Abstract
Although salt stress mainly disturbs plant root growth by affecting the biosynthesis and signaling of phytohormones, such as gibberellin (GA) and auxin, the exact mechanisms of the crosstalk between these two hormones remain to be clarified. Indole-3-acetic acid (IAA) is a biologically active auxin molecule. In this study, we investigated the role of Arabidopsis GA20-oxidase 2 (GA20ox2), a final rate-limiting enzyme of active GA biosynthesis, in IAA-directed root growth under NaCl stress. Under the NaCl treatment, seedlings of a loss-of-function ga20ox2-1 mutant exhibited primary root and root hair elongation, altered GA4 accumulation, and decreased root Na+ contents compared with the wild-type, transgenic GA20ox2-complementing, and GA20ox2-overexpression plant lines. Concurrently, ga20ox2-1 alleviated the tissue-specific inhibition of NaCl on IAA generation by YUCCAs, IAA transport by PIN1 and PIN2, and IAA accumulation in roots, thereby explaining how NaCl increased GA20ox2 expression in shoots but disrupted primary root and root hair growth in wild-type seedlings. In addition, a loss-of-function pin2 mutant impeded GA20ox2 expression, indicating that GA20ox2 function requires PIN2 activity. Thus, the activation of GA20ox2 retards IAA-directed primary root and root hair growth in response to NaCl stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High salt stress interferes with the growth and development of plant roots, including primary roots, lateral roots, and root hairs. The plant hormones gibberellin (GA) and auxin are involved in the responses to salt stress (Dinneny 2014). GA mediates plant root growth by altering root cell proliferation and elongation (Kuraishi and Muir 1962; Achard et al. 2009; Ubeda-Tomas et al. 2009; Colebrook et al. 2013), and indole-3-acetic acid (IAA) is the principal biologically active auxin. Auxin regulates root growth in a dose-dependent manner, and physiological concentrations of IAA are capable of promoting plant root growth and development (Pierik and Testerink 2014). Although high salinity controls root growth, the critical mechanisms involved in the functional integration between GA and IAA remain to be elucidated.
The regulation of GA biosynthesis affects plant growth and development (Tanimoto 2012), and the specificities of the biosynthetic enzymes involved in this process are generally understood. Among the more than 130 GA metabolites, only a few, including GA1, GA3, GA4, and GA7, have biological activities, while the other non-bioactive GAs act as precursors for the bioactive forms or are deactivated metabolites in plants (Yamaguchi et al. 2008; Petricka et al. 2012; Daviere and Achard 2013). The diversity of GA metabolites indicates that the acquisition of bioactive GA requires a series of very complex processes that involve various enzymes. Most of the genes encoding GA biosynthetic enzymes have been cloned and characterized. The final rate-limiting enzymes are a set of GA20-oxidases (GA20oxs) that belong to the 2-oxoglutarate-dependent dioxygenase family. The genome of the model plant Arabidopsis thaliana L. has five GA20ox genes, namely GA20ox1–5. Various studies have examined differences in expression patterns and physiological roles in this small gene family. Each of the five members has a spatiotemporal expression profile (Phillips et al. 1995; Garcíamartínez et al. 1997; Rebers 1999; Carrera and Prat 1999) and is considered to play specific roles in regulating physiological or developmental programs (Sakakibara 2005). In vitro studies showed that GA20ox1, GA20ox2, and GA20ox3 catalyze all of the steps in the conversion of the C20 intermediate GA12 to GA9, which is the immediate precursor of active GA4 (Phillips et al. 1995). GA20ox3 functions almost entirely redundantly with GA20ox1 and GA20ox2 in some developmental phenotypes, with the expression patterns of GA20ox1 and GA20ox2 genes partially overlapping (Rieu et al. 2008; Plackett et al. 2012). In one study, the different physiological roles of GA20ox1 and GA20ox2 were examined by characterizing the phenotypes of the loss-of-function mutants ga20ox1 and ga20ox2 (Rieu et al. 2008). GA20ox1 expression in the loss-of-function ga20ox2-1 mutant was not increased, whereas GA20ox2 expression was strongly up-regulated in the leaves and internodes of ga20ox1 plants (Rieu et al. 2008). GA4 levels were reduced in ga20ox2-1 but not in ga20ox1 (Rieu et al. 2008), indicating that GA20ox2 activity is the main contributor to GA9 and GA4 production (Fernando et al. 2014). In contrast to GA20ox1, the activation of GA20ox2 plays an important role in the modification of Arabidopsis seedling growth through the MADS-box transcription factor Short Vegetative Phase (Fernando et al. 2014). Thus, the activation of GA20ox2 may be relatively specific, but the molecular mechanisms are mostly unknown. Interestingly, GA’s control of root growth occurs particularly in response to salt and drought stresses (Duan et al. 2013; Yu et al. 2013; Colebrook et al. 2013). Although GA20ox2 expression mainly occurs in the shoot apices of Arabidopsis seedlings (Fernando et al. 2014) and the loss of GA20ox2 function results in a decrease in GA metabolites (Plackett et al. 2012), the specificity of GA20ox2 activity in salt-controlled root responses remains to be clarified.
