Abstract
Key message
This paper is the first to directly link two types of ion channel regulation pathway into an emerging and complex CBL–CIPK signal system in wooden plant.
Abstract
In Arabidopsis thaliana, the calcineurin b-like (CBL) 1 gene has been shown to be necessary in response to abiotic stresses. In this study, we identified CBL1 in the woody plant Populus euphratica, designated as PeCBL1. Heterologous expression of PeCBL1 could build the resistance of sensitive phenotypes to low K+ stress in the corresponding Arabidopsis cbl1/cbl9 mutant, and display a salt-sensitive phenotype compared with the mutant. Protein interaction analysis showed that PeCBL1 can interact with PeCIPK24, 25 and 26, and form different complexes of PeCBL–PeCIPK. To further investigate the mechanism of PeCBL1, we analyzed the fluxes of K+ and Na+ in roots of the wild-type Arabidopsis, cbl1/9 mutant, and PeCBL1 transgenic plants under low K+ stress and high Na+ stress. These analyses revealed that, compared to the cbl1/9 mutant, the PeCBL1 transgenic plant roots exhibited a higher capacity to absorb K+ after exposure to low K+ stress, and a lower capacity to discharge Na+ after exposure to salt stress. The results suggest that CBL1 interacts with CIPK24, CIPK25 and CIPK26 to regulate Na+/K+ homeostasis in Populus euphratica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In plants, Ca2+ ions serve as an ubiquitous second messenger in many adaptation and developmental processes, including response to abiotic stress, pathogen defense and adjustment of ion homeostasis (Sanders et al. 2002; White and Broadley 2003; Kim et al. 2009). One unanswered question is how this cation serves as a messenger for numerous signals and confers specific cellular responses (Luan 2009). In response to environmental and developmental stimuli, plant cells can react to spatial and temporal changes in cytosolic calcium concentration. Such stimulus-specific elevations in cytosolic calcium concentration have been termed the “Ca2+ signature”. The next step is to determine which proteins can decode the “Ca2+ signature” and translate it into a downstream response. It has been demonstrated that the decoding process starts with the calcium sensors that can bind Ca2+ ions and thereby alter their own structural properties (Luan 2009). Until now, a diverse array of calcium sensors in plant cells has been identified, including calmodulin (CaM), calcium-dependent protein kinase (CDPK) and calcineurin B-like (CBL). Among these calcium sensors, CDPK combines with Ca2+-binding and kinase activity within a single protein, thereby forming “sensor-response” modules (Sanders et al. 2002; Harper and Harmons 2005). By contrast, CaM and CBL are small calcium sensors; they have no activity domain and have to function by interacting and regulating specific downstream target proteins (Luan et al. 2002). Unlike CaM, which can interact with a diverse array of target proteins, CBL only interacts specifically with a group of Ser/Thr kinases designated as CBL-interacting protein kinases (CIPKs) (Shi et al. 1999; Albrecht et al. 2001).
The increasing number of full-genome sequences of higher plant species enables us to easily identify the members of the CBL–CIPK pathway. The use of bioinformatics strategies revealed the existence of the 10 CBL and 26 CIPK genes in the Arabidopsis genome as well as 10 CBLs and 30 potential CIPKs in the rice genome (Kolukisaoglu et al. 2004; Weinl and Kudla 2009). In addition, the genome of the moss encodes four CBLs and seven CIPKs, and the fern possesses a complement of four CBL members and five CIPKs (Weinl and Kudla 2009). In the Populus genome, 10 CBL and 27 CIPK were identified (Yu et al. 2007; Zhang et al. 2008). All of the plants mentioned above contain several CBLs and CIPKs; thus, one CBL may interact with several CIPKs, and one CIPK also may interact with several CBLs, which theoretically allows for many independent combinations. Protein interaction studies in Arabidopsis have revealed a network-like organization of this signaling system in that the selectivity of CBL–CIPK complex formation represents one of the potential mechanisms that generate a stimulus-specific signaling response (Batistic and Kudla 2004).
In Arabidopsis, both CBL1 and CBL9 can interact with CIPK23, forming CBL1–CIPK23 or CBL9–CIPK23 complexes. These complexes can activate a voltage-gated inward K+ channel (AKT1) in a Ca2+-dependent manner and enhance K+ uptake under low K+ conditions (Xu et al. 2006; Li et al. 2006). In addition, the CIPK23 pathway can also phosphorylate one type of nitrate sensor, CHL1, and play an important role in nitrate uptake under low-level nitrate concentrations (Ho et al. 2009). More recently, it has been demonstrated that CBL1 can interact with CIPK7 and is involved in cold response (Huang et al. 2011). The elements of similar CBL–CIPK complexes have been identified by other coworkers. It has been shown that the calcium sensor/protein kinase complexes SOS3/CBL4-SOS2/CIPK24 can regulate the Na+/H+ exchanger, SOS1, which is localized to the plasma membrane; they can also transport sodium ions out of the cell under salt stress (Liu et al. 2000; Xiong et al. 2002). SCABP8/CBL10-SOS2/CIPK24 is also observed to be involved in the salt-stress pathway, which functions mainly in the shoots (Quan et al. 2007; Kim et al. 2007). These results provided growing evidence for crucial functions of different CBL–CIPK complexes in response to abiotic stress signals.
