Abstract
Key message
Our results demonstrate that the flavonoids biosynthetic pathway can be effectively manipulated to confer enhanced plant root growth under water-stress conditions.
Abstract
Abscisic acid (ABA) is one of most important phytohormones. It functions in various processes during the plant lifecycle. Previous studies indicate that ABA has a negative effect on root growth and branching. Auxin is another key plant growth regulator that plays an essential role in plant growth and development. In contrast to ABA, auxin is a positive regulator of root growth and development at low concentrations. This study was performed to help understand whether flavonoids can suppress the effect of ABA on lateral root growth. The recessive TRANSPARENT TESTA GLABRA 1 (ttg1) mutant was characterized on ABA and sucrose treatments. It was determined that auxin mobilization could be altered by modifying flavonoids biosynthesis, which resulted in alterations of root architecture in response to ABA treatment. Moreover, transgenic TTG1-overexpression (TTG1-OX) seedlings exhibited enhanced root length and lateral root number compared to wild-type seedlings grown under normal or stress conditions. Genetic manipulation of the flavonoids biosynthetic pathway could therefore be employed successfully for the improvement of plant root systems by overcoming the inhibition of ABA and some abiotic stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are very susceptible to environmental challenges imposed by biotic and abiotic factors such as pathogens, herbivores, low temperatures and drought. To guard against these stresses, plants undergo developmental changes, exhibiting plasticity in the architecture of shoot and root systems in a given environment. For example, plants generate plenty of lateral roots under normal conditions, while lateral root initiation and elongation are limited under drought conditions to maximize the elongation of the primary root to reach water deep in the soil (Malamy 2005; van der Weele et al. 2000; Xiong et al. 2006). Developmental plasticity has evolved over a long period of time and may contribute to the “real-time adaptation” of plants that allows them to acclimate to specific surroundings.
Roots provide an optimal system for studying developmental plasticity, a characteristic feature of plant growth (Malamy 2005). One of the main determinants of root system architecture is lateral root (LR) initiation and growth. This developmental process is influenced by endogenous and exogenous factors, such as auxin, abscisic acid (ABA), drought stress and osmotica (Fukaki and Tasaka 2009; Deak and Malamy 2005). Auxin, a crucial phytohormone, is produced mainly in the shoot apex and is transported toward the root system via polar auxin transporters (Ljung et al. 2001). Polar flows of auxin control organ development, cell elongation and cell division, resulting in the alteration of shoot and root system architecture in responses such as phototropism, gravitropism and thigmotropism (reviewed in Peer et al. 2011). In contrast, ABA inhibits root growth and functions as a negative regulator in LR emergence (Pilet 1975; Deak and Malamy 2005). Exogenously applied ABA suppresses the emergence of LR primordia from the parent root prior to the activation of the LR meristem (De Smet et al. 2003). ABA-induced LR inhibition reportedly occurs through an auxin-independent pathway (Fukaki and Tasaka 2009). The fact that the application of auxin to medium could not rescue LR inhibition in response to ABA suggests that an ABA-sensitive, auxin-independent checkpoint is involved at the post-emergence stage (De Smet et al. 2003). On the contrary, the Arabidopsis recessive mutant axr2-1/iaa7 is resistant to exogenous ABA whereas the slr-1/iaa14 mutant is hypersensitive to ABA in root growth inhibition assays, suggesting that Aux/IAA-dependent auxin signaling also affects the plant root response to ABA (Fukaki and Tasaka 2009).
Flavonoids are secondary metabolites that are divided into various subclasses, including flavanones, isoflavonoids, anthocyanins, flavonols, catechins, flavones and proanthocyanidins, on the basis of structure. Although they are non-essential for plant growth and development, flavonoids play species-specific roles in nodulation, fertility, the defense system and UV protection (Peer and Murphy 2007). The coordinated up-regulation of flavonoids biosynthesis-related genes has been well established, and several structural genes for flavonoids biosynthesis have been identified in various plant species (Olsen et al. 1993; Shirley et al. 1995). The Arabidopsis thaliana genes TRANSPARENT TESTA 2 (TT2), TRANSPARENT TESTA 8 (TT8) and TTG1 encode the MYB, bHLH and WD40 transcription factors, respectively; these proteins function together in a transcriptional complex to drive the expression of flavonoids biosynthetic genes such as the DIHYDROFLAVONOL 4-REDUCTASE (DFR), LEUCOANTHOCYANIDIN DIOXYGENASE (LDOX) and ANTHOCYANIDIN REDUCTASE (ANR) (Lepiniec et al. 2006; Dubos et al. 2008; reviewed in Francesca et al. 2006). In the other branch of the flavonoids biosynthetic pathway, plants can produce compounds called flavonols. Flavonols is a class of secondary metabolites that belong to the flavonoids group. Flavonols differ from the other flavonoids classes by the degree of oxidation of the central pyran ring (Skerget et al. 2005). In animal cells, flavonols can inhibit the generation of ROS (reactive oxygen species), which results in the protection of cells from oxidative damage (Pollastri and Tattini 2011). Many lines of evidence indicate that flavonols may control plant growth and development by redirecting the polar auxin transport flow (Pollastri and Tattini 2011; Santelia et al. 2008). These compounds are known to function as regulators of auxin transporters, leading to alterations in shoot and root branching (Peer and Murphy 2007).