In addition to GA, auxin/IAA is required for plant root growth. Local IAA levels are vital for plant root morphogenesis (Pierik and Testerink 2014), with the optimum level of IAA generally being determined by the activation of biosynthetic enzymes and transporters. YUCCAs are flavin mono-oxygenase family members that catalyze a rate-limiting step in IAA biosynthesis (Di et al. 2016). The Arabidopsis genome has 10 YUCCA genes, namely YUCCA1–10 (Kasahara 2015). Limited evidence exists, however, for the involvement of IAA biosynthetic enzymes in root growth. In contrast, IAA transporters, especially the plasma membrane IAA polar transporters known as PIN-FORMEDs (PINs), regulate primary root growth and root hair development. The mechanisms of PIN-driven IAA redistribution are generally understood. In roots, PIN1 or PIN2 transports IAA towards the shoot through stele cells or towards the root tip through epidermal cells, respectively (Moubayidin et al. 2010; Willige et al. 2011). The loss-of-function pin1 mutant, accordingly, impairs shoot tissue differentiation and development (Gälweiler et al. 1999), while the loss-of-function pin2 mutant causes diminished IAA accumulation in root cells, reducing root hair growth (Ottenschlager et al. 2003). In addition, the root hair-specific over-expression of PIN1 or PIN2 greatly inhibits root hair growth by depleting IAA levels in root hair cells (Sarnowska et al. 2013). The endodermis buffers local IAA levels through IAA transporters, while local IAA maxima define lateral root initiation (Vermeer et al. 2014; Marhavý et al. 2016). Thus, the PIN-driven IAA distribution may be required for primary root, root hair, and lateral root growth. Yet to be determined, however, is how plants integrate the YUCCA-catalyzed IAA generation with the PIN-driven IAA distribution, especially in root growth and development.
Importantly, the functions of GA and IAA converge in roots to regulate cell expansion and root growth (Benkova and Hejatko 2009). Like the application of the IAA-transport inhibitor 1-N-naphthylphthalamic acid, the pin1 mutant attenuates the effect of GA on root growth (Benkova and Hejatko 2009). In addition, NaCl reduces the expression of PIN1 and PIN2 but does not affect local IAA biosynthesis in roots (Liu et al. 2015). A reasonable explanation is that NaCl disturbs the conjunction of IAA generation and its transport, and thus impairs the optimum IAA concentration needed for root growth. Interestingly, the major GA-responsive tissue in roots is the endodermis (Dinneny 2014), which is a gateway for solutes to sense and respond to Na+ toxicity (Duan et al. 2013; Dinneny 2014). This information raises the question as to whether and how GA20ox2 is involved in such NaCl-controlled root growth. Thus, in this study, whether and how NaCl modifies GA20ox2 gene expression, and whether this modification affects PIN-dependent IAA distribution during root growth and development, were studied. The findings suggest that GA20ox2 has an important role in NaCl-controlled primary root and root hair growth through its mediation of IAA generation and transport.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia-0 was used as the wild-type (WT) in the experiments. T-DNA insertion lines ga20ox2-1 (At5G51810, GABI-KAT734G06) and pin2 (At5G57090, SALK_122916.49.40.x) were purchased from the Arabidopsis Biological Resource Center, and the respective homozygous mutant plants were confirmed by PCR amplification. PIN2-green fluorescent protein (GFP) and DR5-β-glucuronidase (GUS) seeds (Columbia-0 background) were donated by Jian Xu (Sassi et al. 2012).
All of the seeds were collected and stored under identical conditions. Seeds were surface-sterilized with 0.1% HgCl2 for 5 min, washed five times with distilled water, and sown on Murashige–Skoog (MS) medium (0.6% agar and 3% sucrose). The plates were kept at 4 °C for a 3-day vernalization period and then transferred to a growth chamber for a 3-day germination period. Growth room conditions were as follows: 22 ± 2 °C, a 16-h-light/8-h-dark photoperiod, 65% relative humidity, and a light intensity of approximately 100 μmol m−2 s−1. Seedlings were subsequently transferred to fresh MS medium (0.8% agar and 3% sucrose) with or without supplementation. Seedling age (days) was counted from the day of transfer. Plates were placed vertically during seedling growth. In appropriate experiments, seedlings were cut into two sections, roots and shoots. The cutting point was the lower end of the hypocotyls.
Plasmid construction and plant transformation
To construct a GA20ox2-complementation line, the promoter fragment and coding sequence of GA20ox2 were amplified and the resulting product cloned into the pCAMBIA1300 vector. The constructs were introduced into Agrobacterium tumefaciens strain GV3101 and transformed into ga20ox2-1 plants by floral infiltration. To construct a GA20ox2 over-expression vector, full-length GA20ox2 cDNA was amplified and cloned into the pSUPER1300 vector. The constructs were introduced into A. tumefaciens strain GV3101 and transformed into WT plants by floral infiltration. Transformed T1 plants were selected on hygromycin-containing medium. GA20ox2 expression in the transgenic lines was detected by reverse-transcription PCR (RT-PCR). Among the GA20ox2-complementation plant lines, 3 of the 12 transgenic lines had similar levels of GA20ox2 mRNA. Among the GA20ox2 over-expression plant lines, 2 of the 13 transgenic lines had higher levels of GA20ox2 mRNA. These transgenic lines were selected and used in the following experiments. To generate the GA20ox2-GUS construct, the GA20ox2 promoter fragment was amplified and then cloned into the promoter-less GUS expression vector pCAMBIA1381.
The constructs were introduced into A. tumefaciens strain GV3101 and transformed into WT plants by floral infiltration. Transformed T1 plants were selected on hygromycin-containing medium. GA20ox2-GUS/pin2, PIN2-GFP/ga20ox2-1, and DR5-GUS/ga20ox2-1 lines were obtained by crossing. Pollen was transferred from GA20ox2-GUS, PIN2-GFP, and DR5-GUS transformed plants to the mature stigmas of pin2 and ga20ox2-1 plants. T1 plants were self-pollinated and grown to form the T2 generation. T2 plants were screened on hygromycin-containing medium and identified by RT-PCR. Homozygous T3 plants were used in the experiments.
Gene expression analysis
Gene expression was analyzed using RT-PCR or qRT-PCR. Based on Duan et al. (2013) and Han et al. (2014), 7-days-old Arabidopsis seedlings were used in this work. Total RNA was extracted from 100 mg of roots or shoots grown with or without NaCl using a Plant RNA MIDI kit (Life Feng, Shanghai, China). cDNA was synthesized from the RNA using an oligo (dT)18 primer and Moloney murine leukemia virus reverse transcriptase (Promega, http://www.promega.com). For RT-PCR, the volume of each cDNA sample was adjusted to produce the same signal strength for Actin2 after 22–24 cycles, and the products were analyzed by electrophoresis on 1.2% agarose gels. qRT-PCR experiments were performed using gene-specific primers and SYBR Premix (Takara, http://www.takara-bio.eu/) on an ABI 7500 real-time PCR system (Bio-Rad, USA).
