Abstract
Pectobacterium carotovorum subsp. carotovorum causes soft rot disease in various plants, including Chinese cabbage. The simple extracellular leucine-rich repeat (eLRR) domain proteins have been implicated in disease resistance. Rice leucine-rich repeat protein (OsLRP), a rice simple eLRR domain protein, is induced by pathogens, phytohormones, and salt. To see whether OsLRP enhances disease resistance to bacterial soft rot, OsLRP was introduced into Chinese cabbage by Agrobacterium-mediated transformation. Two independent transgenic lines over-expressing OsLRP were generated and further analyzed. Transgenic lines over-expressing OsLRP showed enhanced disease resistance to bacterial soft rot compared to non-transgenic control. Bacterial growth was retarded in transgenic lines over-expressing OsLRP compared to non-transgenic controls. We propose that OsLRP confers enhanced resistance to bacterial soft rot. Monitoring expression of defense-associated genes in transgenic lines over-expressing OsLRP, two different glucanases and Brassica rapa polygalacturonase inhibiting protein 2, PDF1 were constitutively activated in transgenic lines compared to non-transgenic control. Taken together, heterologous expression of OsLRP results in the activation of defense response and enhanced resistance to bacterial soft rot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brassica rapa L. subsp. pekinensis (Chinese cabbage) is one of the most consumed and important vegetables cultivated across 37,200 ha in 2009 in Korea. Bacterial soft rot is the most severe and destructive disease in most vegetables, including Chinese cabbage. Chinese cabbage is highly susceptible to soft rot disease caused by Gram-negative bacterium, Pectobacterium carotovorum subsp. carotovorum (Pcc) (Ren et al. 2001). In addition, control of soft rot disease is difficult due to a wide range of hosts, the ability of the bacteria to survive in plant debris in the soil, and host susceptibility (Vanjildorj et al. 2009). Chemical controls are not currently available for bacterial soft rot.
Pcc causes maceration of parenchymal tissue by increasing the levels of multiple exoenzymes including pectinases, cellulases, and proteases in all organs, ultimately resulting in plant cell death. These cell wall degrading enzymes break pectin down into unsaturated oligogalacturonates and trigger various plant defense responses, including phytoalexin synthesis, proteinase inhibitor synthesis, membrane protein phosphorylation, and a response similar to a hypersensitive response (HR) (Lam et al. 2001; Vlot et al. 2008; Ban et al. 2009).
Plant proteins with extracellular leucine-rich repeat (eLRR) domains are classified into five classes based on their domain organization: polygalacturonase inhibitor protein (PGIP)-like proteins, leucine-rich repeat-extensin-like proteins, receptor-like proteins, receptor-like kinases, and simple eLRR domain proteins (van der Hoorn et al. 2005; Zhou et al. 2009). Simple eLRR domain proteins have been reported in several plant species, such as sorghum, tomato, tobacco, Arabidopsis, and rice (Hipskind et al. 1996; Tornero et al. 1996; Jacques et al. 2006; Jung and Hwang 2007). It is proposed that the sorghum eLRR (SLRR) protein may be involved in protein–ligand binding (Hipskind et al. 1996). The tomato eLRR (LeLRP) protein is induced in diseased plants and is processed during pathogen infection (Tornero et al. 1996). The tobacco eLRR (NtLRP1) protein is localized in the endoplasmic reticulum and proposed to be a negative regulator of the elicitin-mediated HR (Jacques et al. 2006). The pepper eLRR (CaLRR1) protein is induced by pathogens and expressed in the phloem of Capsicum annuum cells (Jung et al. 2004). The CaLRR1 protein interacts with hypersensitive-induced reaction (HIR) protein, CaHIR1, and suppresses cell death induced by CaHIR1 (Jung and Hwang 2007). Recently, it was demonstrated that the rice eLRR protein (OsLRR1) enters the endosomal pathway and interacts with OsHIR1. Over-expression of OsLRR1 in Arabidopsis confers enhanced resistance to the biotrophic pathogen, Pseudomonas syringae, and causes the constitutive expression of defense-related genes, such as PR1, PR2, and PDF1 (Zhou et al. 2009). More recently, OsLRR1 was found to enhance the plasma membrane localization of OsHIR1 (Zhou et al. 2010).
