Abstract
Methane (CH4), one of the most important greenhouse gases, has conventionally been considered as a physiologic inert gas. However, this perspective has been challenged by the observation that CH4 has diverse biological functions in animals, such as anti-inflammatory, antioxidant, and anti-apoptosis. Meanwhile, it has now been identified as a possible candidate of gaseous signaling molecule in plants, although its biosynthetic and metabolic pathways as well as the mechanism(s) of CH4 signaling have not fully understood yet. This paper aims to review the available evidence for the biological roles of CH4 in regulating plant physiology. Although currently available reports do not fully support the notion of CH4 as a gasotransmitter, they do show that CH4 might be produced by an aerobic, non-microbial pathway from plants, and plays important roles in enhancing plant tolerance against abiotic stresses, such as salinity, drought, heavy metal exposure, and promoting root development, as well as delaying senescence and browning. Further results showed that CH4 could interact with reactive oxygen species (ROS), other gaseous signaling molecules [e.g., nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S)], and glutathione (GSH). These reports thus support the idea that plant-produced CH4 might be a component of a survival strategy of plants. Finally, the possibility of CH4 application in agriculture is preliminarily discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methane (CH4) is a ubiquitous, colorless, odorless, and volatile gaseous molecule. Normally, this gas is considered to be a significant greenhouse gas with potential to substantially impact the planet’s climate. CH4 is intrinsically non-toxic to animals, but at high concentrations, it will cause headaches and even asphyxia (Boros et al. 2015). Conventionally, CH4 is considered to be biologically inactive. However, a previous review suggested that CH4 could exhibit a wide range of protective effects in many human disease models, and thus was proposed to be a new functional gas with the possible medical applications (Liu et al. 2012). For example, methane-rich saline can ameliorate ischemia/reperfusion injury of small intestine (Boros et al. 2012), liver (Ye et al. 2015; Wang et al. 2017), myocardium (Chen et al. 2016), kidney (Meng et al. 2018), and sepsis-induced injury (Jia et al. 2018; Li et al. 2019) via anti-oxidation, anti-inflammation, and anti-apoptosis pathways. It also has been reported that CH4 has analgesic effects for monoarthritis in a rat model of chronic inflammatory pain (Zhou et al. 2018).
Previously, it was proposed the six criteria for identifying a gasotransmitter (Wang 2014) and it was shown that CH4 is a gasotransmitter candidate similar to nitric oxide (NO), carbon oxide (CO), and hydrogen sulfide (H2S). Its gaseous characteristics, high membrane permeability, endogenous production and catabolism in mammals, and biological effects elicited by exogenous donors further highlighted the potential as a gasotransmitter (Ghyczy et al. 2008; Liu et al. 2012; Boros et al. 2015). Increasingly, studies of CH4 have been performed in plants, so the evidence was steadily accumulated to support this hypothesis. For example, aerobic non-microbial emission of CH4 (Keppler et al. 2006; Wang et al. 2013a, b; Liu et al. 2015; Martel and Qaderi 2017) and its biological activities in plants have been increasingly demonstrated (Cui et al. 2015, 2017; Zhu et al. 2016; Samma et al. 2017; Hu et al. 2018; Mei et al. 2019).
The objective of this review is to summarize the important progress in botanical functions of CH4, including enhancing plants tolerance against stresses (salinity, drought, osmotic stress, and metal exposure, etc.), and regulating plant growth and development (lateral rooting and adventitious root development, etc.). These positive effects of CH4 in plants indicated that it might be used in agricultural production as a novel plant growth regulator and not only for medical treatment.
CH4 synthesis and emission
Microbial CH4 production
CH4 can be produced through abiotic or biotic pathways. CH4 emissions from the three main abiotic pathways (volcanic activities, geothermal systems, and water–rock interactions) are considered to be insignificant, accounting for about 1% of the total global amount (Emmanuel and Ague 2007; Fiebig et al. 2009). In contrast, approximately 99% of CH4 in the atmosphere is derived from decomposition of organic compounds and microbial CH4 production that accounts more than 70% of CH4 global production (Wang et al. 2013b).
Methanogenic microorganisms are obligate anaerobic members of the archaea that are distinguished from bacteria and eukaryotes, and produce CH4 as a byproduct of metabolism under anaerobic conditions. Methanogens are often present in wetlands, rice paddy fields, landfills, oceans, and digestive tracts of humans and animals (Liu and Whitman 2008). Normally, methanogens existing in the gut are widely considered to be a major cause of CH4 production in ruminants and humans (Costello et al. 2013). Archae have been also isolated from trees that act as a significant contributor of methanogenesis (Covey et al. 2012; Wang et al. 2016; Yip et al. 2019). Methanogens can be divided into three major groups: (1) the first group reduces CO2 to form CH4 along with H2 as an electron donor; (2) the second group decomposes acetate to form CH4; and (3) the third one produces CH4 by reducing methyl-group containing compounds, including methanol, methylated amines, and methylated sulfides (Liu and Whitman 2008).
