Abstract
Key message
We found a histone variant enhances drought tolerance partially via promoting stomatal closure other than osmotic stress resistance, indicating the crucial and complicated contribution of epigenetic regulation to abiotic stress response.
Abstract
Histone variants epigenetically regulate gene transcription through remodeling chromatin. They have been implicated in modulating plant abiotic stress response, however, the role(s) is not well documented. Here, we identified an abiotic stress responsive H2A variant gene TaH2A.7 from wheat. TaH2A.7 shared high identity with H2A homologs and localized to the nucleus. TaH2A.7 overexpression in Arabidopsis significantly enhanced drought tolerance, but had no effect on the response to saline, osmotic and oxidative stresses. TaH2A.7 lowered water loss rate, and promoted ABA-induced stomatal closure. In TaH2A.7 overexpression plants, the mRNA levels of numerous genes involved in the ABA pathway and stomatal movement signaling pathway were elevated, H2O2 level in guard cells was increased, as well. Together, TaH2A.7 can enhance drought tolerance via, at least in part, promoting stomatal closure, and appears to be a promising target for molecular breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histone variants influence gene expression by remodeling chromatin (Talbert and Henikoff 2010). Histone variants are constitutively transcribed, yet vary from the canonical forms with respect to both their primary amino acid sequences and post-translational modifications (Talbert and Henikoff 2010). In plants, altered activity or level of histone variants has been found to be associated with the response to abiotic stress. The H1-S variant of tomato is drought-inducible. Knockdown of H1-S by antisense technology promotes stomatal closure and enhances drought tolerance (Scippa et al. 2004). The H2A.Z variant of Arabidopsis is involved in the response to phosphate starvation (Smith et al. 2010) as well as in the correct perception of ambient temperature (Kumar and Wigge 2010). Major histones differ in the numbers of their variants. H2A variants are the most diverse, H3 has much less variants, while H2B variants are very rare (Kamakaka and Biggins 2005). Although a few variants such as H1-S and H2A.Z have been proven to participate in the abiotic stress response, the involvement of the most of others has not yet been unraveled (Jenks et al. 2014; Zhu et al. 2012).
The regulatory roles of histone variants have been proven to be complicated. Especially, the incorporation of histone variants is determined by a well conserved selection mechanism which ensures the stability and suitability of the chromosomes’ structure (Song et al. 2013). In the incorporation machinery, chaperones and other transcription regulation-associated effectors are essential for recruiting histone variants to chromatin (Henikoff and Ahmad 2005). The alteration in just a few residues can substantially influence the binding capacity of native histones with chaperones (Henikoff and Ahmad 2005; Tachiwana et al. 2008). Thus, the contribution of histone variants to the abiotic stress response appears complicated and has not yet been well elucidated.
Bread wheat (Triticum aestivum) is one of the most important food crops. We previously produced a wheat cultivar SR3 following an asymmetric somatic hybridization event between wheat cultivar JN177 and tall wheatgrass (Thinopyrum ponticum), and it shows superior salinity and drought tolerance (Xia 2009; Xia et al. 2003). The genome scale genetic and epigenetic variation has taken place in SR3 genome (Wang et al. 2015; Xia 2009), which results in a shift of transcriptomic and proteomic profiles (Liu et al. 2012; Peng et al. 2009; Wang et al. 2008). Transcriptomic analyses have shown that a number of probes annotated as histone variants were responsive to salt and osmotic stress, some of which exhibited differential responsive patterns between SR3 and JN177 (Liu et al. 2012). Based on these probes, we isolated a H2A variant gene TaH2A.7, and found that its overexpression in Arabidopsis promoted stomatal closure and offered drought tolerance.
