004 was comprised largely of aggregates 20–30 mm in length. Fecal pellets from salps of this size are predicted from our results to sink at ~700 m day−1. In water 3,500 m deep these pellets would reach the seafloor in 5 days, while on the continental shelf it would take less than a day. The population of larger aggregates observed in 2006 produced faster-sinking pellets which would sink to the bottom in even less time. Previous work indicates that the organic composition of salp fecal material would not be affected in this period of time (Caron et al. 1989), as microbial decomposition in cold Antarctic waters would likely occur at a depressed rate.
Sinking rates of salp fecal pellets show a coherent pattern of sinking velocity with salp size (Fig. 7). These measurements predict that feces from smaller aggregate salps (10–20 mm) would sink 200–400 m day−1. These results are considerably lower than measurements of feces from 13 mm aggregates (Pakhomov et al. 2006), which ranged from 760 m day−1 for loosely packet pellets to over 1,600 m day−1 for compact pellets . The reason for the large discrepancy in these results is not obvious, as comparable methods were used in both studies. Variability in the measured velocities may be attributed to irregular pellet sizes, turbulence inside the graduated cylinder, and/or non-uniform pellet densities. It should be noted, however, that our study measured sinking rates for many fecal pellets, collected from 22 separate aggregate chains originating from multiple stations and a range of temperatures and food availability.
Fecal pellets produced by solitaries were generally larger than fecal pellets of aggregates, but broke up much easier and were fairly irregular in shape. Measured sinking rates were similar to that of aggregate pellets (600–1,600 m day−1). Some larger pellets sank slower than the smaller pellets produced by aggregates, but this may be due to pellet fragmentation resulting in increased surface area. Regardless, fecal material produced by solitary salps would reach the benthos in approximately the same amount of time as aggregate fecal material, on the order of one to several days.
The contribution to vertical flux by salp populations has been estimated in a number of studies (Table 6). The wide variability of these measurements is correlated with the high variability of salp biomass, as well as differences in salp species and environmental variables. By coupling measured defecation rates with measurements of population biomass, our results for S. thompsoni’s contribution to vertical carbon flux were consistent with earlier estimates based on much fewer data. Salp blooms are patchy both spatially and temporally, but when observed in high densities salps have the potential to contribute a large amount of rapidly-sinking fecal matter.
To put defecation and sinking rates for salps into a broader ecological context, it is necessary to compare the potential vertical flux from salps with other major sources. Phytoplankton, copepods and krill also have the potential to contribute large amounts of downward-sinking organic material in the Southern Ocean (Table 7). However, their contribution differs markedly from salp feces in several ways. Much of the organic material derived from primary production is continually recycled in the microbial loop and only a fraction actually reaches deep sediments (Longhurst and Harrison 1989; Froneman et al. 2004), primarily as ungrazed large diatoms that sink after a bloom. When found in high densities, copepods have the potential to defecate large amounts of downward-sinking material. However, copepod feces are much smaller than salp feces and sink much more slowly. These feces are largely remineralized in the euphotic zone (usually by re-ingestion or disintegration by the same grazing copepods), which greatly reduces their potential to reach deep sediments (Fortier et al. 1994; Turner 2002). Euphausiid fecal material is loosely packed and stringy, sinking much less quickly than salp feces. Furthermore, adult krill are normally found further inshore than salps in the Southern Ocean, spatially segregating their contribution to vertical flux (Pakhomov 2004). Comparing the results for S. thompsoni (Table 6) with these other sources (Table 7), it is clear that when abundant, salps need to be considered as a major contributor to the vertical flux of organic matter.
In general, vertical POM flux in the Southern Ocean is lower than found in other major oceanic regions (Honjo 1990). Increased total mass flux is observed at marginal ice zones, exhibiting high seasonal variability and peaking during summer months (Honjo et al. 2000; Collier et al. 2000; Fischer et al. 2002). Some inshore regions such as the Bransfield Strait experience pulses in increased fecal flux, normally associated with high populations of krill or copepods (Schnack-Schiel and Isla 2005). The ice-free regions that S. thompsoni populations inhabit (North of marginal ice and South of the Polar Front) exhibit depressed sedimentation rates (Honjo et al. 2000; Collier et al. 2000), and are usually much deeper (>1,000 m) than marginal ice zones. Deep-water sediment trap measurements made simultaneously at several depths reveal that relatively little material settles beyond the upper 200 m (Collier et al. 2000); therefore, the deep open-water of the Southern Ocean has even lower annual sedimentation. Pulses of fecal material from S. thompsoni blooms could have a considerable impact on benthic communities in these regions, periodically supplying large amounts of fresh organic material to habitats accustomed to relatively little POM input from the surface. However, S. thompsoni is only abundant in the austral summer, so such pulses take place only seasonally. The hypothesized increase in salp dominance in the Southern Ocean due to a depletion of sea ice (Loeb et al. 1997; Atkinson et al. 2004) would be expected to increase the annual contribution to vertical flux by S. thompsoni.