Although many lines of evidence suggest that ABA inhibits LR growth through modulation of Aux/IAA-dependent auxin signaling components, the molecular mechanisms underlying their crosstalk remain elusive. It has been recently reported that ABA triggers biosynthesis of phenolic compounds to activate defense mechanisms against UV stress (Berli et al. 2010). To address whether flavonoids play a role in ABA-induced LR inhibition, we studied ttg1 seedlings, which have impaired flavonoid biosynthesis (Walker et al. 1999), and plants overexpressing TTG1. We reasoned that altered levels of flavonoid accumulation might affect LR growth in response to ABA. LR growth following ABA treatment was reduced in ttg1 seedlings and increased in plants overexpressing TTG1. Our results demonstrate that the flavonoid biosynthetic pathway can be effectively manipulated to confer enhanced plant root growth under water-stress conditions.
Materials and methods
Plant materials and growth conditions
Wild-type Arabidopsis thaliana (ecotype Columbia and Landsberg), the recessive mutant of TRANSPARENT TESTA GLABRA 1 (ttg1), CHSpro::GUS (Oh et al. 2011a) and DR5::GUS lines (Oh et al. 2011b), PAP1-D (CS3884), aba3 (CS157), tt2 (CS83) were employed in these experiments. Seeds were sterilized and stored at 4 °C for 3 days before seeding on half-strength Murashige and Skoog (1/2 × MS) medium containing 2 % (w/v) sucrose (pH 5.7). Plant samples were cultured in a growth chamber under the following conditions: temperature: 23 ± 1 °C; light intensity: 50–55 μmol photons m−2 s−1; light/dark cycle: 16 h/8 h; relative humidity: 70 %. Four-day-old seedlings were transferred to 1/2 × MS media containing the indicated supplements.
Construction and generation of transgenic plants
Transgenic Arabidopsis plants expressing the full-length cDNA of TTG1 (At5g24520) were generated. Briefly, full-length TTG1 cDNA was amplified by PCR and cloned into the pMDC32 vector, which contains the cauliflower mosaic virus (CaMV) 35S promoter. To obtain transgenic plants that overexpress TTG1, wild-type plants were transformed with the Agrobacterium tumefaciens strain GV3101 harboring the construct CaMV35S::TTG1 using the floral dip method (Clough and Bent 1998). Homozygous T4 transgenic (TTG1-OX) seeds were used for all experiments.
RT-PCR
Ten-day-old seedlings of wild-type and TTG1-OX (lines #4 and #5) were used for RNA extraction and cDNA preparation. RNA extraction was performed using the Trizol reagent (Invitrogen, USA) following the manufacturer’s instructions. PCR reactions using the cDNA described above were carried out with specific primer sets (supporting information Table S1) and repeated using different numbers of cycles (30 and 40 cycles).
Anthocyanin measurement
For visualization of anthocyanins, seeds of mutants were grown for 3 days on 1/2 × MS medium containing 50 μM norflurazon (chlorophyll inhibitor). Seedlings were then observed and photographed using a Leica EZ4D microscope.
To quantify the levels of anthocyanins, 7-day-old wild-type, TTG1-OX (lines #4 and #5), ttg1 and PAP1-D seedlings grown on 1/2 × MS medium were used for anthocyanin extraction. Approximately 50 mg of tissue samples were homogenized in liquid nitrogen, and anthocyanin levels were determined as described in Mancinelli et al. (1988) with some modifications. In brief, anthocyanins were extracted overnight at 4 °C in 250 μl of 1 % (v/v) HCl in methanol. A total of 250 μl distilled water and 250 μl chloroform was added to the samples to separate the anthocyanins from the chlorophyll. Samples were then vortexed and centrifuged for 2 min at 3,000×g to remove the chlorophyll. The aqueous phase was examined by measuring the optical density at A 535 and A 650. Anthocyanin levels were calculated using the simple formula (A 535 − A 650)/fresh weight.
Flavonoids staining in seedlings
Seven-day-old seedlings of each mutant and wild-type seedlings were grown and stained as previously described (Sheahan and Rechnitz 1993; Murphy et al. 2000; Lewis et al. 2011) with some modifications. Briefly, seedlings were stained for 7 min using saturated (0.25 %, w/v) DPBA with 0.02 % (v/v) Triton X-100 and were then washed in distilled water for 5 min. Samples were visualized using a Confocal Laser Scanning Microscope (LSM 5 Exciter, Carl-Zeiss) with an Ar-laser (458/488/514 nm) for GFP and a 543 nm HeNe-laser for YFP.