We have been interested in rice-Xanthomonas oryzae pv. oryzae (Xoo) interaction. We isolated the Oryza sativa LRR protein (OsLRP) (GenBank accession number AF364178) from expressed sequence tag (EST) analysis of a cDNA library made with mRNAs from Xoo-infected leaves. While we were doing research on OsLRP in rice, a paper on OsLRR1, the same gene as OsLRP, was published as mentioned above. Therefore, we introduced OsLRP into Chinese cabbage to develop a crop resistant to bacterial soft rot in this study. Transgenic plants were analyzed and challenged against the bacterial soft-rot pathogen, Pcc.
Materials and methods
Plant treatments
Rice seedlings (Oryza sativa cv. Hwachung) were grown in a greenhouse for 3 weeks. Three-week-old rice seedlings were washed and incubated in tap water for 2 days and then treated with 1 mM benzothiadiazole (BTH), 1 mM salicylic acid (SA), 100 μM jasmonic acid (JA), 200 mM NaCl, 100 μM abscisic acid (ABA), respectively. Samples were taken at indicated times in Fig. 1. A strain of Xoo KXO98 incompatible to O. sativa cv. Hwachung was grown in PSA medium (10 g peptone, 10 g sucrose, 1 g sodium glutamate, and 15 g agar per liter) for 2 days and then resuspended in 10 mM MgCl2 to a final OD600 of 0.5. For bacterial inoculations, 21-day-old rice seedlings were challenged by spraying bacterial suspension with atomizer (Ji et al. 2008). Samples were taken at 12, 24, 48 h post-inoculation (hpi) and then were immediately frozen in liquid nitrogen and stored at −80 °C until further analysis.
Schematic representation of OsLRP domains and expression analysis of OsLRP1 in response to various stimuli. a OsLRP contains a signal peptide (SP), a leucine zipper (LZ), and a LRR domain with four leucine-rich repeats. b RT-PCR using rice leaves treated with pathogen (Xoo), phytohormones (SA, JA, and ABA), BTH, NaCl, and non-treated control (NT) was performed. OsActin was used for normalization of each sample. Each treatment is described in “Materials and methods”
Reverse transcription-polymerase chain reaction (RT-PCR)
Leaf samples were ground to powder in liquid nitrogen and total RNAs were prepared for RT-PCR as previously described (Hwang et al. 2008). Total RNA (1 μg) was used for reverse transcription with M-MLV RTase (Promega, Madison, WI, USA). Two microliters of each reverse transcriptase reaction was used for subsequent PCR. The PCR reaction was performed with 28 cycles (95 °C for 30 s, 55–60 °C for 30 s and 72 °C for 30 s) and a final extension for 5 min at 72 °C. The actin gene primer pair (BrActin or OsActin) was used as a control for the same amount of transcripts from all samples in this experiment. The primers used in this study are listed in Table 1.
Isolation of OsLRP and plant transformation vector construction
Full-length cDNA corresponding to OsLRP gene was obtained by EST analysis of a cDNA library made with mRNAs from Xoo-infected leaves. OsLRP gene-specific primers were designed from the sequence of GenBank accession No. AF364178 (Table 1) for gateway cloning (Invitrogen, Carlsbad, CA, USA). PCR was performed with 30 cycles (94 °C for 30 s, 53 °C for 1 min, and 72 °C for 1 min) and a final extension at 72 °C for 7 min. The 922-bp product was cloned into pDONR221 to make an entry clone through the use of BP clonase (Invitrogen, Carlsbad, CA, USA). 35S:OsLRP was made via LR clonase reaction between the OsLRP entry clone and pB2GW7 (Gateway™, Belgium). The map of resulting vector (35S:OsLRP) is shown in Fig. 2.