Non-microbial CH4 production and consumption
Non-microbial CH4 production accounts less than 30% of global CH4 production and has received less attention because of its lower proportion (Wang et al. 2013b). Previously, non-microbial CH4 production has been confirmed in animals (Tuboly et al. 2013), plants (Keppler et al. 2008), fungi (Lenhart et al. 2012), soils (Jugold et al. 2012; Wang et al. 2013a), and the surface of ocean (Bange and Uher 2005). It has also been observed that CH4 can be produced in rat mitochondria under hypoxic conditions. In this, mitochondrial dysfunction elicited significant CH4 production (Ghyczy et al. 2008), which occurs in mammalian cells after inhibition of cytochrome c oxidase (the last enzyme of mitochondrial electron transport chain) by sodium azide (NaN3) (Tuboly et al. 2013).
Conventionally, plants have been considered as pathways for CH4 transfer and emission from soil to atmosphere. It was only in 2006, that direct evidence of non-microbial CH4 emission in the presence of oxygen was first reported (Keppler et al. 2006). However, much suspicion and controversy ensued, until it was subsequently confirmed that aerobic non-microbial CH4 can be produced in the intact and detached plants under various stress conditions, including high temperature (Keppler et al. 2008; Vigano et al. 2008; Bruhn et al. 2009; Abdulmajeed et al. 2017), UV irradiation (Keppler et al. 2008; McLeod et al. 2008; Vigano et al. 2008; Bruhn et al. 2009, 2014; Messenger et al. 2009; Abdulmajeed et al. 2017), physical injury (Wang et al. 2009, 2011; Lenhart et al. 2015), hypoxia (Wang et al. 2011), drought (Qaderi and Reid 2011), low light (Bruggemann et al. 2009; Martel and Qaderi 2017), salinity (Zhu et al. 2016), metal exposure (Cui et al. 2017; Samma et al. 2017; Gu et al. 2018), reactive oxygen species (ROS) (Messenger et al. 2009; Althoff et al. 2010, 2014), and bacterial and fungi infection (Messenger et al. 2009; Hietala et al. 2015).
Identifying the specific precursor(s) of CH4 production in plants is, therefore, a research priority. Various organic compounds containing functional groups, such as methyl (–CH3), methoxyl (–O–CH3), hydroxymethyl (–CH2–OH), thiomethyl (–S–CH3), etc., may serve as the intermediates for CH4 production. Pectin was suggested to be a precursor for plant CH4 production under heating and UV irradiation because of its high degree of methylation (Keppler et al. 2008; McLeod et al. 2008; Vigano et al. 2008; Messenger et al. 2009). Other plant components such as lignin (Vigano et al. 2008, 2009), cellulose (Vigano et al. 2008, 2009), ascorbic acid (AsA) (Althoff et al. 2010), leaf surface waxes (Bruhn et al. 2014), and methionine (Met) (Althoff et al. 2014; Lenhart et al. 2015; Han et al. 2017) have also been confirmed as potential sources for plant CH4 release and production.
Substantive evidence has suggested that ROS, which can be stimulated or intensified under various types of stresses, is a conceivable driver of non-microbial CH4 emissions. It has been demonstrated that aerobic non-microbial CH4 production associated with ROS production, can be limited by ROS removal, and stimulated by the enzymes that inhibit ROS removal (Messenger et al. 2009). ROS may also be involved in CH4 formation from cleavage of pectin and/or lignin (Keppler et al. 2008; Messenger et al. 2009). Interestingly, a strong increase of CH4 generation was discovered in plant cell cultures exposed to NaN3 (Wishkerman et al. 2011). This result is consistent with an increase in NaN3-induced CH4 generation in rat liver cells (Ghyczy et al. 2008). These findings indicated that interference of electron transport chains in mitochondria may be partially involved in CH4 production in both animals and plants. However, Bruhn et al. (2012) subsequently questioned the specificity of NaN3, which can also inhibit the activities of some ROS-removing enzymes, including catalase (CAT) and peroxidase (POD). Together, although ROS may be an important hub for the stimulation of plant CH4 production (Fig. 1), the complete mechanism(s) of plant CH4 production has still to be unequivocally determined.
The proposed mechanisms for CH4 production in plant under abiotic stresses. The stimuli factors are shown as red dash-lined boxes. ROS are considered to play a vital role in CH4 production. AsA ascorbic acid, CO2 carbon dioxide, H2 hydrogen, ROS reactive oxygen species.
A well-studied source of CH4 transfer is aerenchyma, a specialized tissue that forms in root and stems (Drew et al. 2000; Evans 2004). Regarding the mechanism, it was suggested that CH4 in situ moves by diffusion rather than pressurized transport (Pangala et al. 2014). Moreover, transpiration has also been demonstrated to be a mechanism for transporting and emitting CH4 to atmosphere (Rusch and Rennenberg 1998). Transpiration-driven CH4 emission varies with CO2 concentration and stomatal conductance (Garnet et al. 2005). CH4 emission might be organ-specific, and the stem was identified as the largest source (Abdulmajeed et al. 2017; Barba et al. 2019; Covey and Megonigal 2019).
Methanotrophy is a ubiquitous process for CH4 consumption (Covey and Megonigal 2019). According to reports, CH4 was consumed by symbiosis in Sphagnum mosses, part of which was consumed by endophytic methanotrophs (Raghoebarsing et al. 2005). Subsequently, the CH4 uptake by trees of four species, including Betula pubescens, Picea abies, Pinus sylvestris, and Sorbus aucuparia, was observed both in the laboratory and in situ measurements (Sundqvist et al. 2012).