Histochemical GUS assay
GUS assays were performed using GUS solution containing 1 mM X-gluc, 100 mM sodium phosphate buffer (pH 7.0), 10 mM EDTA, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide and 0.1 % Triton X-100. Seedlings were incubated in this solution at 37 °C for 6 h and decolorized with 70 % ethanol before observation using a Leica EZ4D microscope (Jefferson et al. 1987).
Statistical analyses
Each experiment was repeated three times using at least ten samples every time. Statistical analysis was done by one-way ANOVA Tukey’s test at a 95 % confidence level.
Results
The ttg1 seedlings exhibited retarded root growth in response to ABA treatment
To examine whether the alteration of flavonoids can effect root growth in seedlings in response to ABA treatment, ttg1 recessive mutant and wild-type 4-day-old seedlings were transferred to 1/2 × MS medium supplemented with the indicated concentrations of ABA and sucrose (Fig. 1). In the normal level of sucrose applied medium (2 % w/v), the number of LRs of wild-type seedlings decreased when the ABA concentration was increased in the medium (Fig. 1b). However, the lowest concentration of ABA (0.1 μM) facilitated lateral root growth in wild-type seedlings. The ttg1 mutants were much more sensitive to ABA inhibition of LR growth than the wild type at higher concentration of ABA at 4 % sucrose (Fig. 1). We found that the phenotype of the ttg1 mutant in response to ABA was compensated by the overexpression of the TTG1 gene. From this result, we hypothesized that decreasing flavonoids biosynthesis can have a negative effect on root development in response to ABA.
Growth of ttg1 and wild-type (Col-0) seedlings in response to ABA and sucrose. a Seeds were germinated and grown on normal 1/2 × MS medium, and 4-day-old seedlings were transferred to 1/2 × MS medium supplemented with ABA and sucrose, as indicated in the figure. Pictures were taken on the seventh day after transfer. b Numbers of lateral roots (LRs) for ttg1 and Col-0 seedlings from a were plotted. The data shown are from three separate experiments performed at 23 °C (n = 20 per experiment)
Increase of flavonoids biosynthesis resulted in an increase in LR number in response to ABA treatment
Sucrose is a factor that stimulates the accumulation of anthocyanins in plants by altering the expression of genes in the flavonoids biosynthetic pathway (Solfanelli et al. 2006). In this study, we altered the content of sucrose in the medium (from 0 to 8 %) to try to enhance the level of endogenous flavonoids, and we hoped that this alteration would affect the development of the root system upon exposure to ABA (Fig. 1). Indeed, we observed a clear increase in LR number in both wild-type and ttg1 seedlings when the sucrose content was increased from 2 to 8 % in medium lacking ABA (Fig. 1b). Enhancing the sucrose concentration of the medium can also help overcome ABA-mediated inhibition of root system development. Indeed, in the presence of ABA (1 and 10 μM), higher concentrations of sucrose (2–4 %) also led to an increase in LR number (from ~8 to ~10 and ~4 to ~6) (Fig. 1b). However, a higher concentration of sucrose (8 %) along with ABA (1 and 10 μM) resulted in a decrease in the number of LRs in both the wild-type and ttg1 seedlings (Fig. 1b).
To support the above results, we attempted to test the expression of CHS by growing CHSpro::GUS transgenic seedlings and measuring the level of anthocyanins in response to ABA and/or sucrose (Fig. 2). From these results, we realized that an increase in exogenously applied sucrose as positively correlated with the expression of CHS, especially when the sucrose content was shifted from 2 to 4 % in the medium. Moreover, the anthocyanins content was also enhanced as long as increased sucrose as well as ABA concentration was used (Fig. 2b). We also determined the level of anthocyanins in the ABA-deficient mutant, aba3, in response to sucrose and/or ABA (Fig. 2c). Taken together, we found that an increase in flavonoids biosynthesis resulted in the enhancement of LR number in response to ABA treatment.
The effects of sucrose and/or ABA on the expression of CYCB1pro::GUS, CHSpro::GUS and the accumulation of anthocyanins. a Arabidopsis T3 homogenous transgenic seeds harboring CYCB1pro::GUS or CHSpro::GUS were germinated and grown on 1/2 × MS medium for 10 days and transferred to 1/2 × MS medium supplemented with ABA and sucrose as indicated in the figure. Seedlings were incubated for 6 h before visualization of GUS staining. The seedlings shown were typical of three separate experiments performed at 23 °C (n = 10 per experiment). b Seven-day-old wild-type (Col-0) seedlings growing on 1/2 × MS medium were treated with the indicated concentrations of sucrose and ABA for 24 h and the level of anthocyanins was measured. A one-way ANOVA Tukey’s test (P < 0.05) was performed to determine which treatments significantly affected anthocyanin accumulation. Lower-case letters above the column indicate significant differences. Vertical bars indicate standard error (n = 3). c Seven-day-old wild-type (Col-0) and aba3 seedlings growing on 1/2 × MS medium were treated with 8 % sucrose and 10 μM ABA for 24 h and the level of anthocyanins was measured. A one-way ANOVA Tukey’s test (P < 0.05) was performed to determine which treatments resulted in a significant difference in anthocyanin accumulation between wild-type and mutant seedlings. Lower-case letters above the column indicate significant differences. Vertical bars indicate standard error (n = 3)
Increase of flavonols accumulation in TTG1-OX seedlings
The full-length cDNA of TTG1 was constructed under the control of the CAMV35S promoter and transformed into wild-type Arabidopsis thaliana. After three generations of selection, two independent lines (#4 and #5) from the T4 generation were isolated. Genotype checking confirmed that these lines were homozygous and were genuine overexpression mutants (Fig. 3a). The T4 seeds from these lines were used in later experiments.
