Abstract
Pectobacterium carotovorum and Pectobacterium aroidearum represent the primary pathogens causing variable soft rot disease. However, the fundamental defense responses of Pinellia ternata to pathogens remain unclear. Our investigation demonstrated that the disease produced by P. carotovorum is more serious than P. aroidearum. RNA-seq analysis indicated that many cell wall-related genes, receptor-like kinase genes, and resistance-related genes were induced by P. aroidearum and P. carotovorum similarly. But many different regulatory pathways exert a crucial function in plant immunity against P. aroidearum and P. carotovorum, including hormone signaling, whereas more auxin-responsive genes were responsive to P. carotovorum, while more ethylene and gibberellin-responsive genes were responsive to P. aroidearum. 12 GDSL esterase/lipase genes and 3 fasciclin-like arabinogalactan protein genes were specifically upregulated by P. carotovorum, whereas 11 receptor-like kinase genes and 8 disease resistance genes were up-regulated only by P. aroidearum. Among them, a lectin gene (part1transcript/39001) was induced by P. carotovorum and P. aroidearum simultaneously. Transient expression in N. benthamiana demonstrated that the lectin gene improves plant resistance to P. carotovorum. This study offers a comprehensive perspective on P. ternata immunity produced by different soft rot pathogens and reveals the importance of lectin in anti-soft rot of P. ternata for the first time.
Similar content being viewed by others
Background
Pinellia ternata (Thunb.) Breit (P. ternata), whose dry tuber is a traditional Chinese medicine (TCM) in Chinese Pharmacopoeia, has an important function in the treatment of cough, vomiting, infection, inflammation, early pregnancy, and tumors [1,2,3]. It has been employed as a TCM for millennia, traced back to the Han Dynasty in many notable prescriptions. With increased demand, P. ternata has been produced via artificial planting, causing continuous cropping barriers and frequent disease [4].
Among the frequent diseases identified, soft rot is one of the most destructive in P. ternata. Symptomatic plants have small water-soaked spots on leaves that progress into widespread translucent spots, spreading to the stems and tubers [5, 6]. Soft rot is responsible for significant agricultural losses in P. ternata. The pathogens responsible for soft rot in P. ternata were found to be Dickeya fangzhongdai (D. fangzhongdai), Pectobacterium carotovorum (P. carotovorum), Pectobacterium atrosepticum (P. atrosepticum), and Pectobacterium aroidearum (P. aroidearum) [6,7,8,9]. To limit the invasion of soft rot, increasing chemical fungicides were employed to protect P. ternata, leading to pesticide residues and environmental pollution. Therefore, it is essential to understand the defense responses of P. ternata to soft rot invasion to develop green, effective, and economical strategies to control soft rot and breed for disease resistance.
Plant immunity produced by microorganisms can be described by a “zigzag model” [10]. In the early stage of infection, pathogen-associated molecular patterns (PAMPs), recognized by pattern recognition receptor (PRRs) complexes at the plasma membrane, activate downstream immune regulation of the host plant, called PAMPs triggered immunity (PTI) [11]. Recognition of PAMPs leads to a series of signaling events, commonly referred to as the basal defense response, which activates the plant’s systemically acquired resistance [12]. In this process, mitogen-activated protein kinase (MAPK), receptor kinase, and phosphorylase are also activated, reactive oxygen species (ROS) are generated, accompanied by hormone biosynthesis and callose deposition [13]. However, when pathogens exhibit a tendency to adapt to the host plant, a series of virulence factors that interfere with the PTI of the plant, called effectors, contribute to pathogen infection. Meanwhile, a series of resistance proteins (R proteins) shows a predominant role in plant immunity. R proteins activate downstream immune regulation by recognizing pathogen effectors, limiting the infection and diffusion of pathogens [14]. The recognition of effectors is specific in effector-triggered immunity (ETI), which supports the hypothesis of “gene to gene” to defend different pathogens specifically.
Plant resistance to soft rot does not necessarily depend on a single resistance gene but activates defense systems mediated by SA, JA, and ethylene following recognition of damage-associated molecular patterns (DAMPs), including oligogalacturonide (OG) fragments [15]. PRRs identify the invasion of pathogens, and activate the hormone signal to promote the expression of downstream defense genes (CPK, CML, RBOH, MPK3, and MPK4) alongside the biosynthesis of indole-thioglucoside and other secondary metabolites, promoting plant resistance [16, 17]. Phenylalanine ammonia lyase-dependent and salicylic acid-mediated host resistance is the core of plant immunity [18]. Throughout this process, ferredoxin-like protein (pflp) is essential in conferring resistance against soft rot disease by accelerating the rapid production of H2O2, callose deposition, and hypersensitivity reaction [19,20,21]. Transcription factors also play important roles in plant resistance. Overexpression of WRKY12 increases the resistance to soft rot pathogen by transcriptional activation of defense-related genes [22, 23], but could also be a negative regulator of plant immunity against pathogens [24]. Transcription factors, plant hormones, MAPK signal transduction, and resistance-related genes form a comprehensive defense system in plants against soft rot disease.