Plant support of microbial methanogenesis and methanotrophy
Plant root exudates and litter residues are important organic carbon sources (Phillips et al. 2011). Vegetation type and species composition have significant effects on methanogenic archaea diversity and activity, as well as CH4 production (Ström et al. 2005; Godina et al. 2012). Vascular plants produce root exudates and easily degradable litter that provide abundant substrate to methanogenic archaea, which may substantially increase CH4 emissions (Ström et al. 2005; Whalen 2005). Meanwhile, plant-transported O2 diffuses into the rhizosphere (Armstrong et al. 2006), where CH4 is consumed by methanotrophic bacteria (Sorrell et al. 2002; Raghoebarsing et al. 2005). However, in the presence of O2, methanogenesis can be suppressed (Fritz et al. 2011). It was also observed that in cushion plant lawns, CH4 emissions were absent because of high root densities coincided with high soil oxygen, but in Sphagnum lawns, CH4 emissions were substantial. Subsequent study suggested that methanotrophs were members of the nitrogen-fixing communities in all wood decay stages (Mäkipää et al. 2018). Together, plants might play important roles in regulating the production, oxidation, and export of CH4. However, it still requires more evidence to underlie its regulation mechanism.
Roles for CH4 in plants
Plant tolerance against abiotic stress
Ample evidence has clearly demonstrated the protective effects of CH4 on various stresses in plants (Table 1). The protection achieved by CH4 in animals has been proposed to be mediated by reducing oxidative stress (Boros et al. 2012; Wang 2014). Rapid overproduction of ROS is triggered by abiotic stresses, thus resulting in lipid peroxidation and oxidative damage (Møller et al. 2007). To counteract these toxic stress metabolites, plants possess defense systems that include antioxidant enzymes, superoxide dismutase (SOD), ascorbate peroxidase (APX), CAT, and POD. In addition, non-enzymatic components, such as AsA, glutathione (GSH), and glucose metabolism, could detoxify ROS to enhance plant tolerance against stress (Foyer and Noctor 2011; Noctor et al. 2012; Uzilday et al. 2014). It has been identified that there is a correlation between exposure to CH4 and increase of antioxidant enzyme activity as well as their gene expression, thereby reestablishing redox homeostasis (Cui et al. 2015, 2017; Zhu et al. 2016).
Drought and salinity stress are considered as major limitations for crop productivity. CH4 can alleviate polyethylene glycol (PEG) stress, a solute for mimicking water deficiency by inducing osmotic stress, through regulating ROS status by improving sugar, AsA, and GSH homeostasis (Han et al. 2017). Subsequent result discovered that NO might be involved in CH4-ameliorated seed germination inhibition triggered by PEG, and CH4-reestablished redox homeostasis is NO-dependent (Zhang et al. 2018). Salinity stress also imposes an ionic imbalance somewhat like osmotic stress and certain nutrition disorders (Zhu 2001, 2003; Kurniasih et al. 2013), resulting in the inhibition of seed germination and seedling growth, and a decline in productivity (Turner et al. 2013). CH4-rich water (MRW) may also reestablish ionic homeostasis by increasing K+ and Ca2+ contents, and by decreasing Na+ content (Zhu et al. 2016). It was also observed that heme oxygenase1/carbon monoxide (HO1/CO) might be involved in CH4-alleviated salinity stress in Medicago sativa.
Metal exposure is an acute problem of crop production in some production areas. More importantly, it poses a serious threat to animal and human health (Jarup and Akesson 2009). Metal exposure causes severe inhibition in seed germination and plant growth, even to the extent of plant death (Foy et al. 1978). Biochemical and genetic evidence shows that metal imposes oxidative stress by inducing excessive levels of ROS production (Cui et al. 2017; Samma et al. 2017; Gu et al. 2018). Methane generally provides protective effects toward plant metal toxicity through at least two mechanisms: reducing metal accumulation and reestablishing redox homeostasis in plant cells. This hypothesis has been supported by strong evidence. For instance, CH4 noticeably alleviated an excess of copper (Cu)-induced inhibition of the seed germination and seedling growth of Medicago sativa (Samma et al. 2017). This was in accordance with a reduction in the accumulation of Cu and Cu-induced proline, concomitant with an increase of α/β-amylase activities and total sugar content. The reestablishment of redox homeostasis was also observed.
Application of a CH4 solution can also block cadmium (Cd) accumulation by modulating the expression levels of miR159 and miR167, as well as their target genes ABC transporter and Nramp6 in root tissues (Gu et al. 2018). Besides, CH4 reestablishes redox and GSH homeostasis, which contributes to ameliorate Cd toxicity in M. sativa. In addition, CH4 could suppress aluminum (Al) accumulation by regulating the expression of organic acid metabolism and transport genes, including citrate synthase (CS), malate dehydrogenase1/2 (MDH1/2), aluminum-activated malate transporter1 (ALMT1), and aluminum-activated citrate transporter (AACT), to maintain nutrient homeostasis and improve Al-induced oxidative stress (Cui et al. 2017).