Reduced level of anthocyanins in TTG1-OX seedlings. Full-length TTG1 cDNA was cloned under the control of the CaMV 35S promoter to generate TTG1-OX seeds. a The overexpression of TTG1 was examined using RT-PCR. Actin is shown as the internal loading control. Ten-day-old seedlings were grown on normal 1/2 × MS medium before total RNA extraction for RT-PCR. b Phenotypes of wild-type, ttg1 and TTG1-OX seeds or seedlings. Seed coat color (300 mg seeds of each) and the accumulation of anthocyanins (n = 50 per each experiment) are shown. For visualization of anthocyanins, seeds of mutants were grown for 3 days on 1/2 × MS medium containing 50 μM norflurazon (inhibitor of chlorophyll formation). Seedlings then were then observed, and pictures were taken using a Leica EZ4D microscope. Purple color indicates anthocyanin accumulation. c Ten-day-old seedlings grown on normal 1/2 × MS medium were ground, and anthocyanins were extracted in aqueous solution and subjected to measurement. The seeds or seedlings shown were typical of three separate experiments performed at 23 °C. A one-way ANOVA Tukey’s test (P < 0.05) was performed to indicate significant differences in anthocyanin accumulation between the wild-type and mutants. Lower-case letters (a, b, c) were marked above the column to reflect significant differences. Vertical bars indicate standard error (n = 3) (color figure online)
Because ttg1 was reported to exhibit lower levels of accumulation of anthocyanins than the wild-type, we measured anthocyanins levels in TTG1-OX transgenic seedlings. We used the PAP1-D mutant as a positive control because it has been previously reported to accumulate high levels of anthocyanins (Borevitz et al. 2000). As shown in Fig. 3b, c, the seedlings that overexpressed TTG1 were also shown to contain lower anthocyanin levels than the wild-type. This led us to examine the transcript levels of flavonoids biosynthetic genes that were reported to be target genes of TTG1 regulation. In this experiment, the level of DFR transcript was slightly lower in TTG1-OX #4 and TTG1-OX #5 seedlings than in the wild-type, while the expression of FLAVONOL SYNTHASE (FLS1) was enhanced in these mutants (Fig. 4a). We consequently determined the level of flavonols accumulation in seedlings of these mutants. The results, shown in Fig. 5, indicate that levels of kaempferol (green color) and quercetin (yellow color) were enhanced in TTG1-OX seedlings compared to the wild-type and the other mutants for control. As shown in Fig. 5a, the tt2 mutant lacking in the proanthocyanidin biosynthesis served as a negative control. In contrast, ABA-deficient mutant aba3 accumulated slightly more quercetin, which we did not expect (Fig. 5a). We then asked whether ABA and/or sucrose can alter the level of flavonols. As shown in Fig. 5b, ABA and/or sucrose were able to alter the level of quercetin and kaempferol.
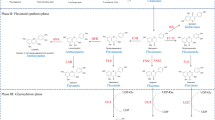
The transcript levels of the DFR and FLS1 genes in wild-type and TTG1-OX seedlings. a The transcript levels of flavonoids biosynthetic genes DFR and FLS1 were determined in wild-type and TTG1-OX #4 and #5 seedlings on normal 1/2 × MS medium. Several rounds of RT-PCR were performed, and PCR results from two experiments using different numbers of cycles are shown. UBIQUITIN-CONJUGATING ENZYME 21 (UBC21) was used as the internal loading control. Ten-day-old seedlings were grown on normal 1/2 × MS medium before total RNA extraction for RT-PCR. b Simplified model of the flavonoids biosynthetic pathway. First, chalcone synthase (CHS) catalyzes the condensation of one molecule of 4-coumaroyl-CoA with three molecules of malonyl-CoA. The later steps in this pathway are catalyzed by a series of enzymes, leading to the production of two main types of final products: anthocyanins and flavonols, i.e., auxin transport inhibitors. (CHI chalcone isomerase, F3H flavonol 3-hydroxylase, F3′H flavonol 3′-hydroxylase, FLS flavonol synthase, DFR dihydroflavonol-4-reductase, LDOX leucoanthocyanidin dioxygenase)
Kaempferol and quercetin (flavonols) accumulation in wild-type and TTG1-OX seedlings. a Seven-day-old wild-type (Col-0 and Ler), TTG1-OX, PAP1-D, ttg1, tt2 and aba3 seedlings grown on normal 1/2 × MS medium were used for DPBA staining for the visualization of kaempferol and quercetin accumulation (Bar 100 μm). The roots shown were typical of three separate experiments performed at 23 °C (n = 10 seedlings per experiment). b Seven-day-old wild-type (Col-0) seedlings were transferred to 1/2 × MS medium supplemented with indicated concentrations of sucrose and ABA for 24 h and used for DPBA staining for the visualization of kaempferol and quercetin accumulation (Bar 100 μm). The roots shown were typical of three separate experiments performed at 23 °C (n = 10 seedlings per experiment)
To examine whether the wild type TTG1 gene can complement the altered root phenotype of the ttg1 mutant, we introduced the 35S::TTG1 construct into ttg1 plants. The TTG1/ttg1 seedlings had longer primary roots than ttg1 seedlings (Fig. S1).