Results
TaH2A.7 was responsive to abiotic stress
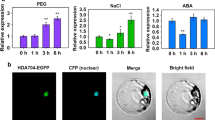
Our previous analysis found a probe with annotation as histone variant H2A, which was induced by osmotic (simulated by PEG treatment) and salt stress in SR3 and JN177, more strongly induced in SR3 (Liu et al. 2012). Here, the responsive patterns of the gene (namely TaH2A.7) matching the probe were further characterized. TaH2A.7 expression was induced by NaCl treatment, more strongly in SR3 than in JN177 (Fig. 1a). As a result of osmotic stress simulated by PEG treatment, the mRNA level of TaH2A.7 was gradually increased, and this increase was more obvious in SR3 (Fig. 1b). The osmotic stress is a key effect of drought, so we further analyzed the expression profile under drought conditions mimicked by water-withholding. This resulted in accumulation of TaH2A.7 transcripts after 2 weeks, to a greater extent in SR3, and this accumulation was more remarkable compared with PEG treatment (Fig. 1c). ABA is often drastically accumulated during abiotic stress. We found the expression profile of TaH2A.7 after ABA treatment mirrored the pattern observed in PEG-treated plants (Fig. 1d). These data suggested that TaH2A.7 participates in the abiotic stress response.
TaH2A.7 was responsive to abiotic stresses. a Expression pattern after 200 NaCl treatment for 24 h. b Expression pattern during 18 % PEG treatment from 0 to 48 h. c Expression pattern during drought treatment by water-withholding for 1–4 weeks. d Expression pattern during 100 mM ABA treatment from 0 to 48 h. Real-time PCR analysis was conducted for detecting expression patterns. Data presented as mean ± standard deviation
TaH2A.7 was a H2A histone variant and localized to the nucleus
We isolated the full-length cDNA of TaH2A.7 from SR3 and JN177. A high frequency of genetic variation had taken place in SR3 compared to JN177 (Wang et al. 2015), but TaH2A.7 had no allelic variation between the two cultivars. Histones are typically basic proteins rich in lysine and arginine. TaH2A.7 contained 7.5 % arginine and 14.4 % lysine, causing the pI value equal to 10.66. TaH2A.7 possessed four α-helices between 26 and 95 amino acid residues long, which could form histone-folding domains, a key characteristic of histones (Fig. S1). TaH2A.7 shared high sequence identity with H2A homologs of other plants. When compared with 13 members of Arabidopsis H2A superfamily, TaH2A.7 shared higher identities to three H2A.W variants HTA7, HTA6 and HTA12 (Yelagandula et al. 2014) (69, 67 and 65 %, respectively) than other members (including H2A.Z variants HTA4, 8, 9 and 11, and H2A.X variants HTA3 and 5) (Deal et al. 2007), which was the basis of TaH2A.7 nomenclature. In the phylogenetic tree based on amino acid sequences (Fig. 2a), TaH2A.7 was clustered with H2A homologs from monocots, within which TaH2A.7 was in the sub-clade containing those from wheat, wheat ancestors Aegilops tauschii (D genome) and Triticum urartu (A genome), and Hordeum vulgare. TaH2A.7 was separated from the clade with H2A homologs from dicots, including HTA12 of Arabidopsis. These H2A homologues were not grouped with HTA6 and HTA7 of Arabidopsis. The transient expression assay showed that the fluorescent signal of transformants expressing GFP alone was dispersed throughout the cell, but the signal produced by the TaH2A.7 fusion accumulated in the nucleus (Fig. 2b), showing that TaH2A.7 functioned in the nucleus.