Plant lectins are proteins with functions in immune regulation, bactericidal responses, and anti-inflammatory reactions, and can be separated into ten classes according to motif conformation, namely the Agaricus bisporus agglutinin (ABA), the Amaranthin domain, the chitinase-related agglutinin (CRA), the Cyanovirin domain, the Galanthus nivalis agglutinin (GNA), the Hevein domain, the Jacalin-related domain, the Legume lectin domain, the Lys M domain, and the Ricin-B domain [25]. Lectins bind to carbohydrates on their surface to damage the cell walls of pathogens, especially bacteria, fungi, and protozoa, preventing the microorganism from attaching to the host cell [26]. A unique mannose-binding plant lectin from Narcissus tazetta bulbs, NTL-125, is a potential antiviral compound of natural origin against SARS-CoV-2 [27]. A lectin extracted from the fish species Misgurnus anguillicaudatus possessed significant agglutinating activity against gram-negative bacteria, resulting from its relationship with lipopolysaccharides (LPS) [28]. Additionally, lectin could also interact with chitin to exert antifungal effects [29]. These mechanisms are common to most lectins and explain how these molecules inhibit the formation of biofilms and bacterial aggregates [30]. Jacalin-like lectins (JRLs) are involved in mediating broad-spectrum disease resistance to monocotyledonous plants by binding to oligosaccharide signatures characteristic of the infection process to relocate the protein towards the location of pathogen attack [31]. Lectin is an important feature of Araceae plants [32], belonging to mannose-binding modules in monocotyledonous plants, which can bind specifically to mannooligosaccharides [33]. However, there is limited research on lectins in P. ternata and their role in plant immunity and resistance to pathogens.
In this study, RNA-seq was employed to characterize candidate genes of P. ternata “Ying Shan” induced by virulent bacteria P. carotovorum QJ-1 and mild virulent bacteria P. aroidearum QS-1. Differentially expressed genes (DEGs) analysis and Gene Ontology (GO) analysis were conducted to identify key genes and core signal pathways in plant immunity. Quantitative real-time PCR (qRT-PCR) and transient expression were conducted to explore the induced expression patterns and potential biological function of key genes. This study provides integrated and basic data regarding the immunity of P. ternata induced by Pectobacterium, laying the foundation for further exploration of the P. ternata-Pectobacterium molecular interaction.
Results
Disease produced by P. carotovorum is more severe than by P. aroidearum
P. carotovorum and P. aroidearum are soft rot pathogens in P. ternata. In our prior work, P. carotovorum QJ-1 and P. aroidearum QS-1 were extracted from symptomatic plants and then configured as a bacterial suspension with a concentration of OD600 = 1.0. Infection of whole P. ternata “Yingshan” plants were conducted to examine their virulence. Water-soaked spots and beating down were observed at 48 hpi following treatment with P. aroidearum and P. carotovorum, respectively, compared to water treatment (Fig. 1A). Additionally, inoculation on detached leaves of P. ternata “Yingshan” was performed, and the disease spot was observed and calculated every 24 h. The results indicated that larger disease lesions were observed with exposure to P. carotovorum compared to P. aroidearum (Fig. 1B and C). It demonstrated that although both P. carotovorum QJ-1 and P. aroidearum QS-1 are pathogens producing soft rot, the pathogenicity of P. carotovorum QJ-1 is significantly higher than P. aroidearum QS-1.
Infection with P. aroidearum and P. carotovorum of P. ternata. (A) Whole plant of P. ternata infected with P. aroidearum and P. carotovorum for 48 h. (B) Detached leaves of P. ternata inoculated with P. aroidearum and P. carotovorum. Images were photographed at 24 hpi and 48 hpi under normal light. (C) Statistic of mean lesion diameter. The disease lesion diameter was measured every 24 h from at least 20 leaves with three replication (one-way ANOVA, **, p < 0.01). Error bars indicate SD. Pa means the infection with P. aroidearum, Pc means the infection with P. carotovorum
RNAseq and differentially expressed gene analysis
The virulence of P. carotovorum is stronger than P. aroidearum, motivating us to evaluate the similarities and differences in immunity response triggered by P. carotovorum and P. aroidearum. To investigate gene expression changes under the treatment of P. aroidearum and P. carotovorum, leaves from inoculated plants for 48 h were collected for RNA isolation and RNA-seq. In total, 269.69 million raw paired-end reads were acquired from 12 RNA samples. Following quality assessment and filtering, 264.4 million clean paired-end reads were retained (Table S1). All clean reads were mapped to the full-length transcript assembled in our previous work using bowtie2, and 21,464 transcripts were constructed in total. The expression of transcripts was computed using rsem. The FPKM value was calculated and shown in Table S1, and the correlation between the 12 samples was shown with a heatmap (Fig. S1). Nine genes were randomly selected for qRT-PCR to validate the expression (Fig. S2).