All of the primers used for vector construction and the gene expression analysis are listed in Fig. S2.
Quantification of primary root and root hair lengths
The lengths of the primary roots of seedlings were measured under a FV1000 microscope (Olympus, Tokyo, Japan). Root hairs were observed as described by Bai et al. (2014). Using a FV1000 microscope and Image J software (http://rsbweb.nih.gov/ij), root hair length and root hair density were determined by measuring the longest root hairs of 10 roots and by counting the number of root hairs within 1 cm of the root tip of each line, respectively.
GUS staining and quantification assay
Histochemical GUS staining was performed according to Han et al. (2014). Seedling roots or shoots were incubated for 6 h in the dark at 37 °C in GUS staining solution (0.1 M sodium phosphate buffer, pH 7.0; 0.05 mM K3[Fe(CN)6]; 0.05 mM K4[Fe(CN)6]; 1 mg/ml X-Gluc; and 0.1% Triton X-100) and then maintained for 3 h in 70% ethanol at 65 °C for the removal of chlorophyll. Photographs were obtained using a Stereo-Zoom microscope and a Nikon Coolpix digital camera.
A GUS quantitative assay was performed by homogenizing 20-mg samples in extraction buffer (50 mM Na3PO4, pH 7.0; 10 mM β-mercaptoethanol; 1 mM Na2EDTA; 0.1% sodium lauryl sarcosine; and 0.1% Triton X-100). Each extract was centrifuged at 13,000×g for 15 min at 4 °C, and the supernatant was used for measurements. Protein concentrations were normalized with Bradford reagent (Bio-Rad). The fluorescence of 4-methylumbelliferyl-β-glucuronide hydrate (Sigma-Aldrich) was measured on a Fluoroskan Ascent FL fluorometer (excitation, 365 nm; emission, 455 nm). Measurements were read every 30 min and fitted to a standard curve. Enzyme activity was calibrated to the 4-methylumbelliferone concentration.
Confocal microscopy
Roots were dissected from 7-days-old WT and ga20ox2-1 seedlings grown on MS medium with or without NaCl. PIN2-GFP reporter activity was analyzed using a Zeiss LSM710 confocal microscope, with image analyses performed using Zeiss 2011 software (excitation, 488 nm; emission, 500–550 nm), and all pictures were acquired with exactly the same confocal settings. All image analyses were repeated at least three times.
Measurement of Na+ content
Each 7-days-old seedling (incubated with or without 120 mM NaCl for 4 h) was cut into just two parts, including root and shoot. After incubation at 110 °C for 10 min, the segments were dried at 70 °C for 48 h. The dried tissues were incinerated at 550 °C for 6 h. Each aliquot of sample ash was dissolved in 0.5 M HCl solution to determine its Na+ content by inductively coupled plasma–atomic emission spectrometry (Perkin-Elmer Optima 2100DV, Shelton, CT, USA).
Measurement of the GA4 content
GA4 levels were monitored according to Fambrini et al. (2011) and Kurepin et al. (2015). Shoot or root material (0.5–1 g) was ground in liquid nitrogen and transferred to a 4-ml EP tube. After the addition of 1 ml cold 80% (v/v) methanol (first-grade chromatographic quality) and 1% (v/v) acetic acid, each tube was incubated at 4 °C for 12 h. The samples were centrifuged at 12,000×g for 10 min at 4 °C. The supernatants were sequentially passed through a column containing C18 adsorbent (Qasis MCX 3 cc, 60 mg), evaporated to dryness under vacuum at 30 °C, and then re-suspended in 200 μl 80% (v/v) methanol and 1% (v/v) acetic acid. Aliquots of 100 μl were tested, and three biological replicates were performed. The analysis was performed on an Applied Biosystems MDS SCIEX 4000 QTRAP liquid chromatograph-tandem mass spectrometry system.
Statistical analysis
Differences in various parameters were compared using Student’s t-test (**P < 0.01, *P < 0.05). Data from at least three biological replicates were analyzed with similar results.
Results
Root morphogenesis of ga20ox2-1 seedlings exposed to NaCl stress
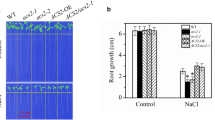
To investigate the possible effects of GA20ox2 expression on the root growth of NaCl-exposed Arabidopsis seedlings, we prepared various seeds with altered GA20ox2 expression levels. The ga20ox2-1 mutant was identified as a T-DNA knock-out mutant (Fig. 1a). Complementation (GA20ox2/ga20ox2-1) and over-expression (GA20ox2-OE) plants were created using transgenic methods and had mRNA levels of the GA20ox2 gene equal to, or significantly exceeding, those of WT seedlings (Fig. 1a).