TaH2A.7 was a H2A homologue and localized to the nucleus. a The phylogenetic tree of TaH2A.7 and H2A proteins from other plants using the neighbour joining method. Ta Triticum aestivum, Ata Aegilops tauschii, Tu Triticum urartu, Hv Hordeum vulgare, Bd Brachypodium distachyon, Si Setaria italica, Zm Zea mays, Mt Medicago truncatula, Sl Solanum lycopersicum, Nt Nicotiana tomentosiformis, Cs Cucumis sativus. b Subcellular localization assay of TaH2A.7, where TaH2A.7 fused with GFP was transiently expressed in onion epidermis
TaH2A.7 overexpression enhanced tolerance to drought but not salt
To primarily confirm the in planta role, TaH2A.7 was transformed into Arabidopsis for ectopic overexpression. The stable integration of TaH2A.7 into the genome and its constitutive expression were confirmed by genomic PCR and real-time PCR, respectively, and two transgenic lines (3-3 and 11-5) producing high transgene transcripts were selected for further analysis (Fig. 3a, b). The transgenic lines (OE) appear phenotypically indistinguishable from the wild-type (WT) and empty vector control transgenic line (VC, to avoid the effect of vector) during the whole life course (data not shown). Given that TaH2A.7 was responsive to salt and drought (Fig. 1), we firstly analyzed the phenotype under these two stressors. When exposed to NaCl, the growth of all seedlings was restricted, and the extent of restriction was similar among OE, WT and VC (Fig. S2). Three weeks of water-withholding resulted in severe wilting phenotypes in the seedlings of WT and VC, while OE plants were only slightly wilted under the same condition; after re-watering, WT and VC seedlings were largely dead, but most of the OE plants survived (Fig. 3c). The survival rates of OE lines were significantly higher than WT and VC (Fig. 3d). These results indicated that TaH2A.7 overexpression offered Arabidopsis drought, but not salt tolerance.
TaH2A.7 overexpression enhanced drought tolerance in Arabidopsis. a The integration of TaH2A.7 into the genome of Arabidopsis was confirmed by genomic PCR. b The transcription of TaH2A.7 in the transgenic lines was confirmed by RT-PCR. c The phenotype of Arabidopsis seedlings by the water-withholding assay. d The survival rate calculated from panel c. Wt Arabidopsis ecotype Col-0; 3-3/11-5 transgenic Arabidopsis lines over-expressing TaH2A.7; VC transgenic Arabidopsis transformed with an empty vector. Data presented as mean ± standard deviation. In d, columns marked without different letters indicate no difference in means using the one-way ANOVA LSD analysis (P < 0.05)
TaH2A.7 overexpression did not affect response to osmotic and oxidative stress
Drought often causes the decrease of water potential in soil and therefore results in osmotic stress. When confronted with osmotic stress, mimicked by applying mannitol, the OE lines had comparable seedling size to WT and VC (Fig. S3A–C). On the other hand, abiotic stress can induce the over-accumulation of intracellular ROS (Mittler 2002), so abiotic stress tolerance is sometimes resulted from the enhanced resistence to oxidative stress. However, in the presence of H2O2, the leaf size and root length of the OE lines were similar when compared to WT and VC (Fig. S3E–G). These results indicate that the improved drought tolerance by TaH2A.7 was not attributed to the enhancement of tolerance to osmotic and oxidative stresses.
TaH2A.7 overexpression increased sensitivity to ABA
ABA is a vital stress response-associated phytohormone, and its drastic elevation is a characteristic of drought response (Abe et al. 2003). Thus, seed germination rate was assayed to investigate the role of TaH2A.7 in ABA pathway. WT, VC and OE lines had comparable seed germination rate under the control condition (Fig. 4a). When applying exogenous ABA, seed germination was delayed. The effect of ABA treatment was stronger in the OE lines than in WT and VC (Fig. 4b, c), suggesting that TaH2A.7 overexpression increased sensitivity to ABA. TaH2A.7 overexpression can increase the mRNA levels of NCED3 and ABA2, two genes involved in ABA synthesis pathway, in response to drought stress, although such regulatory effects were not observed under the control condition (Fig. 4d, e). This was also seen when the transcript abundance of ABI1 and ABI2, two key components of ABA signaling pathway, was compared (Fig. 4f, g). These results demonstrated that TaH2A.7 increased ABA sensitivity via promoting ABA pathway.