Participation in root organogenesis
Root development is of great importance for plants to thrive. Recent studies have shown that CH4 acts as an inducer of root organogenesis (Table 2). Application of MRW can significantly induce the formation of adventitious roots (AR) in cucumber explants. Pharmacological and molecular evidence indicated the involvement of HO1/CO and Ca2+ pathways (Cui et al. 2015). Further studies indicated that NO (Qi et al. 2017), H2S (Kou et al. 2018), and GSH (Jiang et al. 2019) operate as downstream components regulating CH4-induced AR formation. The modulation of the cell division-related gene (CsCDC6), cell cycle regulatory genes (CsDNAJ-1, CsCDPK1, and CsCDPK5), auxin signaling-related genes (CsAux22D-like and CsAux22B-like), and auxin inducible genes (AtCYCB1; 1, AtCDKA; 1, and AtGH3.3, etc.) as well as genes encoding calcium-dependent protein kinases (CDPKs), were also involved. Interestingly, CH4-induced NO-mediated S-nitrosylation and H2S-dependent S-sulfhydrylation, both of which belong to post-translational modification and play important roles in diverse biological processes (Yun et al. 2011; Aroca et al. 2015; Yang et al. 2015), were observed in cucumber explants (Qi et al. 2017; Kou et al. 2018). These results indicated that post-translational modification might be used to explain the mechanism underlying CH4 functions in plants.
Although the origin of lateral roots (LRs) is different from ARs origin, it has been found that they share key elements with other gaseous signaling cascades. Recent study has demonstrated that CH4 can trigger LR formation in alfalfa, rapeseed, Arabidopsis, and tomato (Mei et al. 2019). To date, hydrogen peroxide (H2O2) and H2S have been found to be required for CH4-induced LR formation (Mei et al. 2019; Zhao et al. 2019). Related studies provided the molecular mechanism for CH4-induced tomato LR formation. This model postulates that CH4 triggers a signaling cascade, and results in increased production of downstream molecules, nicotinamide adenine dinucleotide phosphate (NADPH)-dependent H2O2 and l-cysteine desulfhydrase (DES)-dependent H2S, followed by the development of LR formation with the involvement of cell cycle regulatory genes, miRNAs, and their target genes (e.g., ARFs) (Mei et al. 2019; Zhao et al. 2019).
Participation in vegetable postharvest preservation
The postharvest storage of vegetables induces a redox imbalance. CH4 could delay senescence and associated browning of daylily buds via regulating polyphenol oxidase activity to maintain redox homeostasis (Hu et al. 2018). Moreover, the decrease of the unsaturated/saturated fatty acid ratio and energy charge during storage was also attenuated. These results suggested that CH4 can be used in postharvest practice.
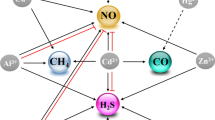
The interaction between CH4 and other signaling molecules
Cross talk between CH4 and ROS
As mentioned above, ROS is one of the CH4 inducers. CH4 production was decreased by the removal of ROS and increased by inhibiting ROS removal enzymes (Messenger et al. 2009). Meanwhile, CH4 can significantly block the increased ROS overproduction through various mechanisms, including increasing antioxidant enzymes activities, reestablishing AsA and GSH homeostasis, and modulating glucose metabolism, thus enhancing plant tolerance against abiotic stresses (Han et al. 2017; Samma et al. 2017; Gu et al. 2018). Furthermore, CH4 could increase NADPH oxidase-dependent H2O2 production, followed by the induction of LR formation in tomato seedlings (Zhao et al. 2019).
Cross talk between CH4 and NO
NO is an essential gasotransmitter involved in multiple physiological functions. CH4 interacts with NO in controlling adventitious rooting (Qi et al. 2017) and combating osmotic stress (Zhang et al. 2018). Using laser confocal scanning microscopy and inhibitor tests, we discovered that endogenous NO synthesis was induced by CH4 via NO synthesis-like (NOS-like) protein and diamine oxidase (DAO), two NO synthetic enzymes. Afterwards, the modulation of target gene expression and post-translational modification were observed during the development of cucumber AR formation (Qi et al. 2017). Above CH4 responses were reversed by 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) and 2,4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) (two NO scavengers), NG-nitro-l-argmethylester hydrochloride (l-NAME) (a mammalian NOS-like enzyme inhibitor), and β-hydroxyethyl hydrazine (β-HEH) (a DAO inhibitor). This evidence showed that CH4-induced AR formation was NO-dependent and partially mediated by NOS-like protein and DAO. Besides, CH4 could enhance the plant tolerance against osmotic stress via maintaining redox homeostasis, and modulating starch metabolism (Zhang et al. 2018). Meanwhile, CH4-triggered NO-dependent S-nitrosylation was observed either. Above results reflect the complexity of CH4 signaling.
Cross talk between CH4 and other gas molecules
Similar to NO, CO might be another second messenger in CH4 signaling. For instance, HO1-dependent CO acts as a downstream component during CH4-induced cucumber AR formation via modulating DNAJ-1 and CDPK1/5 gene expression (Cui et al. 2015). Subsequent studies revealed that H2S was also partially involved in CH4-induced LR and AR formation by regulating the expression of cell cycle regulatory genes, ARFs, and miRNA, and the involvement of S-sulfhydrylation was also suggested (Kou et al. 2018; Mei et al. 2019).