Root morphogenesis of ga20ox2-1 seedlings in response to NaCl treatment. a Comparison of GA20ox2 expression levels among the loss-of-function mutant ga20ox2-1, WT, transgenic GA20ox2/ga20ox2-1 complemented line, and transgenic GA20ox2-OE over-expression line seedlings. b, c Primary root growth status (b) and root length statistics (c) of WT, ga20ox2-1, GA20ox2-OE, and GA20ox2/ga20ox2-1 seedlings in the presence or absence of NaCl. d–f Root hair growth status (d), root hair length (e), and root hair number statistics (f) of WT, ga20ox2-1, and GA20ox2-OE seedlings with or without the NaCl treatment. The red arrows in (d) indicate the beginning of NaCl treatments. Samples selected randomly from three independent experiments are shown. Values are means ± SDs (**P < 0.01, *P < 0.05) of 90 seedlings
We first compared root elongation in the loss-of-function ga20ox2-1 mutant with that in WT seedlings in response to various NaCl concentrations. The 120 mM NaCl treatment significantly altered root elongation in ga20ox2-1 and WT seedlings (Fig. S1). To demonstrate that this alteration resulted from GA20ox2 expression, we compared the effects of 120 mM NaCl on the elongated root of the altered GA20ox2-expression lines. After growth on free MS medium for 7 d post-transfer, the root lengths of ga20ox2-1 seedlings (13.35 ± 1.18 mm) were shorter than those of the WT (17.17 ± 1.29 mm), GA20ox2-OE (19.27 ± 1.73 mm), and GA20ox2/ga20ox2-1 (17.04 ± 1.48 mm) (Fig. 1a, b). Thus, GA20ox2 expression levels are positively correlated with the elongation of primary roots in Arabidopsis seedlings. We expected root lengths of the complementation GA20ox2/ga20ox2-1 lines to be similar to those of the WT. Interestingly, when seedlings were grown on MS medium containing 120 mM NaCl for 7 days, the net elongation of ga20ox2-1 seedling roots was 11.28 ± 1.25 mm, which was longer than that in the WT (9.42 ± 1.09 mm), GA20ox2/ga20ox2-1 (9.39 ± 1.18 mm), and GA20ox2-OE (7.09 ± 0.94 mm) (Fig. 1a, b). Thus, primary root elongation in Arabidopsis seedlings in the presence of NaCl stress was negatively correlated with GA20ox2 expression levels, a result contrary to that of the blank control. These observations suggest that NaCl can modify GA20ox2-associated root elongation and that the loss-of-function mutant ga20ox2’s root growth was insensitive to salt stress in Arabidopsis seedlings.
We also examined how NaCl changed the lengths and densities of ga20ox2-1 root hairs. Under control conditions, no significant differences in the lengths and densities of root hairs were found between the WT and ga20ox2-1 (Fig. 1d–f), whereas GA20ox2-OE root hairs were shorter than those of WT and ga20ox2-1 seedlings (Fig. 1d–f). Under the 120-mM NaCl treatment, root hairs were longer in ga20ox2-1 plants (1.27 ± 0.29 mm) than in the WT (1.13 ± 0.30 mm), while the number of elongated root hairs was significantly elevated in ga20ox2-1 (52.83 ± 8.27 mm) compared with the WT (24.67 ± 4.18 mm). In addition, almost no root hair elongation was found in GA20ox2-OE seedling roots (Fig. 1d–f). These results indicate that the activation of GA20ox2 inhibits the elongation (but not the initiation) of root hairs. We, therefore, hypothesized that the activation of GA20ox2 plays a role in NaCl-modified primary root or root hair growth.
Retardation of active GA accumulation in ga20ox2-1 roots
Because active GA4 regulates root elongation and specifically accumulates in the root tips of Arabidopsis seedlings (Sarnowska et al. 2013), whether this regulation connected active GA4 and Na+ accumulation in ga20ox2-1 shoots or roots was determined. This information will help clarify the mechanism by which NaCl regulates root growth in ga20ox2-1 seedlings.
GA4 levels were monitored by liquid chromatography-mass spectrometry. In the NaCl-free control experiments, GA4 levels (Fig. 2a) in WT roots [1.19 ± 0.20 ng/g fresh weight (FW)] were greater than those in ga20ox2-1 (0.58 ± 0.09 ng/g FW) and GA20ox2-OE (0.61 ± 0.12 ng/g FW) seedling roots. No significant differences in GA4 levels were observed between seedling shoots of the WT and ga20ox2-1, while GA4 levels were greater in GA20ox2-OE seedling shoots than in WT or ga20ox2-1 shoots (Fig. 2a). Thus, root elongation under normal conditions may be positively correlated with GA20ox2 expression and GA4 accumulation in Arabidopsis seedlings. In NaCl-stressed seedlings, GA4 levels in WT roots (0.74 ± 0.15 ng/g FW) were similar to those in ga20ox2-1 roots (0.65 ± 0.13 ng/g FW), while GA4 levels in WT shoots (0.76 ± 0.14 ng/g FW) were significantly depressed compared with those in ga20ox2-1 shoots (0.91 ± 0.13 ng/g FW) (Fig. 2a). Unexpectedly, GA4 accumulated in shoots but not in roots, and this accumulation corresponded to root elongation in NaCl-exposed ga20ox2-1 and WT seedlings. Unlike in the WT and ga20ox2-1, NaCl-stressed GA20ox2-OE seedlings exhibited increased GA4 (Fig. 2a) accumulations in roots (1.51 ± 0.18 ng/g FW) as well as in shoots (2.06 ± 0.25 ng/g FW). In response to NaCl stimuli, GA20ox2-OE seedlings had excessive GA4 accumulations. Thus, GA20ox2 expression may be the basis for the NaCl-modified GA4 accumulation and distribution observed in Arabidopsis seedling shoots and roots.
Na+ levels in various GA20ox2-expressing lines were tested after exposure to NaCl for 4 h. Compared with the blank treatment, the exposure of WT seedlings to NaCl increased Na+ levels in roots and shoots, with similar proportional increases in both. This increase was much greater in GA20ox2-OE plants than in WT seedlings (Fig. 2b). Under a NaCl treatment, however, the Na+ content of ga20ox2-1 roots was lower than that of WT roots, whereas no significant differences were observed in the contents of ga20ox2-1 and WT shoots (Fig. 2b). These results suggest that GA20ox2 expression is involved in Na+ absorption and distribution in NaCl-stressed Arabidopsis seedlings.