TaH2A.7 overexpression promoted ABA pathway and increased sensitivity to ABA. a–c The germination rate in the presence of various concentrations of ABA. d–g The expression levels of genes involved in ABA pathway under the control and drought treatment. Wt Arabidopsis ecotype Col-0; 3-3/11-5 transgenic Arabidopsis lines over-expressing TaH2A.7; VC transgenic Arabidopsis transformed with an empty vector. Data presented as mean ± standard deviation. In d–g columns marked without different lowercase or uppercase letters indicate no difference in means using the one-way ANOVA LSD analysis (P < 0.05)
TaH2A.7 overexpression reduced water loss rate and promoted stomatal closure
Enhanced drought tolerance in plants is often accompanied by reduced transpiration efficiency, which is reflected by lower water loss rate of leaves (Wang et al. 2003). The detached leaves of OE lines had lower rates of water loss than did WT and VC lines (Fig. 5a). The rate of water loss is determined by both stomatal density and aperture (Wang et al. 2003). The OE lines had similar stomatal density to WT and VC (Fig. 5b). Under the control condition, the stomatal aperture of the OE lines was identical to those of WT and VC (Fig. 5c, d). In the presence of exogenous ABA, the stomatal aperture became smaller, with a more obvious change in the OE lines than in WT and VC (Fig. 5c, d). We further measured the transcriptional profiles of several genes associated with stomatal movement. When compared to WT and VC, the expression levels of GORK1 and SLAC1, encoding the key cation and anion channels modulating the turgor of guard cells, were comparable in the OE lines under the control, but were higher after drought treatment (Fig. 5f, g). When compared to WT and VC, the transcript abundance of OST1, the component linking upstream signals and ionic channels was also comparable under control condition, but was more pronounced under drought stress in the OE lines (Fig. 5e). This data indicates that the enhancement of drought tolerance by TaH2A.7 was, at least in part, attributed to its effect on stomatal movement.
TaH2A.7 overexpression decreased water loss rate and promoted stomatal closure in a stress-dependent manner. a Water loss assay. b The stomatal density comparison. c The stomatal aperture assay. d The statistic result of panel c. e–g The expression levels of genes involved in stomatal movement under the control and drought treatment. In a–d The detached leaves of 4-week old seedlings were used. Data presented as mean ± standard deviation. Columns marked without different lowercase or uppercase letters indicate no difference in means using the one-way ANOVA LSD analysis (P < 0.05)
TaH2A.7 overexpression promoted H2O2 accumulation in guard cells
Exposure to ABA can enhance the generation of H2O2 in guard cells, which acts as an key signaling molecule in modulating drought- and ABA-mediated stomatal closure (Kim et al. 2010), so we asked if TaH2A.7 can regulate the production of H2O2 in guard cells. Although H2O2 level in the OE lines is similar to that in WT and VC lines under the well-watered condition, ABA-induced H2O2 accumulation was enhanced by TaH2A.7 overexpression (Fig. 6a, b). H2O2 production in guard cells is governed by NADPH oxidase (NOX) activity (Kwak et al. 2003). In the absence of stress there was no difference in NOX activity among all samples, but much higher upregulation of NOX activity was observed, when challenged by drought, in the OE lines compared to WT and VC (Fig. 6c). Consistently, TaH2A.7 overexpression led to increased mRNA levels of two NOX genes, RBOHD and RBOHF under drought condition (Fig. 6d, e).
TaH2A.7 overexpression promoted H2O2 accumulation in guard cells. a H2O2 levels in guard cells in absence and presence of ABA visualized by fluorescence staining assay. b The statistic comparison of panel b. c The activity of NADPH oxidase. d, e The relative transcriptional level of two NADPH oxidase genes. Data presented as mean ± standard deviation. Columns marked without different lowercase or uppercase letters indicate no difference in means using the one-way ANOVA LSD analysis (P < 0.05)
Discussion
Histones are characterized by being rich in basic amino acids and containing a conserved histone folding domain (Henikoff and Ahmad 2005). TaH2A.7 has a high content of both lysine and arginine, and exhibits high propensity to form histone folding domains (Fig. S1). TaH2A.7 shares higher identities to three H2A.W variants (Yelagandula et al. 2014) than other H2A members (Deal et al. 2007), indicating it is an H2A.W variant. Unlike those of major histones, the mRNAs of histone variants are polyadenylated at 3′-terminal (Henikoff and Ahmad 2005). TaH2A.7 was derived from polyadenylated mRNAs, further indicating that it encodes a H2A variant instead of the major H2A.