Cross talk between CH4 and Ca2+
In rice LR formation, Ca2+ acts downstream of HO1-dependent CO signaling (Hsu et al. 2013). Similarly, exogenous Ca2+ strengthens CH4-triggered cucumber AR formation (Cui et al. 2015), which was further impaired by the addition of its chelator ethylenediaminetetraacetic acid (EDTA) and a Ca2+ channel blocker lanthanum chloride (LaCl3). These observations indicated that the Ca2+ might be involved in CH4-elicited cucumber AR formation.
Cross talk between CH4 and GSH
GSH is an important cellular antioxidant with multiple functions in plants, including redox signaling, antioxidant defense, and root organogenesis (Noctor et al. 2012). Some studies have shown that GSH homeostasis is reestablished by CH4 in plants when subjected to Cd exposure (Gu et al. 2018) and osmotic stress (Han et al. 2017). Recent result showed that γ-glutamyl cysteine synthetase (γ-ECS)-dependent GSH might be required for CH4-induced cucumber AR formation (Jiang et al. 2019).
Conclusion and perspectives
Some literature is now available that preliminarily illustrates the complex and integrated regulation of CH4 synthesis, functions, and its signaling (Figs. 1, 2). Considerable advances have been made in the field of identifying the biological effects of CH4. However, aerobic non-microbial CH4 production still remains to be further and completely elucidated in plants. On the other hand, although direct targets of CH4 in plant cells remain unknown, the molecular mechanism underlying the biological roles of CH4 involves gene expression, miRNA, protein, plant hormone levels, and the regulation of protein post-translational modification.
Possible mechanism related to the botanical effects of CH4. APX ascorbate peroxidase, ARFs auxin response factors, CAT catalase, CO carbon oxide, DHA oxidized ascorbic acid, DHRA dehydroascorbate reductase, γ-ECS gamma-glutamylcysteine synthetase, GR glutathione reductase, GSH glutathione, GSSG oxidized glutathione, HO1 heme oxygenase 1, H2O2 hydrogen peroxide, H2S hydrogen sulfide, MDA malondialdehyde, miRNA microRNA, NO nitric oxide, NOX reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, POD peroxidase, PPO polyphenol oxidase, ROS reactive oxygen species, SOD superoxide dismutase
It is clear that CH4 plays valuable roles in plant development and adaption against environmental stimuli. Since methanotroph activity may be increased under conditions with high concentration of CH4 (Sorrell et al. 2002), and methanotrophs appear to be coupled with N2 fixation (Mäkipää et al. 2018), we speculated that CH4 might have potential capacity to improve soil fertility by changing microbial community and increasing methanotrophic bacteria activity. Certainly, this hypothesis requires further validation.
Finally, it reminds us of some crucial challenges facing current application of CH4. First, because of its innate flammability and difficulty in transport, CH4 fumigation may be impractical for agriculture. Consequently, we proposed the application of MRW irrigation, which may provide a safe, portable, and easy approach. The reason is that the concentration of CH4 in the saturated methane-rich water or methane-rich saline was about 1–1.5 mM (Ye et al. 2015; Zhou et al. 2018; Jiang et al. 2019), far below its lowest explosive concentration (5%; v/v) (Liu et al. 2012). Second, it should be noted that CH4 is a potent climatic change gas, and its potential usage is a challenge.
References
Abdulmajeed AM, Derby SR, Strickland SK, Qaderi MM (2017) Interactive effects of temperature and UVB radiation on methane emissions from different organs of pea plants grown in hydroponic system. J Photochem Photobiol B 166:193–201
Althoff F, Jugold A, Keppler F (2010) Methane formation by oxidation of ascorbic acid using iron minerals and hydrogen peroxide. Chemosphere 80:286–292
Althoff F, Benzing K, Comba P, McRoberts C, Boyd DR, Greiner S, Keppler F (2014) Abiotic methanogenesis from organosulphur compounds under ambient conditions. Nat Commun 5:4205
Armstrong J, Jones RE, Armstrong W (2006) Rhizome phyllosphere oxygenation in Phragmites and other species in relation to redox potential, convective gas flow, submergence and aeration pathways. New Phytol 172:719–731
Aroca Á, Serna A, Gotor C, Romero LC (2015) S-sulfhydration: a cysteine post-translational modification in plant systems. Plant Physiol 168:334–342
Bange HW, Uher G (2005) Photochemical production of methane in natural waters: implications for its present and past oceanic source. Chemosphere 58:177–183