Schematic representation of the 35S-OsLRP construct for transformation into Chinese cabbage and the transcript level of OsLRP transgene in Chinese cabbage. a Linear map of the 35S-OsLRP construct for plant transformation. P35S: cauliflower mosaic virus 35S promoter; T35S: cauliflower mosaic virus 35S terminator. b RT-PCR was performed to analyze the expression pattern of the transgene in transgenic lines (T0). c The photo shows phenotypes of T0 transgenic lines and non-transgenic control (NT)
Transformation of Chinese cabbage
Seeds of Chinese cabbage (Brassica rapa subsp. pekinensis cv. Seoul) were sterilized with 70 % ethanol for 1 min, followed by treatment with 2 % sodium hypochlorite for 20 min. Seeds were placed on Murashige and Skoog (MS) medium containing 30 g/l sucrose and 6 g/l phytagel after washing with sterile distilled water. The plates were maintained at 25 °C under dark conditions for 6 days and transferred into light conditions for 1 day. The cotyledons were excised (1 cm) and placed on CO medium (MS medium containing sucrose 30 g/l, 1 mg/l 1-naphthaleneacetic acid, 5 mg/l 6-benzylaminopurine, 8 mg/l AgNO3, and 8 g/l phytagel) for 3 days under light conditions. Agrobacterium (GV3101) carrying 35S-OsLRPwere grown overnight in 25 ml YEP medium containing 5 μg/ml spectinomycin and harvested by centrifugation. The pellet was resuspended in 30 ml CO liquid media containing 12 mg acetosyringone, and then the cotyledon explants were added to the medium for 15 min under dark conditions. The explants were blotted onto sterile 3 M paper and then placed on CO medium for co-cultivation. After 3 days, the explants were rinsed three times in CO liquid medium containing 250 mg/l cefotaxime and then surface-dried on sterilized filter paper. For selection, the explants were transferred into SM medium (CO medium containing 250 mg/l cefotaxime and 5 mg/l l-phosphinothricin) and a 16/8 h day/night photoperiod for 6 weeks. The calli with multiple shoots on the SM medium were transferred to rooting medium (MS medium containing 30 g/l sucrose, 8 g/l phytagel, 250 mg/l cefotaxime, and 5 mg/l l-phosphinothricin). After 3 weeks, the root-plants were transferred to soil for acclimation.
Disease assay of transgenic Chinese cabbage Pcc stock was inoculated in 5 ml Luria–Bertani (LB) broth and were grown overnight in a 30 °C shaking incubator. The next day, the overnight culture was re-inoculated into fresh 5 ml LB broth in 1/100 dilution and were grown for 6 h under the same condition. After centrifugation of culture, the pellet was re-suspended in 1 mM NaCl to obtain an OD600 value of 0.1. The three leaves from 2-month-old non-transgenic plants (T0 lines) were excised, respectively and placed onto petri dishes. Sterile toothpicks were used to make wound on the midribs of the leaves and 5 μl of the bacterial cell suspension was dropped onto the wound sites. The leaves were then transferred to a plastic box at 25 °C.
Transgenic plants (T1) were challenged with Pcc at 103 cells/ml by infiltration with needless syringe. Three leaf discs from five plants (T1) were harvested from each infiltration area with a 0.8-cm cork borer at 0 and 3 days post-inoculation (dpi), respectively. The discs were ground with a sterile mortar and pestle in 0.3 ml of 10 mM MgCl2 and then diluted and plated on LB media containing 5 mg/ml rifampicin to determine bacterial population density (CFU/cm2). The colonies were counted after the plates were incubated at 28 °C for 3 days. An average of bacterial numbers was made from numbers of colonies from samples of ten different T1 plants and each error bar indicates standard deviation in the graph.
Results and discussion
Expression analysis of OsLRP in response to various stimuli
Initially, we isolated OsLRP by EST analysis of a cDNA library prepared from mRNAs of leaves infected with Xoo. OsLRP is also known as OsLRR1 (Zhou et al. 2009). The eLRR domain proteins, such as SLRR, LeLRR, NtLRP, CaLRR, and OsLRR1, have been implicated in defense responses to biotic stresses in various plant species (Hipskind et al. 1996; Tornero et al. 1996; Jung et al. 2004; Jacques et al. 2006; Jung and Hwang 2007; Zhou et al. 2009, 2010). OsLRP was previously reported to be induced by Xoo and wounding. To analyze the expression patterns of OsLRP in response to various stimuli, including Xoo infection, we carried out RT-PCR analysis (Fig. 1). OsLRP was induced by Xoo as previously reported (Zhou et al. 2009). In addition, OsLRP was up-regulated by biotic stimuli such as BTH, SA, and JA. OsLRP was also up-regulated by abiotic stress, such as salt, but not by ABA, suggesting that OsLRP may be involved in biotic stresses as well as abiotic stresses (Fig. 1).