TaH2A.7 is responsive to abiotic stress (Fig. 1), and its overexpression enhances drought tolerance (Fig. 3). In line with previous findings (Kumar and Wigge 2010; Smith et al. 2010), our work further confirms that histone variants are indeed crucial regulators in modulating the abiotic stress response. Drought tolerance is determined by the capacity of water maintenance, which is orchestrated via water absorption and loss efficiencies, reflected by osmotic stress tolerance capacity and transpiration rate, respectively. TaH2A.7 overexpression has no effect on osmotic stress response (Fig. S3A–C), but lowers water loss rate and promotes ABA-induced stomatal closure (Fig. 5a–d), showing the improved drought tolerance by TaH2A.7 is largely attributed to the reduction of water loss rate via promoting stomatal closure instead of the acceleration of water absorption. It is well known that ABA plays crucial role in drought-driven stomatal closure, and H2O2 is a key factor in ABA-mediated stomatal movement signaling pathway (Munemasa et al. 2011). Here, TaH2A.7 reinforces the ABA pathway (Fig. 4d–g), and elevates H2O2 production in guard cells (Fig. 6), indicating that TaH2A.7 promotes stomatal closure through elevating H2O2 level in guard cells by accelerating ABA pathway. The acceleration of ABA pathway by TaH2A.7 overexpression also results in the increase in ABA sensitivity during germination (Fig. 4). The incorporation of histone variants into chromatin is important for the establishment of an active or inactive transcriptionally poised chromatin structure (Guillemette and Gaudreau 2006). Thus, it is very likely that TaH2A.7 promotes stomatal closure through epigenetic regulation on the key factors of the stomatal movement signaling pathway. Indeed, the mRNA levels of numerous marker genes were altered in OE plants in response to stresses (Figs. 4, 5, 6). Our study, characterizing TaH2A.7 as a key regulator of drought tolerances, provides a unique launching point for us to further investigate the underlying mechanisms of stress responses in plants. Genome-wide identification of the target(s) of TaH2A.7 or TaH2A.7-regulated genes will enable us to elucidate the genetic networks governing drought tolerances, and offer invaluable clues for molecular breeding.
Note that although TaH2A.7 is induced by NaCl and PEG (Fig. 1a, b), its overexpression has no effect on salt and osmotic response (Figs. S2, S3). Chaperones and other transcription regulation-associated effectors are essential for recruitment of histone variants to chromatin (Henikoff and Ahmad 2005). Thus, it could be speculated that the incorporation of TaH2A.7 into chromatin in Arabidopsis depends on the necessary interaction with intermediaries, and these intermediaries are specifically responsive to drought and bring TaH2A.7 to regions containing genes that specifically, at least largely, modulate stomatal movement. This idea is supported by our finding that the induction of the marker genes by TaH2A.7 is only found under drought but not the control condition (Figs. 4, 5, 6). Moreover, histones hold epigenetic marks such as methylation and acetylation which are dependent on developmental and environmental constraints, and they deeply influence gene expression and other chromatin properties. This also affects the role of TaH2A.7 in the response and tolerance to abiotic stresses. Besides, it could not be excluded that the induction of TaH2A.7 by NaCl and PEG is just the response, but has not contributed to enhance tolerance to these stresses.