Induction of GA20ox2 expression by NaCl
To determine how NaCl affects GA20ox2 expression, we created transgenic lines containing the GUS reporter to trace GA20ox2 expression. In the absence of NaCl, histochemical staining showed that GA20ox2 was expressed in various plant tissues, such as cotyledons, microtubules, hypocotyls, sepals (data not shown), and root tips (Fig. 3a), with only very low GA20ox2 levels detected in leaves (Fig. 3a, b). In contrast, the application of NaCl resulted in a significant increase in GA20ox2 expression in shoots, including cotyledons and leaves (Fig. 3a, b). The NaCl-stimulated GUS activity levels in shoots and roots were ~ five- and twofold, respectively, greater than those in the control (Fig. 3b). To verify that NaCl-induced GA20ox2 expression occurs mainly in shoots, we also compared GA20ox2 transcriptional activity levels in shoots with those in roots using qRT-PCR. Compared with the control, the NaCl treatment increased GA20ox2 mRNA levels by ~ 25- and ~ 12-fold in shoots and roots, respectively (Fig. 3c). Thus, NaCl predominantly induces GA20ox2 expression (Fig. 3) and active GA4 accumulation in shoots (Fig. 2), but inhibits primary root and root hair elongation (Fig. 1). A spatial difference exists between the predominant expression of the GA20ox2 gene in shoots and NaCl-inhibited primary root or root hair growth in Arabidopsis seedlings.
Characterization of GA20ox2 expression under the NaCl treatment. a Variations in GA20ox2 expression in 7-days-old seedlings after transfer and growth on MS medium with or without 120 mM NaCl. b Activation of the GA20ox2 promoter-GUS construct in shoot and root tissues of transgenic WT plants grown on MS with or without 120 mM NaCl for 7 days. c GA20ox2 gene expression levels monitored by quantitative real-time PCR in shoots and roots with or without NaCl treatment
Alteration of IAA homeostasis by NaCl in ga20ox2-1 roots
We hypothesized that IAA bridges the above-mentioned spatial gap because IAA is required for NaCl-modified root growth in Arabidopsis seedlings and stimulates the expression of GA biosynthetic genes in various plants (Wolbang and Ross 2001; Wolbang et al. 2004; Frigerio et al. 2006).
To determine whether NaCl-controlled IAA generation and distribution were modified in ga20ox2-1 lines, the transcriptional activities of the IAA biosynthetic genes YUCCA3, YUCCA8, and YUCCA9 were investigated. The mRNA levels of the three genes were reduced by NaCl in WT and ga20ox2-1 seedling roots, but were increased in shoots (Fig. 4a), with this increase being greater in ga20ox2-1 shoots than in WT shoots (Fig. 4a). Then, the NaCl-triggered IAA distribution was examined by monitoring the expression of the IAA reporter gene DR5. In comparison with the control, DR5-GUS expression levels were increased in ga20ox2-1 roots (Fig. 4b). Thus, ga20ox2-1 ameliorated the inhibition of YUCCA gene expression by NaCl in shoot tissues but increased IAA accumulation in root tissues (Fig. 4b).
NaCl-mediated IAA generation and distribution in ga20ox2-1 seedlings. a Expression levels of YUCCA3, YUCCA8, and YUCCA9 genes monitored by RT-qPCR in shoots and roots of ga20ox2-1 and WT seedlings with or without 120 mM NaCl for 7 days. b DR5–GUS-marked IAA levels in shoot and root tissues of ga20ox2-1 and WT seedlings with or without 120 mM NaCl for 7 days. Red circles and arrows indicate DR5-GUS expression in root tips
Alteration of IAA transport by NaCl in ga20ox2-1 roots
Because IAA transport ensures optimum IAA concentrations for root growth (Dinneny 2014) and PIN1 is involved in GA-mediated root elongation (Benkova and Hejatko 2009), while PIN2 promotes cell division in lateral root primordia (Zhao et al. 2011) by maintaining an adequate IAA level in root cells (Ottenschlager et al. 2003), we hypothesized that ga20ox2-1 redistributed IAA by modifying the activity levels of PIN1 and PIN2.
How NaCl affected the activity of PIN1 in ga20ox2-1 seedlings was investigated. In the absence of NaCl, ga20ox2-1 seedlings displayed increased PIN1 expression, and mRNA levels of the PIN1 gene in ga20ox2-1 roots and shoots were ~ 2.5- and ~ 1.6-fold, respectively, that of the corresponding organs in the WT (Fig. 5a). In NaCl-exposed seedlings, mRNA levels of the PIN1 gene consistently declined in WT and ga20ox2-1 roots. In ga20ox2-1 and WT shoots under NaCl treatment, however, PIN1 mRNA levels increased, with level in the former being ~ 5.2-fold that of the latter (Fig. 5a). Thus, ga20ox2-1 may enhance PIN1-dependent IAA transport in shoots relative to the WT in response to NaCl stress.
Control of PIN1 and PIN2 by NaCl in ga20ox2-1 seedling roots. a, b Expression levels of PIN1 (a) and PIN2 (b) detected by RT-qPCR in shoots and roots of ga20ox2-1 and wild-type (WT) seedlings with or without 120 mM NaCl for 7 days. c, d GFP fluorescence (c) and statistics of relative fluorescence intensity (d) of PIN2-GFP in root tissues of ga20ox2-1 and WT seedlings with or without 120 mM NaCl for 7 days. e, f Expression levels of the GA20ox2 gene (e) and GA20ox2 promoter-GUS activity (f) in pin2 mutant seedlings with or without 120 mM NaCl for 7 days
These parallel experiments demonstrated that ga20ox2-1 under control conditions depressed PIN2 transcription levels in roots and increased them in shoots compared with the WT (Fig. 5b). After the NaCl treatment, the WT exhibited an enhanced PIN2 expression level in shoots but not in roots. Unlike the WT, ga20ox2-1 significantly increased PIN2’s expression in the roots of NaCl-exposed seedlings, which displayed PIN2 mRNA levels that were ~ 4.6-fold that of the control. However, PIN2 expression was not significantly modified in ga20ox2-1 shoots (Fig. 5b). Thus, ga20ox2-1 enhanced PIN2-dependent IAA transport in roots compared with the WT.