We characterized one CBL member from Populus euphratica. Further studies demonstrated that it can interact with PeCIPK24, 25, 26 and form the complexes of PeCBL1–PeCIPK24, PeCBL1–PeCIPK25 and PeCBL1–PeCIPK26. Analyzing phenotypes and their fluxes of K+ and Na+ showed that the complexes play an important role in response to low K+ stress and high Na+ stress.
Materials and methods
cDNA cloning and sequence analysis of PeCBL1 from Populus euphratica
Based on the sequences of PtCBL1 (Genbank accession number: EF148841), we designed primers for cloning PeCBL1 from Populus euphratica (Supplementary Materials 1). cDNA syntheses were performed using mRNA Selective PCR Ver. 1. 1 Kit (TaKaRa) and cDNA was amplified (94 °C, 1 min; respective Tm, 50 s; 72 °C, 1 min; in a 50-μL volume) for 30 cycles using the TaKaRa Ex Taq Kit. The cDNA product was inserted into the pMD18-T vector for sequencing. The total RNA in our experiments was extracted from 2-year-old seedlings of Populus euphratica obtained from Alashan in Inner Mongolia of China, according to the method described by Chang et al. (1993). The alignment of the CBL putative amino sequences was generated using the “multiple sequence alignment” method with DNAMAN software. The phylogenetic tree of the CBL family was drawn by the Neighbor-joining bootstrap method (bootstrap analysis with 1,000 replicates) using MEGA 5.10 software based on the multiple alignments of the protein amino acid sequences of all CBLs. The CBL genes of Arabidopsis were obtained from TAIR database (http://www.arabidopsis.org/) and the CBL genes of Populus trichocarpa were from NCBI database referred to the accession number by Zhang et al. (2008).
Generation of constructs
The constructs of yeast two-hybrid interaction assays were generated by inserting specifically digested PCR products of PeCBL1, PeCIPKs into the activation domain (pGADT7) or the DNA-binding domain (pGBKT7) vectors. For generation of BiFC constructs, we cloned PeCBL1 via XbaI and SalI into the pSPYCE(M) vector resulting in PeCBL1-YC; and PeCIPK24, 25, 26, and 27 via BamHI and KpnI into the pSPYNE(R)173 vector resulting in PeCIPK-YN. To create the constructs of PeCBL1 for the analysis of subcellular localization transient expressing in Nicotiana benthamiana and for over-expressing in Arabidopsis, we first cloned these genes into the pDONOR vector, constructed the entry clone by the Gateway system (BP reaction, Invitrogen), and then exchanged the genes into the pBIB::GFP (GW) vector under the CAMV35S promoter by the LR reaction resulting in pBIB::GFP-PeCBL1 (Supplementary materials 1). PeCBL1 were also cloned into the pBI121 vector fusing with GFP (resulting in pBI121::PeCBL1 vector) to analyze the subcellular localization in onion epidermal cells. The detailed primers for PCR in this work are listed in Supplementary Materials 2.
Subcellular localization and BiFC assay analysis of PeCBL1
For subcellular localization and BiFC assays in N. benthamiana leaf cells, the methods were performed as described previously (Waadt and Kudla 2008). We introduced, respectively, the fusion construct pBIB::GFP-PeCBL1, PeCBL1-YC, and PeCIPKs-YN into Agrobacterium tumefaciens strain GV3101. The GV3101 bacteria carrying the construct pBIB::GFP (as control) and the fusion construct pBIB::GFP-PeCBL1 together with the p19 strain were, respectively, infiltrated into 5- to 6-week-old N. benthamiana leaves to analyze subcellular localization, and the bacteria carrying BiFC constructs (PeCBL1-YC and PeCIPKs-YN) were used together with the p19 strain for infiltration to analyze the interaction. After the plants were incubated for 4 days, the leaves were prepared for microscopic analysis with fluorescence (GFP or YFP). The protoplasts for subcellular localization from leaves were prepared for microscopic analysis of the fluorescence distribution. The protoplasts preparation was performed as described by Batistič et al. (2008). For subcellular localization analysis of PeCBL1 in onion epidermal cells, the fusion construct pBI121::PeCBL1 was introduced into onion epidermal cells by the particle bombardment method using the PDS-1000 System (Bio-Rad) at 1,100 psi helium pressure. pBI121 vector was also introduced as a control. GFP expression was observed by a fluorescence microscope after incubation for 24 h at 22 °C.
Yeast two-hybrid analysis
The BD fusion constructs were first introduced into the yeast strain AH109 by the lithium acetate method (yeast two-hybrid system user manual by Clontech). Transformants were spread on SC-Trp media plates and allowed to grow for 2–3 days for screening purposes. And then the yeast strain growth on SC-Trp media (carrying BD vectors) was used to transform the AD fusion vectors. The yeast strain was then screened on SC-Trp/Leu media plates after it was grown for 2–3 days to obtain the transformants carrying AD and BD vectors. Finally, the transformants carrying AD and BD vectors were streaked on an SC-Leu-Trp-His-Ade plate for 3–5 days to determine the interactions between PeCBL and PeCIPKs. To confirm the interaction results, the transformants carrying AD and BD vectors were also transferred to the Waterman filter paper to perform β-galactosidase assay.