Barba J, Bradford MA, Brewer PE, Bruhn D, Covey K, van Haren J, Megonigal JP, Mikkelsen TN, Pangala SR, Pihlatie M, Poulter B, Rivas-Ubach A, Schadt CW, Terazawa K, Warner DL, Zhang Z, Vargas R (2019) Methane emissions from tree stems: a new frontier in the global carbon cycle. New Phytol 222:18–28
Boros M, Ghyczy M, Érces D, Varga G, Tőkés T, Kupai K, Torday C, Kaszaki J (2012) The anti-inflammatory effects of methane. Crit Care Med 40:1269–1278
Boros M, Tuboly E, Mészáros A, Amann A (2015) The role of methane in mammalian physiology—is it a gasotransmitter? J Breath Res 9:14001
Bruggemann N, Meier R, Steigner D, Zimmer I, Louis S, Schnitzler J (2009) Nonmicrobial aerobic methane emission from poplar shoot cultures under low-light conditions. New Phytol 182:912–918
Bruhn D, Mikkelsen TN, Obro J, Willats WGT, Ambus P (2009) Effects of temperature, ultraviolet radiation and pectin methyl esterase on aerobic methane release from plant material. Plant Biol 11:43–48
Bruhn D, Møller IM, Mikkelsen TN, Ambus P (2012) Terrestrial plant methane production and emission. Physiol Plant 144:201–209
Bruhn D, Mikkelsen T, Rolsted M, Egsgaard H, Ambus P (2014) Leaf surface wax is a source of plant methane formation under UV radiation and in the presence of oxygen. Plant Biol 16:512–516
Chen O, Ye Z, Caoc Z, Manaenko A, Ning K, Zhai X, Zhang R, Zhang T, Chen X, Liu W, Sun X (2016) Methane attenuates myocardial ischemia injury in rats through anti-oxidative, anti-apoptotic and anti-inflammatory actions. Free Radic Biol Med 90:1–11
Costello BPJD, Ledochowski M, Ratcliffe NM (2013) The importance of methane breath testing: a review. J Breath Res 7:024001
Covey KR, Megonigal JP (2019) Methane production and emissions in trees and forests. New Phytol 222:35–51
Covey KR, Wood SA, Warren RJI, Lee X, Bradford MA (2012) Elevated methane concentrations in trees of an upland forest. Geophys Res Lett 39:L15705
Cui W, Qi F, Zhang Y, Cao H, Zhang J, Wang R, Shen W (2015) Methane-rich water induces cucumber adventitious rooting through heme oxygenase1/carbon monoxide and Ca2+ pathways. Plant Cell Rep 34:435–445
Cui W, Cao H, Yao P, Pan J, Gu Q, Xu S, Wang R, Ouyang Z, Wang Q, Shen W (2017) Methane enhances aluminum resistance in alfalfa seedlings by reducing aluminum accumulation and reestablishing redox homeostasis. Biometals 30:719–732
Drew MC, He CJ, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5:123–127
Emmanuel S, Ague JJ (2007) Implications of present-day abiogenic methane fluxes for the early Archean atmosphere. Geophys Res Lett 34:L15810
Evans DE (2004) Aerenchyma formation. New Phytol 161:35–49
Fiebig J, Woodland AB, D’Alessandro W, Püttmann W (2009) Excess methane in continental hydrothermal emissions is abiogenic. Geology 37:495–498
Foy CD, Chaney R, White CM (1978) The physiology of metal toxicity in plants. Annu Rev Plant Physiol 29:511–566
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Fritz C, Pancotto VA, Elzenga JTM, Visser EJW, Grootjans AP, Pol A, Iturraspe R, Roelofs JGM, Smolders AJP (2011) Zero methane emission bogs: extreme rhizosphere oxygenation by cushion plants in Patagonia. New Phytol 190:398–408
Garnet KN, Megonigal JP, Litchfield C, Taylor GE (2005) Physiological control of leaf methane emission from wetland plants. Aquat Bot 81:141–155
Ghyczy M, Torday C, Kaszaki J, Szabo A, Czobel M, Boros M (2008) Hypoxia-induced generation of methane in mitochondria and eukaryotic cells—an alternative approach to methanogenesis. Cell Physiol Biochem 21:251–258
Godina A, McLaughlin JW, Webster KL, Packalen M, Basiliko N (2012) Methane and methanogen community dynamics across a boreal peat land nutrient gradient. Soil Biol Biochem 48:96–105
Gu Q, Chen Z, Cui W, Zhang Y, Hu H, Yu X, Wang Q, Shen W (2018) Methane alleviates alfalfa cadmium toxicity via decreasing cadmium accumulation and reestablishing glutathione homeostasis. Ecotoxicol Environ Saf 147:861–871
Han B, Duan X, Wang Y, Zhu K, Zhang J, Wang R, Hu H, Qi F, Pan J, Yan Y, Shen W (2017) Methane protects against polyethylene glycol-induced osmotic stress in maize by improving sugar and ascorbic acid metabolism. Sci Rep 7:46185
Hietala A, Dorsch P, Kvaalen H, Solheim H (2015) Carbon dioxide and methane formation in norway spruce stems infected by white-rot fungi. Forests 6:3304–3325
Hsu YY, Chao Y, Kao CH (2013) Cobalt chloride-induced lateral root formation in rice: the role of heme oxygenase. J Plant Physiol 170:1075–1081
Hu H, Liu D, Li P (2018) Methane delays the senescence and browning in daylily buds by re-established redox homeostasis. J Sci Food Agric 98:1977–1987
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharm 238:201–208