Next, GFP-tagged PIN2 protein was monitored in root tissues. In NaCl-exposed WT seedling roots, the fluorescence intensity of the GFP protein was decreased compared with that in the blank treatment (Fig. 5c, d). In contrast to the WT, ga20ox2-1 increased GFP fluorescence intensity under an NaCl treatment (Fig. 5c, d). The protein level data further implied that ga20ox2-1 relieved the inhibition of NaCl on PIN2 transport activity, thereby facilitating IAA redistribution in roots.
To provide further evidence for the interaction of GA20ox2 and PIN2 in NaCl-controlled root growth, whether PIN2 activation affects GA20ox2 expression was examined. In contrast to the notable elevation of GA20ox2 mRNA levels in WT seedlings, especially in shoots, under NaCl treatment (Figs. 3c, 5e), the transcriptional activity of GA20ox2 in loss-of-function mutant pin2 seedlings (including shoots and roots) was almost zero in the absence or presence of NaCl (Fig. 5e). Furthermore, histochemical staining was used to determine GUS-tagged GA20ox2 levels. In marked contrast to the control, NaCl deepened the blue staining of WT seedlings (especially in shoots; Fig. 5f); however, almost no blue staining was apparent in pin2 seedlings (including shoots and roots) regardless of whether a salt treatment was applied (Fig. 5f). These low GA20ox2 levels were consistent with the decline in GA20ox2 transcriptional activity. Thus, GA20ox2 histochemical staining and GA20ox2 transcriptional activity levels demonstrated that PIN2 regulates GA20ox2 activity.
Discussion
Active GAs often have limited effects on root growth and development that are dependent on IAA transport and accumulation (Niu et al. 2013). To understand the mechanism by which the biosynthesis factor GA20ox2 mediates salt-controlled plant root growth and development, in this study the relationship between GA20ox2 expression and IAA generation and distribution during NaCl-inhibited root growth in Arabidopsis seedlings were assessed. The results indicate a specific role for GA20ox2 in NaCl-controlled root growth.
GA20ox2 expression may be involved in NaCl-controlled root growth of Arabidopsis seedlings because GA20ox2 expression times, levels, and tissue localization may satisfy root growth requirements for young Arabidopsis seedlings. The GA20ox2 mRNA levels, among the five family members, are greatest in 3- and 7-days-old Arabidopsis seedlings, especially in roots (Rieu et al. 2008). When the IAA-permeable analog 1-naphthalene acetic acid was applied to mediate root elongation, the greatest expression of the GA20ox2 gene was observed in 6-days-old Arabidopsis seedlings (Wolbang and Ross 2001). In the present study, root length gradually increased as GA20ox2 expression increased in 7-days-old ga20ox2-1, WT, and transgenic GA20ox2-OE plants under NaCl-free conditions (Fig. 1). Thus, root elongation was positively correlated with GA20ox2 expression levels in young Arabidopsis seedlings. Applications of NaCl, however, transformed this positive correlation into a negative one (Fig. 1). This sharp contrast is a strong indication that GA20ox2 expression may act as a signaling element in NaCl-inhibited root growth of Arabidopsis seedlings, or, alternatively, that NaCl inhibits primary root growth by increasing GA20ox2 expression (Fig. 3).
The regulation of GA20ox2 expression and active GA levels are involved in NaCl-inhibited primary root or root hair growth. NaCl-triggered GA20ox2 expression (Fig. 3) appeared to correspond to the decreased root elongation (Fig. 1) and GA4 accumulation (Fig. 2a) of WT seedlings. In contrast, the reduction in GA20ox2 expression in ga20ox2-1 seedlings depressed NaCl-increased GA4 accumulation (Fig. 2a) and thus ameliorated NaCl-inhibited root elongation (Fig. 1). Thus, NaCl-induced GA20ox2 expression was negatively correlated with primary root growth in Arabidopsis seedlings. The number and elongation of root hairs, however, may be associated with GA20ox2-dependent GA4 levels because increased GA4 accumulation reduces the number of root hairs in Arabidopsis seedlings (Jiang and Fu 2008), while GA20ox2 expression promotes the production of GA4 and its precursor, GA9 (Phillips et al. 1995; Fernando et al. 2014). In our study, NaCl-induced GA20ox2 expression (Fig. 3) was negatively correlated with GA4 accumulation (Fig. 2a) and root hair density (Fig. 1). GA20ox2 expression inhibits the elongation of root hairs but not their initiation (Fig. 1), which explains the decline in root hair numbers. Thus, NaCl inhibits root hair elongation by elevating GA20ox2 expression and GA4 accumulation.
GA20ox2 expression may mediate Na+ uptake and distribution during the NaCl-inhibited primary root or root hair growth of Arabidopsis seedlings. The root endodermis, which executes very early adaptive responses through ions or channel proteins (Vermeer et al. 2014), acts as a gateway for solutes to sense and respond to Na+ toxicity (Duan et al. 2013; Dinneny 2014). Interestingly, the root endodermis is the major GA-responsive tissue (Dinneny 2014). The suppression of GA20ox2 expression (Fig. 1) and GA4 accumulation (Fig. 2) in the ga20ox2-1 mutant accordingly decreased Na+ absorption and accumulation in root tissues (Fig. 2b). As a consequence, the tolerance of ga20ox2-1 root or root hair growth to NaCl increased (Fig. 1). Thus, GA20ox2 expression can alter the sensitivity of Arabidopsis seedling root or root hair growth to NaCl stress.