Generation of transgenic Chinese cabbage lines
The simple eLRR domain proteins have been implicated in disease resistance. It is previously reported that over-expression of OsLRR1 confers enhanced resistance to pathogen in Arabidopsis (Zhou et al. 2009). To develop Chinese cabbage resistance to bacterial soft rot, OsLRP was introduced into Chinese cabbage. The plant expression vector (35S:OsLRP) for OsLRP was made using the pB2GW7vector (Fig. 2) (Hwang et al. 2011). OsLRP is located downstream of 35S promoter. The 35S-OsLRP construct was introduced into Chinese cabbage by Agrobacterium-mediated transformation. We obtained more than 10 independent regenerated plants. Finally, we obtained four independent transgenic lines by genomic PCR using bar-specific primers (data not shown). Two independent lines were further analyzed. To determine whether OsLRP is over-expressed in Chinese cabbage, we carried out RT-PCR. As shown in Fig. 2a, OsLRP was over-expressed in transgenic lines #13 and #40. OsLRP was not detected in non-transgenic controls. Transgenic plants over-expressing OsLRP showed normal phenotypes compared to non-transgenic controls (Fig. 2c).
Over-expression of OsLRP results in enhanced resistance to bacterial soft rot
Since over-expression of OsLRP resulted in enhanced resistance to Pseudomonas syringae pv. syringae in Arabidopsis, we tried to challenge T0 transgenic plants with the soft-rot pathogen, Pcc. We made wounds by toothpicks and then dropped 5 μl of a cell suspension (OD600 of 0.1). Photos were taken at 1 day post-inoculation (dpi) (Fig. 3a). Transgenic lines #27 and #40 did not show soft-watery symptoms around infection sites, whereas non-transgenic controls showed severe soft-rot symptoms around infection sites. Transgenic plants over-expressing OsLRP conferred enhanced resistance to bacterial soft rot. To further confirm whether transgenic plants over-expressing OsLRP shows resistance to bacterial soft rot, we challenged T1 transgenic plants with Pcc (103 cells/ml) by infiltration. Samples were taken at 0 and 3 dpi and bacterial numbers were counted. The black bar in Fig. 3b shows the number of bacterial cells in the samples taken at 0 dpi, while the gray bar shows the number of bacterial cells in the samples taken at 3 dpi. The number of bacteria in the transgenic lines over-expressing OsLRP was reduced approximately 20-fold compared to non-transgenic controls, indicating that OsLRP transgenic T1 plants showed enhanced resistance to bacterial soft rot, as did T0 plants. Taken together, OsLRP conferred reduction of the bacterial growth and symptom of bacterial soft rot.
Disease severity against Pcc in transgenic lines. a Wounds were generated on the midribs of the leaves of transgenic plants (T0), and then 5 μl bacterial suspension were dropped on wound. The photo was taken at 1 day after the inoculation, and arrows indicate the position of inoculation. b Transgenic plants were inoculated with Pcc by infiltration. Bacterial numbers were counted at the indicated times
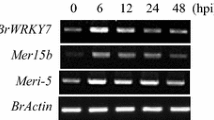
OsLRP activates defense response in Chinese cabbage
Chinese cabbage over-expressing OsLRP exhibits enhanced resistance to Pcc. To determine whether OsLRP activates defense response in Chinese cabbage, we carried out RT-PCR for defense-associated genes (Fig. 4). We obtained defense marker genes from B. rapa ssp. pekinensis from an EST collection of the B. rapa genomics team in our institute. Two glucanase genes were induced by Pcc (Kim and Hwang, unpublished results). As shown in Fig. 4, two different glucanase genes (MERI5B and Meri-5) and PDF1 were up-regulated in OsLRP transgenic lines #13 and #40. In addition, BrPGIP2 was also constitutively expressed in OsLRP transgenic lines (Fig. 4). It is previously known that BrPGIP2 is induced upon Pcc infection. Moreover, over-expression of BrPGIP2 exhibits enhanced resistance to bacterial soft rot (Hwang et al. 2010). These results proposed that OsLRP activates defense response in Chinese cabbage and confer enhanced resistance to bacterial soft rot. LeLRP and SLRR had been implicated in defense signaling (Tornero et al. 1996; Hipskind et al. 1996). It has been suggested that NtLRP and CaLRR1 are modulators of HIR1-mediated cell death. It has also been suggested that the OsLRR1 and OsHIR1 systems are well conserved in rice (Zhou et al. 2009). Heterologous expression of OsLRR1 in Arabidopsis was shown to confer enhanced resistance to P. syringae and activate defense responses (Zhou et al. 2009). Cell death is promoted in transgenic lines over-expressing OsLRR1. The authors proposed that OsLRR1 is also modulator of cell death (Zhou et al. 2009). Hence, we also checked whether cell death was promoted in transgenic Chinese cabbage overexpressing OsLRP. Athsr genes are known to be marker of hypersensitive cell death (Lacomme and Dominique 1999; Pandey et al. 2005). Athsr3 was constitutively expressed in transgenic Chinese cabbage over-expressing OsLRP, indicating that OsLRP also functions as a modulator of cell death in Chinese cabbage. According to the phylogenetic tree of LRR homologs in various plant species, OsLRP is very close to AtLRR1. We also found a Brassica homolog of LRR1 through a search of the National Center for Biotechnology Information (NCBI) database. OsLRP showed a 44 % similarity with putative BrLRR1 (GenBank accession number EU424347.1). Even though there are no reports on the BrLRR-BrHIR system, it appears to be conserved in Chinese cabbage. Heterologous expression of OsLRP enhances resistance to pathogens in Chinese cabbage and Arabidopsis, perhaps due to cell death regulation that results from the activation of defense response. To demonstrate whether the BrLRR1-BrHIR system is conserved in Chinese cabbage, study is required.