Jia Y, Li Z, Feng Y, Cui R, Dong Y, Zhang X, Xiang X, Qu K, Liu C, Zhang J (2018) Methane-rich saline ameliorates sepsis-induced acute kidney injury through anti-inflammation, antioxidative, and antiapoptosis effects by regulating endoplasmic reticulum stress. Oxid Med Cell Longev. https://doi.org/10.1155/2018/4756846
Jiang X, He J, Cheng P, Xiang Z, Zhou H, Wang R, Shen W (2019) Methane control of adventitious rooting requires γ-glutamyl cysteine synthetase-mediated glutathione homeostasis. Plant Cell Physiol 60:802–815
Jugold A, Althoff F, Hurkuck M, Greule M, Lenhart K, Lelieveld J, Keppler F (2012) Non-microbial methane formation in oxic soils. Biogeosciences 9:5291–9301
Keppler F, Hamilton J, Brass M, Rockmann T (2006) Methane emissions from terrestrial plants under aerobic conditions. Nature 439:187–191
Keppler F, Hamilton JTG, McRoberts WC, Vigano I, Brass M, Rockmann T (2008) Methoxyl groups of plant pectin as a precursor of atmospheric methane: evidence from deuterium labelling studies. New Phytol 178:808–814
Kou N, Xiang Z, Cui W, Li L, Shen W (2018) Hydrogen sulfide acts downstream of methane to induce cucumber adventitious root development. J Plant Physiol 228:113–120
Kurniasih B, Greenway H, Colmer TD (2013) Tolerance of submerged germinating rice to 50–200 mM NaCl in aerated solution. Physiol Plantarum 149:222–233
Lenhart K, Bunge M, Ratering S, Neu TR, Schuettmann I, Greule M, Kammann C, Schnell S, Mueller C, Zorn H, Keppler F (2012) Evidence for methane production by saprotrophic fungi. Nat Commun 3:1046
Lenhart K, Althoff F, Greule M, Keppler F (2015) Technical note: methionine, a precursor of methane in living plants. Biogeosciences 12:1907–1914
Li Z, Jia Y, Feng Y, Cui R, Miao R, Zhang X, Qu K, Liu C, Zhang J (2019) Methane alleviates sepsis-induced injury by inhibiting pyroptosis and apoptosis in vivo and in vitro experiments. Aging 11:1226–1239
Liu Y, Whitman WB (2008) Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci 1125:171–189
Liu W, Wang D, Tao H, Sun X (2012) Is methane a new therapeutic gas? Med Gas Res 2:25
Liu J, Chen H, Zhu Q, Shen Y, Wang X, Wang M, Peng C (2015) A novel pathway of direct methane production and emission by eukaryotes including plants, animals and fungi: an overview. Atmos Environ 115:26–35
Mäkipää R, Leppänen SM, Munoz SS, Smolander A, Tiirola M, Tuomivirta T, Fritze H (2018) Methanotrophs are core members of the diazotroph community in decaying Norway spruce logs. Soil Biol Biochem 120:230–232
Martel AB, Qaderi MM (2017) Light quality and quantity regulate aerobic methane emissions from plants. Physiol Plant 159:313–328
McLeod A, Fry S, Loake G, Messenger D, Reay D, Smith K, Yun B (2008) Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol 180:124–132
Mei Y, Zhao Y, Jin X, Wang R, Xu N, Hu J, Huang L, Guan R, Shen W (2019) l-Cysteine desulfhydrase-dependent hydrogen sulfide is required for methane-induced lateral root formation. Plant Mol Biol 99:283–298
Meng Y, Jiang Z, Li N, Zhao Z, Cheng T, Yao Y, Wang L, Liu Y, Deng X (2018) Protective effects of methane-rich saline on renal ischemic-reperfusion injury in a mouse model. Med Sci Monit 24:7794–7801
Messenger DJ, McLeod AR, Fry SC (2009) The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ 32:1–9
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Pangala SR, Gowing DJ, Hornibrook ERC, Gauci V (2014) Controls on methane emissions from Alnus glutinosa saplings. New Phytol 201:887–896
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194
Qaderi MM, Reid DM (2011) Stressed crops emit more methane despite the mitigating effects of elevated carbon dioxide. Funct Plant Biol 38:97–105
Qi F, Xiang Z, Kou N, Cui W, Xu D, Wang R, Zhu D, Shen W (2017) Nitric oxide is involved in methane-induced adventitious root formation in cucumber. Physiol Plant 159:366–377
Raghoebarsing AA, Smolders A, Schmid MC, Rijpstra W, Wolters-Arts M, Derksen J, Jetten M, Schouten S, Damste J, Lamers L, Roelofs J, den Camp H, Strous M (2005) Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 436:1153–1156
Rusch H, Rennenberg H (1998) Black alder [Alnus glutinosa (L.) Gaertn.] trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil 201:1–7
Samma MK, Zhou H, Cui W, Zhu K, Zhang J, Shen W (2017) Methane alleviates copper-induced seed germination inhibition and oxidative stress in Medicago sativa. Biometals 30:97–111
Sorrell BK, Downes MT, Stanger CL (2002) Methanotrophic bacteria and their activity on submerged aquatic macrophytes. Aquat Bot 72:107–119
Ström L, Mastepanov M, Christensen TR (2005) Species-specific effects of vascular plants on carbon turnover and methane emissions from wetlands. Biogeochemistry 75:65–82
Sundqvist E, Crill P, Molder M, Vestin P, Lindroth A (2012) Atmospheric methane removal by boreal plants. Geophys Res Lett 39:L21806