Arabidopsis transformation and phenotype identification
For plant transformation, Agrobacterium tumefaciens GV3101 carrying the pBIB::GFP-PeCBL1 was transformed into Arabidopsis mutant cbl1/cbl9 (SALK_110426 and SALK_142774 hybrid) by the floral dip method (Clough and Bent 1998). The T1 transformants were planted in soil and sprayed with 0.0002 % (v v−1) BASTA. The genotype of surviving plants was further confirmed by PCR. For phenotype identification, transgenic, homozygous T3 seeds, the corresponding mutant, and the wild-type plants were surface-sterilized in half-strength MS media and stratified at 4 °C for 2 days to obtain uniform germination. Four days later, the seedlings were transferred to modified 1/2 MS media containing 100 μM K+ (KH2PO4 replaced by NH4H2PO4 and KNO3 partially replaced by NH4NO3) or 1/2 MS media supplemented with 120 mM NaCl. We allowed 7 days of new growth before analyzing the phenotypes in the presence of 100 μM K+ media and 14 days in the presence of 120 mM NaCl media. For quantitative root elongation data, we marked the position of the root tip immediately after transfer. At the end of the experiment, root elongation was quantified by measuring with a ruler. For the seed germination test, the seeds were propagated on 1/2 MS medium supplemented with 120 mM NaCl or on modified 1/2 MS medium containing 100 μM K+. For germination test, approximately 200 seeds each from the wild type, the cbl1/9 mutant, and the PeCBL1 line were planted in triplicate on 1/2 MS medium, modified 1/2 MS media containing 100 μM K+ and 1/2 MS media supplemented with 120 mM NaCl. The percentage of germinated seeds that developed green cotyledon was calculated in all assays 5 days after germination. And three different PeCBL1 lines together with cbl1/9 and the wild type were repeated in the germination experiment.
Determination of Na+ and K+ content
For measurement of K+ in plants, 4-day-old seedlings growing on vertical 1/2 MS medium were transferred to liquid culture containing low K+ (100, 50 and 20 μM) for 7 days, or high Na+ (100, 120 and 150 mM) for 48 h. The seedlings were then collected and rinsed briefly with distilled water, dried at 300 °C for 24 h, and weighed. The samples were digested with 1 M HNO3 and then boiled for 30 min. The [Na+] and [K+] concentrations of the samples were determined using an atomic absorption spectrophotometer. The measurement was performed independently for three times and the results were presented as average values.
Measurement of net K+ and Na+ flux with the NMT
K+ and Na+ flux measurements were conducted using the roots of 6-day-old Arabidopsis seeds that had been surface-sterilized in half-strength MS media. Seeds were stratified at 4 °C for 2 days, and then transferred to 22 °C for 6 days. In a typical protocol, the Arabidopsis plants were transferred to 150 mM NaCl and 50 μM K+ half-strength modified MS media (KH2PO4 replaced by NH4H2PO4 and KNO3 partially replaced by NH4NO3) for 1 day.
Net flux of K+ and Na+ were measured noninvasively using the NMT (BIO-IM, Younger USA LLC, Amherst, MA, USA). The concentration gradients of the target ions were measured by moving the ion-selective microelectrode between two positions close to the plant material in a preset excursion with a distance of 30 μm, a whole cycle being completed in 6.33 s.
Pre-pulled and silanized glass micropipettes (2–4 μm aperture, XYPG120-2; Xuyue Science and Technology Co., Ltd.) were first filled with a backfilling solution (K+ 100 mM KCl, Na 250 mM NaCl) to a length of approximately 1 cm from the tip. Then, the micropipettes were front filled with approximately 180 μm columns for K+ and 15–25 μm columns for Na+ of selective liquid ion-exchange cocktails (LIXs; K: Fluka 60398; Na: Fluka 71178, Fluka Chemie GmbH, Buchs, Switzerland). An Ag/AgCl wire electrode holder (XYEH01-1; Xuyue Science and Technology Co., Ltd.) was inserted at the back of the electrode to make electrical contact with the electrolyte solution. DRIREF-2 (World Precision Instruments) was used as the reference electrode. Ion-selective electrodes of the following target ions were calibrated prior to flux measurements:
-
1.
K+: 0.1, 0.5 and 1.0 mM (K+ concentration was 0.5 mM in the measuring solution); and
-
2.
Na+: 0.5, 0.9 and 5.0 mM (Na+ concentration was usually 0.9 mM in the measuring solution).
Only electrodes with Nernstian slopes of >50 mV per decade were used in our study. Ion flux was calculated by Fick’s law of diffusion:
where J represents the ion flux in the x-direction, dc/dx is the ion concentration gradient (dx represents the distance of microelectrode movement between a near point and far point and was 30 μm in our experiments), and D is the ion diffusion constant in a particular medium. Data and image acquisition, preliminary processing, control of the electrode positioner and stepper-motor-controlled fine focus of the microscope stage were performed with imflux software.
Ionic fluxes were calculated with mageflux, developed by Xu (http://xuyue.net/mageflux).
Data analysis
The quantitative data in this study used SPSS software to undertake statistical analysis. Differences among treatments were analyzed by one-way ANOVA using the LSD test at P < 0.05.