Enhancement of root development of TTG1-OX seedlings in response to ABA and drought treatment
Because ttg1 seedlings showed retarded root growth on medium supplemented with ABA, we examined the response of TTG1-OX seedlings to ABA treatment. As expected, the TTG1-OX seedlings exhibited stronger root growth in ABA medium than did the wild-type (Fig. 6a, b). Moreover, we also observed that TTG1-OX seedlings produced many more LRs and longer primary roots than did the wild-type upon exposure to drought stress (Fig. 6a, b). We employed the PAP1-D mutant as a positive control for increased flavonoids biosynthesis. The PAP1-D seedlings were transferred to ABA medium under the same experimental conditions used for TTG1-OX seedlings. The results clearly showed that both the PAP1-D and TTG1-OX seedlings exhibited the same root development response upon exposure to ABA (Fig. 6a). The expression levels of some stress-marker genes were therefore examined to confirm whether these mutants indeed exhibited increased levels of stress tolerance. However, as shown in Fig. 6c, the results showed that transcript levels of COLD-REGULATED 15A (Cor15A) and KIN1 were quite similar in wild-type and TTG1-OX seedlings. This demonstrates that the enhanced root growth of TTG1-OX seedlings exposed to stress or ABA treatment were not due to a simple mediation of stress-tolerance signaling pathways.
Growth phenotypes of wild-type, TTG1-OX, PAP1-D and ttg1 seedlings grown on 1/2 × MS medium supplemented with 10 μM ABA or 300 mM mannitol. a Four-day-old seedlings were transferred to test medium and allowed to grow for 10 days before being photographed. The seedlings shown were typical of three separate experiments performed at 23 °C (n = 15 seedlings per experiment). b The numbers of LRs in these seedlings were counted under a microscope. The data shown are from three separate experiments performed at 23 °C (n = 10 per experiment). One-way ANOVA Tukey’s test (P < 0.05) was performed to indicate a significant difference in LR number between wild-type and mutants per treatment. Lower-case letters (a, b, c) were marked above the column to reflect significant differences. Vertical bars indicate standard error (n = 30). c The transcript levels of the stress-marker genes Cor15A and KIN1 were determined for wild-type and TTG1-OX #4 and #5 seedlings on normal 1/2 × MS medium. Several rounds of RT-PCR were performed, and PCR results from two experiments using different numbers of cycles are shown. UBC21 was used as the internal loading control. Ten-day-old seedlings were grown on normal 1/2 × MS medium before total RNA extraction for RT-PCR
Differences in root architecture of TTG1-OX seedlings are related to auxin activity
Auxin is the most important phytohormone for root development. The TTG1-OX seedlings showed enhanced root architecture not only in response to ABA treatment but also under normal conditions (Fig. 6a, b). The above results also indicate that this enhanced root development did not occur through the enhancement of stress-marker gene expression (Fig. 6c). Additionally, flavonols, which are auxin polar transport inhibitors, showed increased accumulation in TTG1-OX seedlings. For these reasons, we hypothesized that the expression of auxin-related genes is altered in TTG1-OX seedlings. In fact, the transcript levels of IAA3 and IAA17 were lower in transgenic seedlings (Fig. 7). The repression of these genes has been shown to affect root system development and adaptation (Kim et al. 2006; Tian and Reed 1999). Next, we intended to examine the auxin sensitivity upon ABA treatment in combination with sucrose (Fig. 8). To do this, transgenic seedlings containing an auxin marker construct (DR5::GUS) were treated with ABA and sucrose (Fig. 8). These results demonstrate that ABA and sucrose have an antagonistic relationship with regard to the auxin sensitivity of plant cells (Fig. 8).