Tuboly E, Szabo A, Garab D, Bartha G, Janovszky A, Eros G, Szabo A, Mohacsi A, Szabo G, Kaszaki J, Ghyczy M, Boros M (2013) Methane biogenesis during sodium azide-induced chemical hypoxia in rats. Am J Physiol Cell Physiol 304:C207–C214
Turner NC, Colmer TD, Quealy J, Pushpavalli R, Krishnamurthy L, Kaur J, Singh G, Siddique KHM, Vadez V (2013) Salinity tolerance and ion accumulation in chickpea (Cicer arietinum L.) subjected to salt stress. Plant Soil 365:347–361
Uzilday B, Turkan I, Ozgur R, Sekmen AH (2014) Strategies of ROS regulation and antioxidant defense during transition from C3 to C4 photosynthesis in the genus Flaveria under PEG-induced osmotic stress. J Plant Physiol 171:65–75
Vigano I, van Weelden H, Holzinger R, Keppler F, McLeod A, Rockmann T (2008) Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components. Biogeosciences 5:937–947
Vigano I, Rockmann T, Holzinger R, van Dijk A, Keppler F, Greule M, Brand WA, Geilmann H, van Weelden H (2009) The stable isotope signature of methane emitted from plant material under UV irradiation. Atmos Environ 43:5637–5646
Wang R (2014) Gasotransmitters: growing pains and joys. Trends Biochem Sci 39:227–232
Wang Z, Gulledge J, Zheng J, Liu W, Li L, Han X (2009) Physical injury stimulates aerobic methane emissions from terrestrial plants. Biogeosciences 6:615–621
Wang Z, Keppler F, Greule M, Hamilton JTG (2011) Non-microbial methane emissions from fresh leaves: effects of physical wounding and anoxia. Atmos Environ 45:4915–4921
Wang B, Hou L, Liu W, Wang Z (2013a) Non-microbial methane emissions from soils. Atmos Environ 80:290–298
Wang Z, Chang SX, Chen H, Han X (2013b) Widespread non-microbial methane production by organic compounds and the impact of environmental stresses. Earth Sci Rev 127:193–202
Wang Z, Gu Q, Deng F, Huang J, Megonigal JP, Yu Q, Lu X, Li L, Chang S, Zhang Y, Feng J, Han X (2016) Methane emissions from the trunks of living trees on upland soils. New Phytol 211:429–439
Wang L, Yao Y, He R, Meng Y, Li N, Zhang D, Xu J, Chen O, Cui J, Bian J, Zhang Y, Chen G, Deng X (2017) Methane ameliorates spinal cord ischemia-reperfusion injury in rats: antioxidant, anti-inflammatory and anti-apoptotic activity mediated by Nrf2 activation. Free Radic Biol Med 103:69–86
Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22:73–94
Wishkerman A, Greiner S, Ghyczy M, Boros M, Rausch T, Lenhart K, Keppler F (2011) Enhanced formation of methane in plant cell cultures by inhibition of cytochrome c oxidase. Plant Cell Environ 34:457–464
Yang H, Mu J, Chen L, Feng J, Hu J, Li L, Zhou J, Zuo J (2015) S-nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol 167:1604–1753
Ye Z, Chen O, Zhang R, Nakao A, Fan D, Zhang T, Gu Z, Tao H, Sun X (2015) Methane attenuates hepatic ischemia/reperfusion injury in rats through antiapoptotic, anti-inflammatory, and antioxidative actions. Shock 44:181–187
Yip DZ, Veach AM, Yang ZK, Cregger MA, Schadt CW (2019) Methanogenic Archaea dominate mature heartwood habitats of Eastern Cottonwood (Populus deltoides). New Phytol 222:115–121
Yun B, Feechan A, Yin M, Saidi NBB, Le Bihan T, Yu M, Moore JW, Kang J, Kwon E, Spoel SH, Pallas JA, Loake GJ (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478:161–264
Zhang Y, Su J, Cheng D, Wang R, Mei Y, Hu H, Shen W, Zhang Y (2018) Nitric oxide contributes to methane-induced osmotic stress tolerance in mung bean. BMC Plant Biol 18:207
Zhao Y, Zhang Y, Liu F, Wang R, Huang L, Shen W (2019) Hydrogen peroxide is involved in methane-induced tomato lateral root formation. Plant Cell Rep 38:377–389
Zhou S, Zhou Y, Ji F, Li H, Lv H, Zhang Y, Xu H (2018) Analgesic effect of methane rich saline in a rat model of chronic inflammatory pain. Neurochem Res 43:869–877
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Zhu K, Cui W, Dai C, Wu M, Zhang J, Zhang Y, Xie Y, Shen W (2016) Methane-rich water alleviates NaCl toxicity during alfalfa seed germination. Environ Exp Bot 129:37–47
Acknowledgements
The authors would like to thank the financial support from the National Natural Science Foundation of China (Grant No. 31572116 and 31771696) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20181317). We would like to thank Dr. Evan Evans (University of Tasmania; tassiebeerdr@gmail.com) for the English editing of this paper.
Author information
Authors and Affiliations
Contributions
LL, SW, and WS wrote and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Neal Stewart.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Wei, S. & Shen, W. The role of methane in plant physiology: a review. Plant Cell Rep 39, 171–179 (2020). https://doi.org/10.1007/s00299-019-02478-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02478-y