The mechanisms underlying GA20ox2’s regulation of NaCl-stressed root growth involve IAA generation and distribution. In addition to 1-naphthalene acetic acid-facilitated GA20ox2 expression during Arabidopsis seedling root elongation (Wolbang and Ross 2001), GA’s promotion of root growth (Benkova and Hejatko 2009; Eilon et al. 2013) depends on IAA generation (Benkova and Hejatko 2009). GA alone does not elongate roots if IAA generation is disrupted by decapitation at the shoot apices of Arabidopsis seedlings (Benkova and Hejatko 2009). A similar mechanism appears to have operated in this study. Compared with the WT, the loss of GA20ox2 expression in the ga20ox2-1 mutant was inimical to root growth (Fig. 1), possibly because of the decrease in YUCCA expression (Fig. 4) and IAA accumulation in roots (Fig. 5). Under NaCl treatment, in contrast, the amelioration of root elongation in ga20ox2-1 seedlings (Fig. 1) was inconsistent with the activation of YUCCAs in shoots (Fig. 4) as well as IAA accumulation in roots (Fig. 5). Thus, IAA generation plays an important role in the involvement of GA20ox2 in NaCl-controlled root growth.
The influence of NaCl on ga20ox2-1 root growth may result from the regulation of IAA transport and redistribution because local IAA levels in root cells direct plant root morphogenesis (Pierik and Testerink 2014), and optimized IAA distribution is required for GA-mediated root growth and development (Niu et al. 2013). IAA redistribution is managed by PIN family members, and the collaboration between PIN1 and PIN2 recycles IAA from shoots to roots (Dinneny 2014). Considering that shoot apex-derived IAA promotes root growth by integrating PIN1 activity with GA-response signaling (Fu and Harberd 2003) and that PIN1 is expressed predominantly in shoot tissues (Gälweiler et al. 1999), ga20ox2-1 may alleviate PIN1 activity in shoot tissues (Fig. 4a) and IAA accumulation in roots (Fig. 4b), maintaining in turn root and root hair growth (Fig. 1) under NaCl-stress conditions. This maintenance also requires PIN2 activity (Figs. 4a, 5) because IAA-induced primary root or root hair growth depends on PIN2 expression (Gou et al. 2010). Furthermore, because GA can control PIN turnover during the root elongation of Arabidopsis seedlings (Moubayidin et al. 2010; Willige et al. 2011) and ga20ox2-1 reduced GA accumulation in seedling roots in our study (Fig. 2), ga20ox2-1 seedlings accordingly ameliorated PIN2 activity (Figs. 4, 5) in NaCl-stressed seedling roots. A similar scenario was described in transgenic GA-deficient Populus lines. In particular, a GA deficiency in transgenic Populus roots facilitated the formation of new lateral root primordia by amending PIN9 activity and IAA accumulation in root tissues (Gou et al. 2010).
In addition to the ga20ox2-1-induced alteration in PIN2 activity, the loss-of-function pin2 mutant decreased GA20ox2 expression with or without the NaCl treatment (Fig. 5). Thus, NaCl reduces root meristem growth by inactivating PINs (Liu et al. 2015). These observations suggest that crosstalk occurs between GA20ox2 and PIN2 during NaCl control of root growth and development, increasing the complexity of GA-regulated root growth (Niu et al. 2013).
The expression of GA20ox2 facilitates NaCl’s inhibition of primary root or root hair growth, with this facilitation being dependent on YUCCA-catalyzed IAA generation and PIN1/2-driven IAA redistribution in Arabidopsis seedlings. The relationships among these factors are outlined in Fig. 6.
References
Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GT, Genschik P (2009) Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr Biol 19(14):1188–1193
Bai L, Ma X, Zhang G, Song S, Zhou Y, Gao L, Miao Y, Song CP (2014) A receptor-like kinase mediates ammonium homeostasis and is important for the polar growth of root hairs in Arabidopsis. Plant Cell 26(4):1497–1511
Benkova E, Hejatko J (2009) Hormone interactions at the root apical meristem. Plant Mol Biol 69(4):383–396
Carrera E, Prat S (1999) Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in Potato. Plant Physiol 119(2):765–774
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2013) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217(1):67–75
Daviere JM, Achard P (2013) Gibberellin signaling in plants. Development 140(6):1147–1151
Di DW, Zhang C, Luo P, An CW, Guo GQ (2016) The biosynthesis of auxin: how many paths truly lead to IAA? Plant Growth Regul 78(3):275–285
Dinneny JR (2014) A gateway with a guard: how the endodermis regulates growth through hormone signaling. Plant Sci 214:14–19
Duan L, Dietrich D, Ng CH, Chan PM, Bhalerao R, Bennett MJ, Dinneny JR (2013) Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 25(1):324–341
Eilon S, Roy W, Yi Z, Cristina C, Eirini K, Joanne C, Tsien RY, Mark E (2013) Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proc Natl Acad Sci USA 110(12):4834–4839
Fambrini M, Mariotti L, Parlanti S, Picciarelli P, Salvini M, Ceccarelli N, Pugliesi C (2011) The extreme dwarf phenotype of the GA-sensitive mutant of sunflower, dwarf2, is generated by a deletion in the ent-kaurenoic acid oxidase1 (HaKAO1) gene sequence. Plant Mol Biol 75(4–5):431–450
Fernando A, Aimone P, Stefano T, Julieta M, Maida RB, José Luis GM, Fabio F, Veronica G, Kater MM, George C (2014) SHORT VEGETATIVE PHASE reduces gibberellin biosynthesis at the Arabidopsis shoot apex to regulate the floral transition. Proc Natl Acad Sci USA 111(26):2760–2769
Frigerio M, Alabadi D, Perez-Gomez J, Garcia-Carcel L, Phillips AL, Hedden P, Blazquez MA (2006) Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol 142(2):553–563
Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421(6924):740–743
Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1999) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282(5397):2226–2230