The transcript levels of the IAA3 and IAA17 genes in wild-type and TTG1-OX seedlings. Several rounds of RT-PCR were performed, and PCR results from two experiments using different numbers of cycles are shown. UBC21 was used as the internal loading control. Ten-day-old seedlings were grown on normal 1/2 × MS medium before total RNA extraction for RT-PCR
The effects of sucrose and ABA on the GUS expression in DR5::GUS transgenic seedlings. Four-day-old DR5::GUS transgenic seedlings grown on normal 1/2 × MS medium were transferred to 1/2 × MS medium supplemented with the indicated concentrations of ABA and sucrose and incubated for 6 h before GUS staining. The seedlings shown were typical seedlings from three separate experiments performed at 23 °C (n = 10 per experiment)
Taken together, we can speculate that genetic modification of the flavonoids biosynthetic pathway somehow affects auxin activation, resulting in changes in root architecture.
Discussion
It is well established that modifications in root system architecture are consequences of a balance between stimulatory and repressive signaling cascades. For example, ABA is proposed to inhibit the formation of LRs from primordia, while auxin accumulation is important for lateral root initiation and elongation (Deak and Malamy 2005). Although root architecture is primarily determined by phytohormones such as auxin and ABA, recent reports suggest that flavonoids exert a critical influence on root architecture as well. According to a previous report, the root architectures of several mutants devoid of certain genes that function in flavonoids biosynthesis are quite different from those of the wild-type (Buer and Djordjevic 2009). For example, tt4 and tt8 seedlings showed fewer LRs when compared to wild-type (Buer and Djordjevic 2009). Moreover, there is an array of convincing evidence that there is a link between flavonoids and auxin polar transport (Murphy et al. 2000; Santelia et al. 2008; Peer and Murphy 2007). By using tt4 seedlings, Murphy et al. (2000) indicated that roots of tt4 released more auxin into the medium than did the wild-type (Murphy et al. 2000). Recently, it was proposed that flavonoids affect the redirection of the auxin stream by exerting a specific influence on the location of PIN (PIN-FORMED) (Santelia et al. 2008). Based on these reports, we hypothesize that ABA may modulate the biosynthesis of flavonoids to alter the direction of auxin polar transport in an attempt to suppress LR growth when plants are suffering from drought stress. TTG1 is an important transcription factor involved in flavonoids biosynthesis. Moreover, TTG1 functions in various processes during plant morphogenesis including root hair and trichome formation (Gonzalez et al. 2008; Walker et al. 1999; Zhang et al. 2003); thus, we exploited the ttg1 mutant to examine its response to ABA with regard to LR growth (Fig. 1). On 0 % sucrose medium, ABA inhibition of LR growth was not observed, while on medium containing higher concentrations of sucrose along with ABA, the ttg1 mutants differed considerably from the wild-type. Moreover, the number of LRs increased with increasing levels of sucrose (2–4 %), despite the presence of ABA (1 μM). According to Deak and Malamy (2005), more than 4.5 % sucrose in the medium may produce abnormal effects on plant growth due to high osmotic stress. A significant decrease in the number of LRs in the ttg1 mutant demonstrates that this mutant is highly sensitive to ABA. It appears that the increased biosynthesis of flavonoids resulting from sucrose treatment might lead to decreased sensitivity to ABA in both wild-type and ttg1 plants (Fig. 1). This reasoning was further supported by our observation of the regulation of the CHS gene that we studied using transgenic plants harboring the GUS reporter gene under the control of the CHS promoter (Fig. 2a). The CHS gene is fairly sensitive to sucrose, leading to increased flavonoids biosynthesis (Solfanelli et al. 2006). However, GUS activity was severely suppressed by the presence of ABA plus 4 % sucrose in the medium (Fig. 2a). Notably, the inhibitory effect of ABA on GUS activity was not obvious if the seedlings were incubated in medium containing more than 4 % sucrose (Fig. 2a). Our results are in accordance with the previous studies that showed that sucrose can enhance flavonoids biosynthesis, leading to an alteration of endogenous flavonols accumulation that in turn affects root architecture (Solfanelli et al. 2006; Peer et al. 2011).