Results
Isolation and sequence analysis of PeCBL1 from Populus euphratica
Database searches together with bioinformatics analysis revealed ten CBLs from the Populus genome. One CBL member that encoded proteins with the highest similarity to Arabidopsis CBL1 was isolated from Populus euphratica and named PeCBL1 (The GenBank accession numbers of CBL are described in Supplementary Materials 3). Analyzing their phylogenetic relationship with all CBL members in Arabidopsis and Populus revealed that PeCBL1 were distributed with PtCBL1 and clustered in one subgroup with AtCBL1 (Fig. 1a), indicating that they share a common ancestor. By aligning the amino acid sequences of PeCBL1, PtCBL1 and AtCBL1, we found that the structures are significantly conserved. The protein identity of PeCBL1 and AtCBL1 shared 84.51 %, and only two amino acids were different between PeCBL1 and PtCBL1 (Fig. 1b).
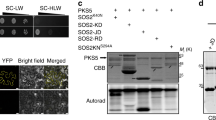
Character and sequence analysis of PeCBL1 from Populus euphratica. a Phylogenic analysis PeCBL1 with all CBL members from Populus (Populus trichocarpa) and Arabidopsis. The CBL genes of Arabidopsis were obtained from TAIR database (http://www.arabidopsis.org/) and the CBL genes of P. trichocarpa were from NCBI database referred to the accession number by Zhang et al. (2008). Sequence phylogram was constructed by Neighbor-joining bootstrap method (bootstrap analysis with 1,000 replicates) using MEGA 5.10 software based on the multiple alignments of the protein amino acid sequences of all CBLs. b Alignment of the deduced amino acids sequences of PeCBL1, PtCBL1 and AtCBL1. The amino acids with an entire homology are the white letters on a black background, and those shared non-identical conserved identity are shadowed by green color. c Sub-cellular analysis of PeCBL1 in onion epidermal cell. Green fluorescence (GFP) signals were observed throughout the cell transformed with the control pBI121-GFP plasmid and the GFP signal in the cell transformed with pBI121::PeCBL1 plasmid was concentrated on cell membrane. d Sub-cellular location of PeCBL1 in the protoplasts of Nicotiana benthamiana. Confocal GFP images throughout the protoplasts transformed with pBIB::GFP was used as a control. The signal treated with pBIB::GFP-PeCBL1 was only located on the protoplasts membrane
Previous studies showed that AtCBL1 is localized to the cell membrane of the plant (Xu et al. 2006). To examine whether the subcellular localization of PeCBL1 is also tagged to the cell membrane, we fused it with GFP under the control of the CAMV35S promoter and transiently expressed them in onion epidermal cells and in N. benthamiana protoplasts. Confocal microscopy revealed that the fluorescence distribution of the GFP signal was concentrated only at the cell membrane (Fig. 1c, d). The cells transformed with the control plasmid blank CAMV35S-GFP (pBIB::GFP or pBI121::GFP) were used as the controls, whereas green fluorescent signals were observed throughout the cells. The results mean that PeCBL1 may be a regulator of the protein located on the cell membrane.
Constitutive expression of PeCBL1 in Arabidopsis cbl1/cbl9 mutant complements the low K+ sensitivity of the mutant, but the transgenic plants show sensitivity to salt stress.
As mentioned previously, PeCBL1 is a homolog of AtCBL1. In Arabidopsis, it has been demonstrated that AtCBL1 is involved in the low K+ signal transduction pathway (Xu et al. 2006; Li et al. 2006). To further analyze the function of PeCBL1, we overexpressed it in the cbl1/cbl9 mutant of Arabidopsis. The five lines genotype was further confirmed by PCR and three lines were selected to analyze their phenotype in response to low K+ stress (Fig. 2a). Before identifying the phenotype, we first examined the growth status on 1/2 MS medium. The seeds of wild type, PeCBL1 and cbl1/9 were surface-sterilized in half-strength MS media to grow for 4 days and then the seedlings were transferred to new 1/2 MS media for 7 days growth. We found no difference in the phenotype and in the primary root elongation test between them. In a quantitative low external K+ test, the cbl1/cbl9 mutant exhibited severe stunting, while the wild-type and PeCBL1 plants had similar and higher rates of root growth, and more lateral roots in the presence of low external K+ for 7 days. The results indicated that the transgenic plants as well as the wild type plants showed significantly higher tolerance to low K+ than did the cbl1/cbl9 mutant. We also investigated the phenotype of these plants in response to salt stress. Interestingly, we found that after 14 days of 120 mM salt treatment, the wild type and PeCBL1 appear yellow and showed little primary root elongation shorter than cbl1/cbl9; even though the difference of the root elongation between PeCBL1 and cbl1/9 did not reach the significant level at P < 0.05 (Fig. 2b, c). This indicated that PeCBL1 maybe a negative regulator of salt response. The finding was not consistent with the previous result in Arabidopsis (Cheong et al. 2003). They reported that cbl1 mutant is hypersensitive to salt and suggested that CBL1 functions as a positive regulator of salt response in plant. But using our phenotype identification methods, we found that cbl1/9 double mutant showed higher tolerance to salt treatment than wild type. As we know, CBL9 is a paralogous to CBL1 and has redundant roles in some ways, e.g., response to low K+ stress (Xu et al. 2006; Li et al. 2006), and also has different roles in some ways, e.g., response to ABA signal (Pandey et al. 2004; Cheong et al. 2007). Why cbl1/9 and cbl1 do not have the same phenotype under salt stress? To explain this, further investigated is required. Furthermore, we examined the low K+ and high Na+ tolerance of these plants at seed germination. Statistical analysis showed that, on the modified 1/2 MS medium supplemented with 100 μM of K+, the seed germination rates of the PeCBL1 transgenic lines as well as the wild type were much higher than the cbl1/cbl9 mutant; but lower on the 120 mM Na+ medium (Fig. 2d). For example, on medium containing 100 μM K+, the transgenic lines overexpressing PeCBL1 showed 88 % and wild type showed 87 % germination in 5 days, whereas 80 % of cbl1/9 seeds had germinated. On medium containing 120 mM Na+, 90.5 % of cbl1/9 seeds germinated within 5 days, whereas PeCBL1-overexpressing seeds germination rate had only achieved 81 % and wild type achieved 80.5 %. These results indicated that PeCBL1 can act like AtCBL1 in the cbl1/cbl9 mutant and complement the low K+ sensitive phenotype. In addition, PeCBL1 may act a negative regulator involved in salt-stress response.