The incorporation of histone variants is determined by a well conserved selection mechanism which ensures the stability and suitability of chromosome structure (Song et al. 2013). The alteration in just a few residues can greatly influence the ability of native histones to interact with chaperones (Henikoff and Ahmad 2005; Tachiwana et al. 2008) which consequently affects the preference towards a certain histone variant (Song et al. 2013). Thus, it cannot be excluded that the ectopic expression of a wheat gene in Arabidopsis may be a possible cause for no contribution of TaH2A.7 to salt tolerance, because the wheat chaperones that help TaH2A.7 modulate salt tolerance-associated genes are absent in Arabidopsis. SR3 epigenome, at least those aspects related to cytosine methylation, differ substantially from that of its progenitor cultivar JN177 (Xia 2009). DNA methylation regulates gene transcription in concert with histone modification and incorporation of histone variants (Henikoff and Ahmad 2005). Thus, the differential response of TaH2A.7 to abiotic stress between SR3 and JN177 (Fig. 1) may result from the difference in epigenetic regulation of their (abiotic stress responsive) targets between two cultivars or from the difference in cis-acting factors binding to the promoter region of TaH2A.7, then is associated with the high salt and drought tolerance ability of SR3. The significance of TaH2A.7, as an epigenetic regulator, is the possibility for its use in crop molecular breeding.
Materials and methods
Wheat growth and treatment
Wheat seedlings were grown to the three-leaf stage in half-strength Hoagland’s solution under a 16 h photoperiod (day temperature 22 °C, night temperature 20 °C) and 60 % relative humidity, and were then transferred to half-strength Hoagland’s solution with none, 200 mM NaCl, 18 % PEG, or 100 μM ABA for 0–48 h. Wheat seedlings in soils were withheld for 1–4 weeks. The leaves were sampled for RNA isolation at a set time of the day.
Cloning and sequence analysis
The probe was subjected to BLASTn analysis against wheat EST database hosted by NCBI, and all matching ESTs were assembled into a contig using CAP3 (Huang and Madan 1999), and gene-specific primer pairs designed from this assembly targeting the open reading frame (ORF). The ORF was amplified from cDNAs of SR3 to JN177. Phylogenetic analyses of the predicted peptide sequence relied on the Neighbor-Joining method implemented in both CLUSTALX and MEGA3 (Kumar et al. 2004; Larkin et al. 2007). Secondary structure was predicted using PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/).
Quantitative real-time PCR (qPCR) and semi quantitative RT-PCR (sqRT-PCR)
Total RNA was extracted with the TRIzol reagent (Takara) and then treated with RNase-free DNase (Promega). The cDNA first strand was synthesized using an M-MLV kit (Invitrogen). The sqRT-PCR cycling regime comprised an initial denaturation (95 °C/5 min), followed by 28 cycles of 94 °C/30 s, 55 °C/30 s, 72 °C/45 s, with a final extension of 72 °C/5 min. The cDNA was used as the template for 20 μL qPCR solution containing 10 μL 2 × SYBR Premix Ex Taq mix (Takara), 0.2 μM forward and 0.2 μM reverse primers, 1 μL of a 1:10 dilution of the cDNA first strand, and the cycling regime comprised a denaturation step of 95 °C/2 min, followed by 45 cycles of 95 °C/10 s, 60 °C/20 s and 72 °C/20 s. A melting curve analysis was performed over the range 80–95 °C at 0.5 °C intervals. Relative gene expression levels were detected using the 2−ΔΔCT method (Livak and Schmittgen 2001). A positive control was provided by a parallel analysis based on the Arabidopsis TUBULIN gene (AT1G04820), with three independent replicates per experiment.
Subcellular localization assay
TaH2A.7 ORF, lacking the termination codon, was introduced into the pBI221 vector, creating an in-frame histone/GFP fusion transgene under the control of the CaMV 35S promoter. The fusion or an empty vector was transiently transformed into onion epidermis cells using a gene gun. After incubation at 21–23 °C for 16 h, the GFP signal was detected by confocal fluorescence microscopy.
Construction of transgenic Arabidopsis
TaH2A.7 ORF was cloned into the pSTART vector driven by the CaMV 35S promoter, and the construct was transformed into Arabidopsis Col-0 using the floral dipping method (Clough and Bent 1998). Homozygous transgenic lines were selected by kanamycin screening and confirmed by genomic PCR. The transcription of the transgenes in A. thaliana was confirmed by RT-PCR.