The ttg1 mutant is more sensitive to ABA than wild-type. This led us to test our hypothesis using the transgenic TTG1-OX seedlings. At first, we expected to see enhanced anthocyanin biosynthesis in the TTG1-OX seedlings, which turned out not to be the case (Fig. 3). Even more surprising, the anthocyanin level was diminished in the transgenic seedlings (Fig. 3c). The roots of ttg1 mutants accumulate more quercetin, which belongs to the flavonols group, than wild-type plants (Buer and Djordjevic 2009). In contrast, the levels of kaempferol and quercetin were reduced in the tt4 and tt7 mutant seedlings (Lewis et al. 2011). Indeed, TTG1-OX accumulated kaempferol and quercetin rather than anthocyanins (Figs. 3c, 5). Therefore, we hypothesized that the increase in LR number in TTG1-OX is a consequence of altered levels of flavonols accumulation (Figs. 4, 5). Dihydroflavonol 4-reductase (DFR, EC 1.1.1.219) is a rate-limiting enzyme involved in the biosynthesis of anthocyanins (Martens et al. 2003). Anthocyanins and catechins are no longer synthesized when the DFR gene is deactivated in barley and Arabidopsis, which indicates that this gene is vital for the production of anthocyanins (Olsen et al. 1993; Shirley et al. 1995). The decreased level of the DFR transcript in TTG1-OX seedlings in comparison to the level in wild-type seedlings may be responsible for the reduced accumulation of anthocyanins in the transgenic seedlings (Figs. 3c, 4a). Similarly, the DFR transcript is reduced in TT2 overexpression (TT2-OX) seedlings (Nesi et al. 2001). It remains unclear why the accumulation of the DFR gene transcript is lower in TTG1-OX and TT2-OX seedlings than in wild-type seedlings. The enhanced level of TTG1 and TT2 protein in TTG1-OX and TT2-OX seedlings, respectively, may interfere with the structure of the transcriptional complex comprising TTG1, TT2 and TT8. It is plausible that a complex with only two components forms. Whereas the level of the DFR transcript was reduced in TTG1-OX plants, the level of the FLS1 transcript was greatly increased. This may explain the enhanced levels of flavonols (kaempferol and quercetin) in TTG1-OX seedlings in comparison to levels in wild-type seedlings (Figs. 4a, 5).
The role of ABA in flavonoid accumulation is controversial, because the level of anthocyanins is enhanced by ABA treatment in some plants (Jiang and Joyce 2003; Jeong et al. 2004), and reduced in others (Guruprasad and Laloraya 1980; Ozeki and Komamine 1986). As demonstrated in our study (Fig. 5b), ABA appears to increase the kaempferol and quercetin at 2 % sucrose. However, in the presence of 4 or 8 % sucrose, the accumulation of flavonols in response to ABA shows concentration dependent manner, indicating that the regulation of flavonoid biosynthesis is very complicated. The precise function of ABA in the regulation of flavonoid biosynthesis requires further study.
Abscisic acid is produced at a high level when plants are exposed to abiotic stresses such as water stress (Jiang and Zhang 2002). It seems that the enhanced number of LRs in TTG1-OX was not a result of the elicitation of the abiotic stress-signaling pathway, because the transcript levels of stress-responsive marker genes such as the Cor15A and the KIN1 were not altered in TTG1-OX under normal conditions (Fig. 6c). A previous report showing that ABA inhibits LR growth at the post-emergence step (De Smet et al. 2003) led us to examine the auxin sensitivity of wild-type seedlings in response to ABA. We found that the transcript levels of auxin responsive genes such as IAA3 and IAA17 were fairly decreased in TTG1-OX compared to wild-type (Fig. 7). We also treated transgenic seedlings containing an auxin marker construct (DR5::GUS) with ABA and sucrose (Fig. 8). These results demonstrate that ABA and sucrose have an antagonistic relationship in the auxin sensitivity of plant cells (Fig. 8). It is possible that the interference of the auxin transport system that occurred in the TTG1-OX seedlings through an alteration of flavonols biosynthesis could change local auxin accumulation, leading to an increase in LR growth in the presence of ABA. Although the molecular mechanism underlying the ability of low concentrations of ABA to facilitate lateral root growth remains unclear, our results demonstrate that ABA regulates the accumulation of anthocyanins and flavonols, which may alter the proper distribution of auxin in root systems.
Water scarcity is a frequently occurring abiotic stress that has a decisive effect on plant growth and development and that limits crop productivity (Sadras and Milroy 1996; Ceccarelli and Grando 1996). Taken together, our results offer the biotechnological potential for increasing crop yield in which the genetic manipulation of the transcription factor involved in the flavonoids biosynthetic pathway could confer enhanced plant root growth under water-stress conditions in the field.