Overexpression of PeCBL1 in Arabidopsis cbl1/cbl9 mutant complements the low K+ sensitivity, but show sensitivity to salt stress. a Identification of the transgenic plants of PeCBL1 by PCR method. The Arabidopsis mutant plant was as the negative control (N) and the expression levels of actin gene in Arabidopsis was used as a constitutively expressed control. b Phenotype analysis of PeCBL1 under low K+ (100 μM) and salt (120 mM Na+) stress. Wild-type, cbl1/9 and PeCBL1 transgenic seedlings grown on 1/2 MS medium for 4 days, and then transferred to 1/2 MS medium for 7 days, low K+ medium for 7 days and high Na+ medium for 14 days. c The primary root growth of the wild-type, transgenic plants and Arabidopsis mutant under 1/2 MS for 7 days, low K+ for 7 days and high salt medium for 14 days. For quantitative root elongation data, we marked the position of the root tip immediately after transfer. At the end of the experiment, root elongation was quantified by measuring with a ruler. d Seed germination rates in the wild type, cbl1/9 and 3 PeCBL1 lines on 1/2 MS medium, or modified 1/2 MS medium containing 100 μM K+ or 120 mM NaCl. Germination was scored at 5 days after incubation. Results are presented as average values and error bars represent the standard deviation. The different letters (a and b) in the same column indicate statistically significant differences at P < 0.05 according to the LSD test (color figure online)
PeCBL1 may influence Na+/K+ ions homeostasis under low K+ and salt stress
Because PeCBL1 may be involved in low K+ and salt stress, we also investigated whether the accumulation levels of Na+ and K+ were different among the three genotype plants under the stress treatment. As shown in Fig. 3, when the seedlings were grown on 1/2 MS medium, the PeCBL1 transgenic plants as well as the wild type accumulated more K+ than the corresponding Arabidopsis mutants (cbl1/cbl9). Under low K+ stress, the K+ content was reduced and the Na+ content was accumulated in all of the seedlings. But the reduction of K+ levels in the PeCBL1 transgenic plants as well as wild type plants was lower than the corresponding Arabidopsis mutants. Under salt stress, Na+ levels increased and K+ content decreased in all of the seedlings. In addition, we found that the Na+ levels in wild-type plants as well as in the PeCBL1 transgenic seedlings were accumulated at higher levels than the corresponding Arabidopsis mutants (cbl1/9). It indicates that the overexpression of PeCBL1 can complement the phenotype of cbl1/cbl9 and positively control K+ acquisition under low K+ stress; reversely, it may negatively influence the discharging Na+ pathway under high Na+ stress.
Comparison of K+ and Na+ content in wild-type, cbl1/9 and PeCBL1 seedlings under low K+ and high Na+ stress. 4-Day-old seedlings growing on vertical 1/2 MS medium were transferred to liquid culture containing low K+ (100, 50 and 20 μM) for 7 days, or high Na+ (100, 120 and 150 mM) for 48 h. The seedlings were then collected for K+ and Na+ assay. a The K+ contents in whole seedlings under 1/2 MS or different intensity of low K+ stress. b The Na+ contents in whole seedlings under 1/2 MS or different intensity of low K+ stress. c The K+ contents in whole seedlings under 1/2 MS or different intensity of high Na+ stress. d The Na+ contents in whole seedlings under 1/2 MS or different intensity of high Na+ stress. Results are the average from three independent replicates. Error bars represent the standard deviation and the different letters (a and b) in the same column indicate statistically significant differences at P < 0.05 according to the LSD test
Protein interaction analysis of PeCBL1 with PeCIPKs via yeast two-hybrid and BiFC systems
In Arabidopsis, it has been demonstrated that AtCBL1 can interact with AtCIPK23, forming CBL1–CIPK23 complexes and regulate low K+ responses. To determine whether these complexes also exist in Populus euphratica, yeast two-hybrid assays were conducted to identify the particular PeCIPKs that interact with PeCBL1 (the GenBank accession numbers of CIPK are described in Supplementary Materials 3). As shown in Fig. 4a, PeCBL1 can not only interact with the paralogous gene pair PeCIPK24 and 25, which are homologous with AtCIPK23, but also with PeCIPK26, which is a homolog with AtCIPK24 (SOS2). The in vivo interaction between CIPKs and CBL1 was also confirmed using BiFC assays. The results are the same as those in the yeast two-hybrid assay (Fig. 4b).