Arabidopsis treatment and phenotype assay
Surface-sterilized Arabidopsis seeds were plated on half strength Murashige and Skoog (MS) agar medium, kept in the dark at 4 °C for 3 days to break dormancy, and subsequently transferred to a 16 h photoperiod at 22 °C for 2 days. Uniformly germinated seeds were transferred onto 1/2 MS plates containing various concentrations of NaCl, mannitol or H2O2 for 12 days. Four-week old Arabidopsis seedlings in soil were water-deprived for 10 days, followed by re-watering for 5 days, and survival rate was calculated. A germination assay was conducted by plating surface-sterilized seeds on half strength MS agar medium containing various concentrations of ABA. After holding for 3 days at 4 °C in the dark, the plates were transferred to a 16 h photoperiod at 22 °C for 5 days. The emergence of the radicle was taken as representation of a successfully germinated seed. Germination rates were expressed as a proportion of seeds that had successfully germinated. To detect the expression of marker genes, four-week old Arabidopsis were water-deprived for 5 days, and RNA was extracted from leaves of stressful and non-stressful plants for real-time PCR. All experiments were replicated three times.
Water loss rate
To measure water loss, leaves were detached from 5-week old Arabidopsis, weighed and placed in a controlled atmosphere (relative humidity 60 %, air temperature 22 °C). Their weight was measured for every 60 min up to 360 min. The rate of water loss was calculated from the loss of weight compared to the initial fresh weight. Each sample consisted of leaves harvested from three separate plants.
NADPH oxidase activity measurement
NOX activity was measured according to the method of Grace and Logan (1996). ~0.3 g leaves of Arabidopsis seedlings was homogenized in the buffer solution containing 1 mL 50 mM KH2PO4 and 0.1 mM EDTA. Protein concentration was determined using a Bradford Coomassie brilliant blue staining assay (Bradford 1976), which was used to calculate the activities of enzymes in a certain volume of protein extraction solution. The reaction was started when 0.2 mM NADPH was added, and NOX activity was calculated by monitoring the decrease in absorbance at 340 nm of a reaction containing 100 mM Tris–HCl, pH 8.0, 1 mM EDTA, and 0.2 mM NADPH (the molar extinction coefficient for NADPH: 6.22 × 103 M−1 cm−1).
Stomatal density and aperture measurement
Stomatal aperture was quantified following Pei et al (1997). Fully expanded young leaves were removed before dawn from 5-week old plants. Leaves were maintained in the light in 20 mM KCl, 1 mM CaCl2, 5 mM Mes-KOH (pH 6.2) for 2.5 h, after which either 50 µM ABA or ethanol was added; measurements were made after the elapse of 2.5 h. The abaxial epidermis was peeled to make slides, and photographed randomly under the microscope. Stomatal aperture was quantified using ImageJ software. For measuring stomatal density, abaxial epidermis was directly peeled from leaves, and photographed randomly under the microscope. The amounts of stoma per square millimeter were calculated.
Measurement of H2O2 level in guard cells
A fluorescence-based assay for H2O2 was conducted according to Pei et al (2000). Either wheat leaves or epidermal stripes peeled from Arabidopsis were dipped in fluorophore dichlorofluorescein (H2DCF), and the resulting fluorescent signals were visualized by confocal microscopy. The signal was quantified using the ImageJ software.
Author contribution statement
W.X., Y.L. and Z.C. carried out most experiments and analyzed data. M.W. and G.X. designed the experiment and supervised the study. M.W. wrote the manuscript. G.X. revised the manuscript.