References
Andersen V (1986) Effects of temperature on the filtration rate and percentage of assimilation rate of Salpa fusiformis Cuvier (Tunicata: Thaliacea). Hydrobiologia 137:135–140
Andersen V (1998) Salp and pyrosomid blooms and their importance in biogeochemical cycles. In: Bone Q (ed) The biology of pelagic tunicates. Oxford University Press, Oxford, pp 125–137
Andersen V, Nival P (1988) A pelagic ecosystem model simulating production and sedimentation of biogenic particles: role of salps and copepods. Mar Ecol Prog Ser 44:37–50
Atkinson A, Siegel V, Pakhomov EA, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Bone Q, Carre C, Chang P (2003) Tunicate feeding filters. J Mar Biol Ass UK 83:907–919
Bruland KW, Silver MW (1981) Sinking rates of fecal pellets from gelatinous zooplankton (salps, pteropods doliolids). Mar Biol 63:295–300
Caron DA, Madin LP, Cole JJ (1989) Composition and degredation of salp fecal pellets: implications for vertical flux in oceanic environments. J Mar Res 47:829–850
Casareto B, Nemoto T (1986) Salps of the Southern Ocean (Australian Sector) during the 1983–84 summer, with special reference to the species Salpa thompsoni, Foxton 1961. Mem Natl Polr Res 40:221–239
Chiba S, Ishimaru T, Hosie GW, Wright SW (1999) Population structure change of Salpa thompsoni from austral mid-summer to autumn. Polar Biol 22:341–349
Collier R, Dymond J, Honjo S, Manganini S, Francois R, Dunbar R (2000) The vertical flux of biogenic and lithogenic material in the Ross Sea: moored sediment trap observations 1996–1998. Deep Sea Res Pt II 47:3491–3520
Dagg MJ, Urban-Rich J, Peterson JO (2003) The potential contribution of fecal pellets from large copepods to the flux of biogenic silica and particulate organic carbon in the Antarctic Polar Front region near 170°W. Deep Sea Res Pt II 50:675–691
Dubischar CD, Bathmann UV (2002) The occurrence of faecal material in relation to different pelagic systems in the Southern Ocean and its importance for vertical flux. Deep Sea Res Pt II 49:3229–3242
Ducklow HW, Baker K, Martinson DG, Quetin LB, Ross RM, Smith RC, Stammerjohn SE, Vernet M, Fraser W (2007) Marine pelagic ecosystems: the West Antarctic Peninsula. Phil Trans R Soc B 362:67–94
Fischer G, Gersonde R, Wefer G (2002) Organic carbon, biogenic silica and diatomn fluxes in the marginal winter sea-ice zone and in the Polar Front Region: interannual variations and differences in composition. Deep Sea Res Pt II 49:1721–1745
Fortier L, Le Fèvre J, Legendre L (1994) Export of biogenic carbon to fish and to the deep ocean: the role of large planktonic microphages. J Plankt Res 16:809–839
Foxton P (1966) The distribution and life-history of Salpa thompsoni Foxton with observations on a related species, Salpa gerlachei Foxton. Disc Rep 34:1–116
Froneman PW, Pakhomov EA, Balarin MG (2004) Size-fractionated phytoplankton biomass, production and biogenic carbon flux in the eastern Atlantic sector of the Southern Ocean in late austral summer 1997–1998. Deep Sea Res Pt II 51:2715–2729
Hamner WH (1975) Underwater observations of blue-water plankton: logistics, techniques, and safety procedures for divers at sea. Limnol Oceanogr 20:1045–1051
Harbison GR, McAlister VL (1979) The filter-feeding rates and particulate retention efficiencies of three species of Cyclosalpa (Tunicata: Thaliacea). Limnol Oceanogr 24:517–528
Haddock SHD, Heine JN (2005) Scientific blue-water diving. California Sea Grant College Program Report No. T-057
Honjo S (1990) Particle fuxes and modern sedimentation in the polar oceans. In: Smith WO (ed) Polar oceanography, vol. II. Academic Press, New York, pp 322–353
Honjo S, Francois R, Manganini S, Dymond J, Collier R (2000) Particle fluxes to the interior of the Southern Ocean in the Western Pacific sector along 170 W. Deep Sea Res Pt II 47:3521–3548
Huntley ME, Sykes PF, Marin V (1989) Biometry and trophodynamics of Salpa thompsoni foxton (Tunicata: Thaliacea) near the Antarctic Peninsula in austral summer, 1983–1984. Polar Biol 10:59–70
Iseki K (1981) Particulate organic matter transport to the deep sea by salp fecal pellets. Mar Ecol Prog Ser 5:55–60
Kremer P, Madin LP (1992) Particle retention efficiency of salps. J Plankt Res 14:1009–1015