Analyzing the interaction of PeCBL1 with PeCIPK24, 25, 26 and 27 by yeast 2-hybrid and BiFC system. a Comparative yeast 2-hybrid interaction analysis of PeCBL1 and PeCIPKs. The yeast strain AH109 containing the indicated plasmid combinations were grown on SC-Leu/Trp and SC-Leu-Trp-His-Ade medium. The β-galactosidase assay on filter paper was also performed. The growth on SC-Leu-Trp-His-Ade medium and the blue coloration on filter paper indicated the interaction. The blank pGAD7 and pGBKT7 vector was, respectively, used as control. b BiFC assays of PeCBL1 and PeCIPKs in vivo. The instruct of PeCBL1-YC with PeCIPKs-YN was coexpressed in N. benthamiana leaf cells and confocal images of YFP fluorescence in cell indicated the interaction. The coexpression of pSPYCE (M) with PeCIPKs-YN was used as control (right images in each panel) (color figure online)
As is understood in Arabidopsis, CBL1–CIPK23 complexes are involved in the low K+ signal transduction pathway. Here, we found that CIPK24 and 25 in Populus euphratica can interact with CBL1; this finding indicated that the low K+ pathway regulation by CBL–CIPK complexes also exists in Populus euphratica. However, this raises the question: if the CBL1–CIPK26 complexes exist in Populus euphratica, how do they work in the stress signal transduction pathway? Based on the phenotypes of the over-expressing PeCBL1 in the Arabidopsis mutant, the interaction results led us to hypothesize that the interactions of CBL and CIPK may play an important role in maintaining the Na+/K+ ion balance in response to ions stress.
PeCBL1 can increase the capacity of net K+ flux and the negatively influence the Na+ efflux under high Na+ and low K+ stress
To verify the hypothesis above, we first used the noninvasive micro-test technique (NMT) to measure steady flux profiles of K+ in the root of 7-day-old Arabidopsis wild-type, cbl1/cbl9 mutant and PeCBL1 transgenic plants, respectively (Fig. 5). The results showed that, under normal growth conditions, the net K+ efflux in the Arabidopsis cbl1/cbl9 mutant roots was slightly higher than the WT and PeCBL1 transgenic plants. Under high Na+ and low K+ stress, however, the capacity of net K+ influx in all three kinds of plants was differentially induced (Fig. 5a). It is noteworthy that, in the PeCBL1 transgenic plants, a significantly induced K+ influx response was measured. In comparison, the cbl1/cbl9 mutant showed much smaller K+ influx than wild-type and PeCBL1 plants. Moreover, the root K+ influx in the mutant shoots showed an invariable pattern, with a stable level of increase after high Na+ and low K+ stress treatment.
Effects of low K+ stress (50 μM K+ for 1 days) and high Na+ stress (150 mM NaCl for 1 day) on net K+ and Na+ fluxes in roots of the wild type, cbl1/9 and PeCBL1. A continuous flux recording of 10–12 min was conducted for each plant in corresponding measuring solutions (pH 6.0). Each result is the mean of six individual plants and bars represent the standard error of the mean. The different letters (a, b and c) in the same column indicate statistically significant differences at P < 0.05 according to the LSD test. a The mean flux of K+ under 1/2 MS, low K+ stress and high Na+ stress. b The mean flux of Na+ under 1/2 MS, low K+ stress and high Na+ stress
Interestingly, the NMT data showed that the pattern of Na+ fluxes in roots of the cbl1/cbl9 mutant differed from that in WT and PeCBL1 after an exposure to 150 mM NaCl (24 h). The high Na+ stress caused an obvious net Na+ efflux, ranging from 250 to 1,260 pmol cm−2 s−1 in the measured regions of cbl1/cbl9 mutant roots, but Na+ fluxes in WT and PeCBL1 roots only varied within 110 to 340 pmol cm−2 s−1 (Fig. 5b). The results indicated that deleting CBL1 and CBL9 genes in Arabidopsis affected the capacity to discharge Na+ and PeCBL1 can recover the phenotype of Arabidopsis knockout mutants. In addition, we found the capacity to discharge Na+ was weakened among the three phenotypes under low K+ stress (Fig. 5b). The NMT results signify that the CBL–CIPK pathway plays an important role in response to high Na+ and low K+ stress.
Discussion
The pathway response to the low K+ is conserved in Populus euphratica and Arabidopsis
The Arabidopsis mutant system could be a useful tool for identifying the function of the genes from Populus. Here, we identified one CBL member that is a homolog with AtCBL1. It can interact with PeCIPK24/25 and the results were consistent with those found in Arabidopsis. In addition, in a previous paper, we identified the interaction of PeCIPK24 and PeKC1 (Zhang et al. 2010). These interactions suggest that the complexes of CBL1–CIPK23 (24) in both Arabidopsis and Populus euphratica are conserved, and that the state of the complexes may reflect a function of the ancient CBL–CIPK system in regulating the low K+ (Weinl and Kudla 2009). That means we can use the Arabidopsis mutant system to identify the function of some CBL and CIPK gene members from Populus euphratica. But for genes (e.g., PeCBL8, Zhang et al. 2008) that are not as well conserved as in Arabidopsis in evolution, Arabidopsis mutants most likely cannot be used to characterize their function.