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Deal RB, Topp CN, McKinney EC, Meagher RB (2007) Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19:74–83
Grace SC, Logan BA (1996) Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 112:1631–1640
Guillemette B, Gaudreau L (2006) Reuniting the contrasting functions of H2A.Z. Biochem Cell Biol 84:528–535
Henikoff S, Ahmad K (2005) Assembly of variant histones into chromatin. Ann Rev Cell Dev Biol 21:133–153
Huang X, Madan A (1999) CAP3: a DNA sequence assembly program. Genome Res 9:868–877
Jenks MA, Hasegawa PM, Chinnusamy V, Dalal M, Zhu JK (2014) Epigenetic regulation of abiotic stress responses in plants. Jenks MA, Hasegawa PM (eds) Plant abiotic stress, 2nd edn. Wiley, pp 203–229
Kamakaka RT, Biggins S (2005) Histone variants: deviants? Genes Dev 19:295–316
Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2 and Ca2+ signaling. Ann Rev Plant Biol 61:561–591
Kumar SV, Wigge PA (2010) H2A.Z-containing nucleosomes mediate the thermosensory response in arabidopsis. Cell 140:136–147
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment Brief. Bioinformatics 5:150–163
Kwak JM et al (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Larkin MA et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Liu C, Li S, Wang M, Xia GM (2012) A transcriptomic analysis reveals the nature of salinity tolerance of a wheat introgression line. Plant Mol Biol 78:159–169
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25:402–408
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Munemasa S, Mori IC, Murata Y (2011) Methyl jasmonate signaling and signal crosstalk between methyl jasmonate and abscisic acid in guard cells. Plant Signal Behav 6:939–941
Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9:409–423
Pei ZM et al (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406:731–734
Peng Z, Wang M, Li F, Lv H, Li C, Xia G (2009) A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Mol Cell Proteom 8:2676–2686
Scippa GS, Michele MD, Onelli E, Patrignani G, Chiatante D, Bray EA (2004) The histone-like protein H1-S and the response of tomato leaves to water deficit. J Exp Bot 55:99–109
Smith AP, Jain A, Deal RB, Nagarajan VK, Poling MD, Raghothama KG, Meagher RB (2010) Histone H2A.Z regulates the expression of several classes of phosphate starvation response genes but not as a transcriptional activator. Plant Physiol 152:217–225
Song Y, Seol JH, Yang JH, Kim HJ, Han JW, Youn HD, Cho EJ (2013) Dissecting the roles of the histone chaperones reveals the evolutionary conserved mechanism of transcription-coupled deposition of H3.3. Nucleic Acids Res 41:5199–5209
Tachiwana H, Osakabe A, Kimura H, Kurumizaka H (2008) Nucleosome formation with the testis-specific histone H3 variant, H3t, by human nucleosome assembly proteins in vitro. Nucleic Acids Res 36:2208–2218
Talbert PB, Henikoff S (2010) Histone variants—ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol 11:264–275
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wang MC, Peng ZY, Li CL, Li F, Liu C, Xia GM (2008) Proteomic analysis on a high salt tolerance introgression strain of Triticum aestivum/Thinopyrum ponticum. Proteomics 8:1470–1489
Wang M, Liu C, Xing T, Wang Y, Xia G (2015) Asymmetric somatic hybridization induces point mutations and indels in wheat. BMC Genom 16:807
Xia G (2009) Progress of chromosome engineering mediated by asymmetric somatic hybridization. J Genet Genom 36:547–556
Xia GM, Xiang FN, Zhou AF, Wang H, Chen HM (2003) Asymmetric somatic hybridization between wheat (Triticum aestivum L.) and Agropyron elongatum (Host) Nevishi. Theor Appl Genet 107:299–305
Yelagandula R et al (2014) The histone variant H2A.W defines heterochromatin and promotes chromatin condensation in Arabidopsis. Cell 158:98–109
Zhu Y, Dong A, Shen WH (2012) Histone variants and chromatin assembly in plant abiotic stress responses. Biochimica et Biophysica Acta (BBA) Gene Regul Mech 1819:343–348
Acknowledgments
This research was supported by the Natural Science Foundation of China (31171175, 31570258), and the National Transgenic Project (2014ZX08002002-003). We grateful to Dr Jian Ding at Harvard Medical School and Gavinda Sangha at Magdalen College Oxford for the writing improvement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Pedro Puigdomenech.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, W., Li, Y., Cheng, Z. et al. A wheat histone variant gene TaH2A.7 enhances drought tolerance and promotes stomatal closure in Arabidopsis. Plant Cell Rep 35, 1853–1862 (2016). https://doi.org/10.1007/s00299-016-1999-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1999-6