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Longhurst AR, Harrison WG (1989) The biological pump: profiles of plankton production and consumption in the upper ocean. Prog Oceanogr 22:47–123
Madin LP (1974) Field observations on the feeding behavior of salps (Tunicata: Thaliacea). Mar Biol 25:143–147
Madin LP (1982) Production, composition and sedimentation of salp fecal pellets in oceanic waters. Mar Biol 67:39–45
Madin LP, Cetta CM (1984) The use of gut fluorescence to estimate grazing by oceanic salps. J Plankt Res 6:475–491
Madin LP, Diebel D (1998) Feeding and energetics of Thaliaceans. In: Bone Q (ed) The biology of pelagic tunicates. Oxford University Press, Oxford, pp 125–137
Madin LP, Kremer P (1995) Determination of the filter-feeding rates of salps (Tunicata, Thaliacea). ICES J Mar Sci 52:583–595
Madin LP, Purcell JE (1992) Feeding, metabolism, and growth of Cyclosalpa bakeri in the subarctic Pacific. Limnol Oceanogr 37:1236–1251
Madin LP, Purcell JE, Miller CB (1997) Abundance and grazing effects of Cycosalpa bakeri in the subarctic Pacific. Mar Ecol Prog Ser 157:175–183
Madin LP, Kremer P, Wiebe PH, Purcell JE, Horgan EH, Nemazie DA (2006) Periodic swarms of the salp Salpa aspera in the slope water off the NE United Staes: biovolume, vertical migration, grazing and vertical flux. Deep Sea Res I 53:804–819
Masque P, Isla E, Sanchez-Cabeza JA, Palanques A, Bruach JM, Puig P, Guillen J (2002) Sediment accumulation rates and carbon fluxes to bottom sediments at the Western Bransfield Strait (Antarctica). Deep Sea Res II 49:921–933
Matsueda H, Handa N, Inoue I, Takano H (1986) Ecological significance of salp fecal pellets collected by sediment traps in the Eastern North Pacific. Mar Biol 91:421–431
Morris RJ, Bone Q, Head R, Braconnot JC, Nival P (1988) Role of salps in the flux of organic matter to the bottom of the Ligurian Sea. Mar Biol 97:237–241
Nishikawa J, Tsuda A (2001) Diel vertical migration of the tunicate Salpa thompsoni in the Southern Ocean during summer. Polar Biol 24:299–302
Pakhomov EA (2002) Salp/krill interactions in the Southern Ocean: spatial segregations and implications for the carbon flux. Deep Sea Res II 49:1881–1907
Pakhomov EA (2004) Salp/krill interactions in the eastern Atlantic sector of the Southern Ocean. Deep Sea Res II 51:2645–2660
Pakhomov EA, Perissinotto R, McQuaid CD (1994) Comparative structure of the macrozooplankton/micronekton communities of the Subtropical and Antarctic Polar Fronts. Mar Ecol Prog Ser 111:155–169
Pakhomov EA, Dubischar CD, Strass V, Brichta M, Bathmann UV (2006) The tunicate Salpa thompsoni ecology in the Southen Ocean. I. Distribution, biomass, demography and feeding ecophysiology. Mar Biol 149:609–623
Perissinotto R, Pakhomov EA (1998) The trophic role of the tunicate Salpa thompsoni in the Antarctic marine ecosystem. J Mar Syst 17:361–374
Schnack-Schiel SB, Isla E (2005) The role of zooplankton in the pelagic-benthic coupling of the Southern Ocean. Sci Mar 69:39–55
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis, 2nd edn. Bull Fish Res Bd Can 167
Suzuki H, Sasaki H, Fukuchi M (2001) Short-term variability in the flux of rapidly sinking particles in the Antarctic marginal ice zone. Polar Biol 24:697–705
Turner JT (2002) Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat Microb Ecol 27:57–102
Wiebe PH, Madin LP, Haury LR, Harbison GR, Philbin LM (1979) Diel vertical migration by Salpa aspera and its potential for large-scale particulate organic matter transport to the deep sea. Mar Biol 53:249–255
Acknowledgments
We wish to thank the many people who worked in support of this project as divers, laboratory assistants and technicians. Included in this group are Erich Horgan, Jeff Godfrey, Jeff Mercer, Kerri Scolardi, Wally Fulweiler, Kelly Rakow and Sandy and Izzie Williams. We are grateful to the crew of the ASRV Laurence M. Gould for their support and the comments of three anonymous reviewers. This study was funded by NSF grants OPP-0338290 to P. Kremer and OPP-0338090 to L. Madin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Irigoien.
Rights and permissions
About this article
Cite this article
Phillips, B., Kremer, P. & Madin, L.P. Defecation by Salpa thompsoni and its contribution to vertical flux in the Southern Ocean. Mar Biol 156, 455–467 (2009). https://doi.org/10.1007/s00227-008-1099-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1099-4