Specificity of CBL–CIPK complex formation represents their variable function under stress
In both the Populus and Arabidopsis genome, there are 10 CBLs and over 25 CIPKs. One CBL can interact with several CIPKs and one CIPK can also interact with several CBLs, which theoretically allow for many independent complexes. Protein interaction studies with cbl and cipk mutant phenotype analysis revealed that the CBL–CIPK complexes can selectively target specific downstream proteins and generate a stimulus-specific signaling response. In Arabidopsis, it has been demonstrated that multiple complexes are involved in the stress signaling response. For example, CBL1 and CBL9 share a high degree of homology, and both of these proteins can interact with CIPK23; however, CBL1 integrates response to drought and other osmotic stress. In contrast, CBL9 mediates response to the phytohormone abscisic acid (Albrecht et al. 2003; Pandey et al. 2004; Hedrich and Kudla 2006). Thus, although CBL1 and CBL9 can both target CIPK23 to the plasma membrane to activate AKT1, it is tempting to speculate that CBL9–CIPK23 complexes might mediate the ABA-dependent aspect of AKT1 regulation. Conversely, the ABA-independent signals may be processed by CBL1–CIPK23 complexes (Xu et al. 2006; Li et al. 2006). In addition, it has been shown that both the SOS3/CBL4-SOS2/CIPK24 complexes and the SCABP8/CBL10-SOS2/CIPK24 complexes are involved in the salt signal transduction pathway, but each of the complexes plays an additional and unique role in the plant salt-stress response (Halfter et al. 2000; Kim et al. 2007; Quan et al. 2007). In this study, using a yeast two-hybrid and a BiFC assay, we identified three complexes between CBLs and CIPKs from Populus euphratica, including CBL1–CIPK24, CBL1–CIPK25 and CBL1–CIPK26. We found that the orthologs of AKT1 (PeKC1 and PeKC2) and SOS1 (PeSOS1) also exist in the Populus genome database. In addition, we showed that PeCIPK24 can interact with PeKC1 and PeKC2 (Zhang et al. 2010) and that PeCIPK26 (orthologs of AtSOS2) can interact with PeCBL4 (orthologs of AtSOS3) and PeSOS1 by yeast two-hybrid assay (unpublished data), implying that these channels operate by the same mechanism in Populus as well. Among the complexes, CBL1–CIPK24 is involved in low K+ stress by regulating PeKC1 or 2, and CBL4–CIPK26 is involved in the salt signal transduction pathway by regulating SOS1. We suggest that different complexes can sense the calcium signature that corresponds to stress and then regulate the specific downstream response. In these processes, CBL may play important roles in recruiting CIPK to the target protein.
The CBL–CIPK signal transduction pathway may play an important role in maintaining Na+/K+ homeostasis
Plant roots take up numerous minerals from the soil. Some minerals (e.g., K+) are essential nutrients and others (e.g., Na+) are toxic for plant growth and development. Either low K+ or high Na+ levels in the soil represent a stress condition that severely affects plants’ life and agricultural production. The K+/Na+ ratio and homeostasis often determine the plant growth rate (Luan 2009). Earlier observations indicated that CBL–CIPK signal transduction pathways can cope with low K+ or high Na+ stress conditions by regulating the activity of ion transporting proteins, AKT1 and SOS1. But how do plant cells control the homeostasis of the K+/Na+ ions under low K+ or salt-stress conditions? In Arabidopsis, CBL1 can interact with CIPK1, 2, 3, 5, 9, 15, 23, 24 and form different complexes. Given that AtCIPK1, AtCIPK9 and AtCIPK23 have been shown to be important for low K+ tolerance and that AtCIPK24 was required for high salt tolerance, CBL1 might be one of the elements mediating crosstalk between the high salt and low K+ stress. We demonstrated that disruption of CBL1/9 function could render the mutant plants hypersensitive to low K+ stress and tolerant of high salt stress and that expression of PeCBL1 in these lines helps the plants recover. When CBL1/9 was knocked out in Arabidopsis, the signal balance was destroyed. To cope with low K+ stress, plants activate the shaker-like channel AKT1 or other potassium channels via the CBL–CIPKs pathway, which improves K+ ion uptake capacity. However, because CBL1 competes with CBL4 to interact with CIPK24 (CIPK26 in Populus), the regulation of SOS1 regulated by SOS3/CBL4-SOS2/CIPK24 and the capacity of extruding Na+ will be reduced. In contrast, to cope with the salt (high Na+) stress, plants activate SOS1 by upregulating the SOS3/CBL4-SOS2/CIPK24 pathway, thus improving the capacity to extrude Na+ ions. Simultaneously, CIPK24 competes with CIPK23 to interact with CBL1; the SOS3/CBL4-SOS2/CIPK24 pathway negatively influences the activation of AKT1 by influencing the CBL1–CIPK23 signal pathway. In Populus euphratica, we only investigated four interactions of PeCBL1 with CIPKs and there are several other CIPK members that need to be further identified. In plants, CBL and CIPK form an interrelated signal network by interacting with each other. Changing levels of any one component in these networks would disrupt the signature balance. Additional work is needed to determine whether other CBLs or CIPKs are involved in the Na+/K+ homeostasis pathway.
In summary, by supplementing the overexpression analysis data through identification of the interaction of CBLs and CIPKs in Populus euphratica, we were able to identify the pathway that is regulated by CBL–CIPK under low K+ and salt stress in Populus. Furthermore, this study directly links two types of ion channel regulation pathways into an emerging and complex CBL–CIPK signal system.