Garcíamartínez JL, Lópezdiaz I, Sánchezbeltrán MJ, Phillips AL, Ward DA, Gaskin P, Hedden P (1997) Isolation and transcript analysis of gibberellin 20-oxidase genes in pea and bean in relation to fruit development. Plant Mol Biol 33(6):1073–1084
Gou J, Strauss SH, Tsai CJ, Fang K, Chen Y, Jiang X, Busov VB (2010) Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 22(3):623–639
Han S, Fang L, Ren X, Wang W, Jiang J (2014) MPK6 controls H2O2-induced root elongation by mediating Ca2+ influx across the plasma membrane of root cells in Arabidopsis seedlings. New Phytol 205(2):695–706
Jiang C, Fu X (2008) Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol 145(4):1460–1470
Kasahara H (2015) Current aspects of auxin biosynthesis in plants. Biosci Biotechnol Biochem 80(1):34–42
Kuraishi S, Muir RM (1962) Increase in diffusible auxin after treatment with gibberellin. Science 137(3532):760–761
Kurepin LV, Park JM, Lazarovits G, Hüner NPA (2015) Involvement of plant stress hormones in Burkholderia phytofirmans-induced shoot and root growth promotion. Plant Growth Regul 77(2):179–187
Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT (2015) Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol 168(1):343–356
Marhavý P, Montesinos JC, Abuzeineh A, Van DD, Vermeer JE, Duclercq J, Rakusová H, Nováková P, Friml J, Geldner N (2016) Targeted cell elimination reveals an auxin-guided biphasic mode of lateral root initiation. Genes Dev 30(4):471–483
Moubayidin L, Perilli S, Ioio RD, Mambro RD, Costantino P, Sabatini S (2010) The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr Biol 20(12):1138–1142
Niu S, Li Z, Yuan H, Pan F, Chen X, Li W (2013) Proper gibberellin localization in vascular tissue is required to regulate adventitious root development in tobacco. J Exp Bot 64(11):3411–3424
Ottenschlager I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100(5):2987–2991
Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Annu Rev Plant Biol 63:563–590
Phillips AL, Ward DA, Uknes S, Appleford NE, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P (1995) Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol 108(3):1049–1057
Pierik R, Testerink C (2014) The art of being flexible: how to escape from shade, salt, and drought. Plant Physiol 166(1):5–22
Plackett AR, Powers SJ, Fernandez-Garcia N, Urbanova T, Takebayashi Y, Seo M, Jikumaru Y, Benlloch R, Nilsson O, Ruiz-Rivero O, Phillips AL, Wilson ZA, Thomas SG, Hedden P (2012) Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 24(3):941–960
Rebers M (1999) Regulation of gibberellin biosynthesis genes during flower and early fruit development of tomato. Plant J 17(3):241–250
Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, Phillips AL, Hedden P (2008) The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J 53(3):488–504
Sakakibara H (2005) Cytokinin biosynthesis and regulation. Vitam Horm 72:271–287
Sarnowska EA, Rolicka AT, Bucior E, Cwiek P, Tohge T, Fernie AR, Jikumaru Y, Kamiya Y, Franzen R, Schmelzer E, Porri A, Sacharowski S, Gratkowska DM, Zugaj DL, Taff A, Zalewska A, Archacki R, Davis SJ, Coupland G, Koncz C, Jerzmanowski A, Sarnowski TJ (2013) DELLA-interacting SWI3C core subunit of switch/sucrose nonfermenting chromatin remodeling complex modulates gibberellin responses and hormonal cross talk in Arabidopsis. Plant Physiol 163(1):305–317
Sassi M, Lu Y, Zhang Y, Wang J, Dhonukshe P, Blilou I, Dai M, Li J, Gong X, Jaillais Y (2012) COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1-and PIN2-dependent auxin transport in Arabidopsis. Development 139(18):3402–3412
Tanimoto E (2012) Tall or short? Slender or thick? A plant strategy for regulating elongation growth of roots by low concentrations of gibberellin. Ann Bot 110(2):373–381
Ubeda-Tomas S, Federici F, Casimiro I, Beemster GT, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett MJ (2009) Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr Biol 19(14):1194–1199
Vermeer JE, von Wangenheim D, Barberon M, Lee Y, Stelzer EH, Maizel A, Geldner N (2014) A spatial accommodation by neighboring cells is required for organ initiation in Arabidopsis. Science 343(6167):178–183
Willige BC, Isono E, Richter R, Zourelidou M, Schwechheimer C (2011) Gibberellin regulates PIN-formed abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell 23(6):2184–2195
Wolbang CM, Ross JJ (2001) Auxin promotes gibberellin biosynthesis in decapitated tobacco plants. Planta 214(1):153–157
Wolbang CM, Chandler PM, Smith JJ, Ross JJ (2004) Auxin from the developing inflorescence is required for the biosynthesis of active gibberellins in barley stems. Plant Physiol 134(2):769–776
Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) VASCULAR-RELATED NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55(4):652
Yu G, Rui W, Choon Wei W, Fei X, Xueliang W, Chan PMY, Cliff T, Lina D, Dinneny JR (2013) A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Proc Natl Acad Sci USA 25(6):2132–2154
Zhao Y, Wang T, Zhang W, Li X (2011) SOS3 mediates lateral root development under low salt stress through regulation of auxin redistribution and maxima in Arabidopsis. New Phytol 189(4):1122–1134
Acknowledgements
We thank Dr. Jian Xu (National University of Singapore, Singapore) for providing PIN2-GFP and DR5-GUS seeds. This work was supported by funding from the National Natural Science Foundation of China to Jing Jiang (Grant Numbers 30971509 and 31271510). We thank Lesley Benyon, PhD, from Liwen Bianji, Edanz Group China (http://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lv, S., Yu, D., Sun, Q. et al. Activation of gibberellin 20-oxidase 2 undermines auxin-dependent root and root hair growth in NaCl-stressed Arabidopsis seedlings. Plant Growth Regul 84, 225–236 (2018). https://doi.org/10.1007/s10725-017-0333-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0333-9