References
Berli FJ, Moreno D, Piccoli P, Hespanhol-Viana L, Silva MF, Bressan-Smith R, Cavagnaro JB, Bottini R (2010) Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ 33:1–10
Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12:2383–2394
Buer CS, Djordjevic MA (2009) Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J Exp Bot 60:751–763
Ceccarelli S, Grando S (1996) Drought as a challenge for the plant breeder. Plant Growth Regul 20:149–155
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:35–43
De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H (2003) An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33:543–555
Deak KI, Malamy J (2005) Osmotic regulation of root system architecture. Plant J 43:17–28
Dubos C, Le Gourrierec J, Baudry A, Huep G, Lanet E, Debeaujon I, Routaboul JM, Alboresi A, Weisshaar B, Lepiniec L (2008) MYBL2 is a new regulator of flavonoids biosynthesis in Arabidopsis thaliana. Plant J 55:940–953
Francesca Q, Antoine B, Loïc L, Erich G (2006) The regulation of flavonoids biosynthesis. In: Erich G (ed) The science of flavonoids. Springer, New York, pp 97–122
Fukaki H, Tasaka M (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69:437–449
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53:814–827
Guruprasad KN, Laloraya MM (1980) Effect of pigment precursors on the inhibition of anthocyanin biosynthesis by BA and ABA. Plant Sci Lett 19:73–79
Jefferson RA, Kavanagh TA, Bevan MV (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jeong ST, Goto-Yamamoto N, Kobayashi S, Esaka M (2004) Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci 167:247–252
Jiang Y, Joyce DC (2003) ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul 39:171–174
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410
Kim H, Park PJ, Hwang HJ, Lee SY, Oh MH, Kim SG (2006) Brassinosteroid signals control expression of the AXR3/IAA17 gene in the cross-talk point with auxin in root development. Biosci Biotechnol Biochem 70:768–773
Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57:405–430
Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BS, Muday GK (2011) Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol 156:144–164
Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28:465–474
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28:67–77
Mancinelli AL, Hoff AM, Cottrell M (1988) Anthocyanin Production in Chl-Rich and Chl-Poor Seedlings. Plant Physiol 86:652–654
Martens S, Knott J, Seitz CA, Janvari L, Yu SN, Forkmann G (2003) Impact of biochemical pre-studies on specific metabolic engineering strategies of flavonoids biosynthesis in plant tissues. Biochem Eng J 14:227–235
Murphy A, Peer WA, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211:315–324
Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L (2001) The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13:2099–2114
Oh JE, Kim YH, Kim JH, Kwon Y, Lee H (2011a) Enhanced level of anthocyanin leads to increased salt tolerance in Arabidopsis PAP1-D plants upon sucrose treatment. J Korean Soc Appl Biol Chem 54:79–88
Oh JE, Kwon Y, Kim JH, Noh H, Hong SW, Lee H (2011b) A dual role for MYB60 in stomatal regulation and root growth of Arabidopsis thaliana under drought stress. Plant Mol Biol 77:91–103
Olsen O, Wang X, von Wettstein D (1993) Sodium azide mutagenesis: preferential generation of A.T → G.C transitions in the barley Ant18 gene. Proc Natl Acad Sci USA 90:8043–8047
Ozeki Y, Komamine A (1986) Effects of growth regulators on the induction of anthocyanin synthesis in carrot suspension cultures. Plant Cell Physiol 27:1361–1368
Peer WA, Murphy AS (2007) Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci 12:1360–1385
Peer WA, Blakeslee JJ, Yang H, Murphy AS (2011) Seven things we think we know about auxin transport. Mol Plant 4:487–504
Pilet PE (1975) Abscisic acid as a root growth inhibitor: physiological analyses. Planta (Berl.) 122:299–302
Pollastri S, Tattini M (2011) Flavonols: old compounds for old roles. Ann Bot 108:1225–1233
Sadras VO, Milroy SP (1996) Soil-water thresholds for the responses of leaf expansion and gas exchange: a review. Field Crops Res 47:253–266
Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml J, Geisler M, Martinoia E (2008) Flavonoids redirect pin-mediated polar auxin fluxes during root gravitropic responses. J Biol Chem 283:31218–31226
Sheahan JJ, Rechnitz GA (1993) Differential visualization of transparent testa mutants in Arabidopsis thaliana. Anal Chem 65:961–963
Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM (1995) Analysis of Arabidopsis mutants deficient in flavonoids biosynthesis. Plant J 8:659–671
Skerget M, Kotnik P, Hadolin M, Hras AR, Simonic M, Knez Z (2005) Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem 89:191–198
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646
Tian Q, Reed JW (1999) Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126:711–721
van der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot 51:1555–1562
Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11:1337–1349
Xiong L, Wang RG, Mao G, Koczan JM (2006) Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol 142:1065–1074
Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130:4859–4869
Acknowledgments
This work was supported by a grant from the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (to Hojoung Lee, 2012; grant #2012-112068-3) and by a grant from the National Research Foundation (to Suk-Whan Hong; grant ##2012-0005857, #2012R1A1A4A01006448).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Y. Lu.
N. H. Nguyen and J. H. Kim contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2012_1382_MOESM1_ESM.png
Figure S1. Growth phenotypes of wild-type (Col-0), TTG1-OX, ttg1 and TTG1/ttg1 seedlings grown on normal 1/2 × MS medium. The 35S::TTG1 construct was introduced into ttg1 plants in a complementation assay. Seedlings were grown on 1/2 × MS medium for 2 weeks before being photographed. Typical seedlings from three separate experiments performed at 23 °C are shown (n = 15 seedlings per experiment) (PNG 591 kb)
Rights and permissions
About this article
Cite this article
Nguyen, H.N., Kim, J.H., Hyun, W.Y. et al. TTG1-mediated flavonols biosynthesis alleviates root growth inhibition in response to ABA. Plant Cell Rep 32, 503–514 (2013). https://doi.org/10.1007/s00299-012-1382-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1382-1