Abbreviations
- ABA:
-
Abscisic acid
- AD:
-
Activation domain
- BD:
-
Binding domain
- BiFC:
-
Bimolecular fluorescence complementation
- CaM:
-
Calmodulin
- CaMV:
-
Cauliflower mosaic virus
- CBL:
-
Calcineurin B-like
- CDPK:
-
Calcium-dependent protein kinase
- CIPK:
-
CBL-interacting protein kinases
- GFP:
-
Green fluorescent protein
- SOS:
-
Salt overly sensitive
- WT:
-
Wild type
- YFP:
-
Yellow fluorescent protein
References
Albrecht V, Ritz O, Linder S, Harter K, Kudla J (2001) The NAF domain defines a novel protein–protein interaction module conserved in Ca2+-regulated kinases. EMBO J 20:1051–1063
Albrecht V, Weinl S, Blazevic D, D’Angelo C, Batistic O, Kolukisaoglu Ü, Bock R, Schulz B, Harter K, Kudla J (2003) The calcium sensor CBL1 integrates plant response to abiotic stresses. Plant J36:457–470
Batistic O, Kudla J (2004) Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK proteins kinase network. Planta 219:915–924
Batistič O, Sorek N, Schültke S, Yalovsky S, Kudlaa J (2008) Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell 20(5):1346–1362
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S (2003) CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 15:1833–1845
Cheong YH, Pandey GK, Grant JJ, Batistic O, Li L, Kim BG, Lee SC, Kudla J, Luan S (2007) Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant Jl 52:223–239
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Halfter U, Ishitani M, Zhu JK (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA 97:3735–3740
Harper JF, Harmon A (2005) Plants, symbiosis and parasites: a calcium signaling connection. Nat Rev Mol Cell Bio 6:555–566
Hedrich R, Kudla J (2006) Calcium signaling network channel plant K+ uptake. Cell 125:1221–1223
Ho CH, Lin SH, Hu HC, Tsay YF (2009) CHL1 functions as a nitrate sensor in plants. Cell 138(6):1184–1194
Huang C, Ding S, Zhang H, Du H, An L (2011) CIPK7 is involved in cold response by interacting with CBL1 in Arabidopsis thaliana. Plant Sci 181(1):57–64
Kim BG, Waadt R, Cheong YH, Pandey GK, Dominguez-Solis JR, Schultke S, Lee SC, Kudla J, Luan S (2007) The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J 52:473–484
Kim MC, Chung WS, Yun D, Cho MJ (2009) Calcium and calmodulin-mediated regulation of gene expression in plants. Mol Plant 2:13–21
Kolukisaoglu Ü, Weinl S, Blazevic D, Batistic O, Kudla J (2004) Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL–CIPK signaling networks. Plant Physiol 134:43–58
Li L, Kim BG, Cheong YH, Pandey GK, Luan S (2006) A Ca2+ signaling pathway regulated a K+ channel for low-K+ response in Arabidopsis. Proc Natl Acad Sci USA 103:12625–12630
Liu J, Ishitani M, Halfter U, Kim CS, Zhu JK (2000) The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA 97:3730–3734
Luan S (2009) The CBL–CIPK network in plant calcium signaling. Trends Plant Sci 14(1):37–42
Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W (2002) Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 14:389–400
Pandey GK, Cheong YH, Kim KN, Luan S (2004) The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell 16:1912–1924
Quan R, Lin H, Mendoza I, Zhang Y, Cao W, Yang Y, Shang M, Chen S, Pardo JM, Guo Y (2007) SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 19:1415–1431
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14:401–417
Shi J, Kim KN, Ritz O, Albrecht V, Gupta R, Harter K, Luan S, Kudla J (1999) Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. Plant Cell 11:2393–2405
Waadt R, Kudla J (2008) In planta visualization of protein interactions using bimolecular fluorescence complementation (BiFC). CSH Protoc. doi:10.1101/pdb.prot4995
Weinl S, Kudla J (2009) The CBL–CIPK Ca2+-decoding signaling network: function and perspectives. New Phytol 184:517–528
White PJ, Broadley PJ (2003) Calcium in plant. Ann Bot 92:487–511
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:165–183
Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125(7):1347–1360
Yu Y, Yin W, Xia X, Zhang H (2007) Comparative genomic analysis of CIPK gene family in Arabidopsis and Populus. Plant Growth Regul 52:101–110
Zhang H, Yin W, Xia X (2008) Calcineurin B-Like family in Populus: comparative genome analysis and expression pattern under cold, drought and salt stress treatment. Plant Growth Regul 56:129–140
Zhang H, Yin W, Xia X (2010) Shaker-like potassium channels in Populus, regulated by the CBL–CIPK signal transduction pathway, increase tolerance to low-K+ stress. Plant Cell Rep 29:1007–1012
Acknowledgments
This work was supported by National Key Technologies R&D Program of China (2011BAD38B01, 2009CB119101), National Natural Science Foundation of China (30972339, and 31070597), the Ministry of Science and Technology of China (2009CB119101), and the Scientific Research and Graduate Training Joint Programs from BMEC (Regulation of Tree WUE).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by L. Jouanin.
H. Zhang and F. Lv contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Lv, F., Han, X. et al. The calcium sensor PeCBL1, interacting with PeCIPK24/25 and PeCIPK26, regulates Na+/K+ homeostasis in Populus euphratica . Plant Cell Rep 32, 611–621 (2013). https://doi.org/10.1007/s00299-013-1394-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1394-5