Expression analysis of defense marker genes in OsLRP transgenic plants. RT-PCR was carried out using OsLRP transgenic plants and non-transgenic controls. Specific primers were used for two glucanases (MERI5B and Meri-5), BrPGIP2, PDF1, Athsr1 shown in Table 1. BrActin is used as a control for the same amount of transcript in each sample
Bacterial soft rot caused by Pcc is a major disease of Chinese cabbage. Some genes that show enhanced resistance to Pcc have been previously reported (Liau et al. 2003; Jung et al. 2008; Vanjildorj et al. 2009; Ban et al. 2009; Hwang et al. 2010). For example, over-expression of the BAA1 gene (encoding bromelain) from pineapple was reported to show resistance to Pcc in Chinese cabbage (Jung et al. 2008). Another example is AiiA encoding N-acyl homoserine lactone-lactonase from Bacillus sp. GH02. Over-expression of AiiA showed enhanced resistance to Pcc (Vanjildorj et al. 2009). This is the first report that LRR homologs show enhanced resistance to the necrotrophic bacterial pathogen, Pcc. LRR homologs will be candidates for engineering plants with disease resistance.
Abbreviations
- OsLRP:
-
Oryza sativa leucine-rich repeat protein
- Pcc:
-
Pectobacterium carotovorum subsp. carotovorum
- HR:
-
Hypersensitive response
- HIR:
-
Hypersensitive-induced reaction
- Xoo:
-
Xanthomonas oryzae pv. oryzae
References
Ban H, Chai X, Lin Y, Zhou Y, Peng D, Zhou Yi, Zou Y, Yu Z, Sun M (2009) Transgenic Amorphophallus konjac expressing synthesized acyl-homoserine lactonase (aiiA) gene exhibit enhanced resistance to soft rot disease. Plant Cell Rep 28:1847–1855
Hipskind JD, Nicholson RL, Goldsbrough PB (1996) Isolation of a cDNA encoding a novelleucine-rich repeat motif from Sorghum bicolor inoculated with fungi. Mol Plant Microbe Interact 9(9):819–825
Hwang SH, Lee IA, Yie SW, Hwang DJ (2008) Identification of an OsPR10a promoter region responsive to salicylic acid. Planta 227(5):1141–1150
Hwang BH, Bae H, Lim HS, Kim K, Kim S, Im MH, Park BS, Kim D, Kim J (2010) Overexpression of polygalacturonase-inhibiting protein 2 (PGIP2) of Chinese cabbage (Brassica rapa ssp. pekinensis) increased resistance to the bacterial pathogen Pectobacterium carotovorum ssp. carotovorum. Plant Cell Tiss Organ Cult 103:293–305
Hwang SH, Yie SW, Hwang DJ (2011) Heterologous expression of OsWRKY6 gene in Arabidopsis activates the expression of defense related genes and enhances resistance to pathogens. Plant Sci 181:316–323
Jacques A, Ghannam A, Erhardt M, de Ruffray P, Baillieul F, Kauffmann S (2006) NtLRP1, a tobacco leucine-rich repeat gene with a possible role as a modulator of the hypersensitive response. MPMI 19(7):747–757
Ji GH, Wei LF, He YQ, Wu YP, Bai XH (2008) Biological control of rice bacterial blight by Lysobacter antibioticus strain 13–1. Biol Control 45(3):286–296
Jung HW, Hwang BK (2007) The leucine-rich repeat (LRR) protein, CaLRR1, interacts with the hypersensitive induced reaction (HIR) protein, CaHIR1, and suppresses cell death induced by the CaHIR1 protein. Mol Plant Pathol 8(4):503–514
Jung EH, Jung HW, Lee SC, Han SW, Heu S, Hwang BK (2004) Identification of a novel pathogen-induced gene encoding a leucine-rich repeat protein expressed in phloem cells of Capsicum annuum. Biochim Biophys Acta 1676(3):211–222
Jung YJ, Choi CS, Park JH, Kang HW, Choi JE, Nou IS, Lee SY (2008) Overexpression of the pineapple fruit bromelain gene (BAA) in transgenic Chinese cabbage (Brassica rapa) results in enhanced resistance to bacterial soft rot. Electron J Biotechnol 11:1–8
Lacomme C, Dominique R (1999) Identification of new early markers of the hypersensitive response in Arabidopsis thaliana. FEBS 459(1999):149–153
Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411:848–853
Lee IA (2005) Isolation and characterization of auxin-regulated gene (BcAXR1) from Chinese cabbage (Brassica campestris). Unpublished thesis (M.A.), The Catholic University of Korea
Liau CH, Lu JC, Prasad V, Hsiao H, You SJ, Lee J, Yang NS, Huang HE, Feng TY, Chen WH, Chan MT (2003) The sweet pepper ferredoxin-like protein (pflp) conferred resistance against soft rot disease in Oncidium orchid. Transgenic Res 12:329–336
Pandey AK, Ger MJ, Huang HE, Yip MK, Zeng J, Feng TY (2005) Expression of the hypersensitive response-assisting protein in Arabidopsis results in harpin-dependent hypersensitive cell death in response to Erwinia carotovora. Plant Mol Biol 59:771–780
Ren JP, Petzoldt R, Dickson MH (2001) Screening and identification of resistance to bacterial soft rot in Brassica rapa. Euphytica 118:271–280
Tornero P, Mayda E, Gómez MD, Cañas L, Conejero V, Vera P (1996) Characterization of LRP, a leucine-rich repeat (LRR) protein from tomato plants that is processed during pathogenesis. Plant J 10(2):315–330
van der Hoorn RA, Wulff BB, Rivas S, Durrant MC, van der Ploeg A, de Wit PJ, Jones JD (2005) Structure–function analysis of Cf-9, a receptor-like protein with extracytoplasmic leucine-rich repeats. Plant Cell 17(3):1000–1015
Vanjildorj E, Song SY, Yang ZH, Choi JE, Noh YS, Park S, Lim WJ, Cho KM, Yun HD, Lim YP (2009) Enhancement of tolerance to soft rot disease in the transgenic Chinese cabbage (Brassica rapa L. ssp. Pekinensis) inbred line Kenshin. Plant Cell Rep 28:1581–1591
Vlot AC, Klessig DF, Park SW (2008) Systemic acquired resistance: the elusive signal(s). Curr Opin Plant Biol 11:436–442
Zhou L, Cheung MY, Zhang Q, Lei CL, Zhang SH, Sun SS, Lam HM (2009) A novel simple extracellular leucine-rich repeat (eLRR) domain protein from rice (OsLRR1) enters the endosomal pathway and interacts with the hypersensitive-induced reaction protein 1 (OsHIR1). Plant Cell Environ 32(12):1804–1820
Zhou L, Cheung MY, Li MW, Fu Y, Sun Z, Sun SM, Lam HM (2010) Rice hypersensitive induced reaction protein 1 (OsHIR1) associates with plasma membrane and triggers hypersensitive cell death. BMC Plant Biol 10:290–299
Acknowledgments
This work was supported in part by two Grants (PJ007850 and PJ008574) from Rural Development Administration (RDA) to Duk-Ju Hwang.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. S. Shin.
Y.H. Park and C. Choi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, Y.H., Choi, C., Park, E.M. et al. Over-expression of rice leucine-rich repeat protein results in activation of defense response, thereby enhancing resistance to bacterial soft rot in Chinese cabbage. Plant Cell Rep 31, 1845–1850 (2012). https://doi.org/10.1007/s00299-012-1298-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1298-9