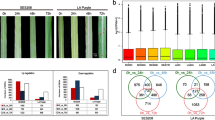
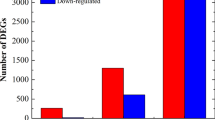
To investigate the differentially expressed genes triggered by P. aroidearum and P. carotovorum, DEGs were identified by EBSeq. A total of 1968 transcripts were upregulated and 570 transcripts were downregulated by P. aroidearum, while 1339 upregulated transcripts and 662 downregulated transcripts were triggered by P. carotovorum (Fig. 2A and B). Many common immunity responses were triggered by P. aroidearum and P. carotovorum, 628 transcripts were upregulated, and 262 transcripts were downregulated commonly (Fig. 2C). To clarify common immunity induced in the progress, the classification of common DEGs was shown in Fig. 2D and Table S2. Many cell wall-related genes were upregulated by the infection of pathogen to against the damage of cell wall, several receptor kinase genes and resistance-related genes were upregulated to defend against the invasion of pathogens.
The DEGs of P. ternata induced by P. aroidearum and P. carotovorum. (A) Volcano plot of the differentially expressed genes induced by P. aroidearum. (B) Volcano plot of the differentially expressed genes induced by P. carotovorum. The red spots mean up-regulated genes, blue spots mean down-regulated genes, black spots mean no-diff genes. Pa means P. aroidearum QS-1 infection and Pc means P. carotovorum QJ-1 infection. (C) Venn graph of DEGs inoculated by P. aroidearum and P. carotovorum. (D) The classification of common DEGs induced by P. aroidearum and P. carotovorum. Pa means P. aroidearum QS-1and Pc means P. carotovorum QJ-1. Common up means common up-regulated genes induced by P. aroidearum and P. carotovorum. Common down/up means common down/up-regulated genes induced by P. aroidearum and P. carotovorum
GO and KEGG analysis of the DEGs triggered by P. aroidearum and P. carotovorum
To assess the common signal pathways regulated by soft rot, GO analysis of 628 common DEGs was conducted (Table S3). Genes involved in cellulose biosynthetic (GO:0030244) and fatty acid biosynthetic (GO:0006633) processes were induced by pathogen infection, and genes associated with channel activity (GO:0015267), transferase activity, transferring acyl groups other than amino-acyl groups (GO:0016747), and cellulose synthase (UDP-forming) activity (GO:0016760) were activated to enhance plant immunity. Moreover, genes associated with membranes (GO:0031225), apoplasts (GO:0048046), and cell walls (GO:0005618) were also highly expressed to defend the pathogen invasion (Fig. 3A). In contrast, Rho guanyl-nucleotide exchange factor activity (GO:0005089), catalase activity (GO:0004096), and oxidoreductase activity, acting on NAD(P)H (GO:0016651) were suppressed under the perception of pathogens (Fig. 3B). These findings demonstrated that various different types of genes and related signaling pathways constitute the basic immune defense of P. ternata to soft rot, genes involved in cell wall, membrane, and oxidoreductase functions are the core of plant immunity.
GO analysis was also conducted on specific DEGs induced by P. carotovorum and P. aroidearum (Fig. S3). The findings indicated that genes involved in hydrolase, hydrolyzing O-glycosyl compounds, xyloglucan: xyloglucosyl transferase activity, and cell wall were upregulated, and genes involved in enzyme inhibitor activity and translation initiation factor activity were downregulated with exposure to P. carotovorum. Hydrolase and xyloglucan: xyloglucosyl transferase plays an essential role in the degradation of the cell walls. Large watery lesions appear on the leaves of P. ternata at 48 hpi, the cells of which are collapsed, releasing enzymes to degrade the cell walls of P. ternata. Additionally, genes involved in fatty acid beta-oxidation, calcium-dependent phospholipid binding, and catalase activity were upregulated upon treatment with P. aroidearum, while genes involved in carbohydrate metabolic process, and integral components of membranes were downregulated. These results suggested that many defense pathways of P. ternata were induced by P. aroidearum to resist pathogen infection, and genes related to the membrane were inhibited by P. aroidearum to facilitate destruction.
KEGG analysis was also conducted to explore the secondary metabolites biosynthesis, the result found that genes involved in ko01130 (Biosynthesis of antibiotics) were significantly induced by P. aroidearum. Genes involved in ko00940 (phenylpropanoid biosynthesis), ko00941 (Flavonoid biosynthesis), ko00945 (Stilbenoid, diarylheptanoid and gingerol biosynthesis), were significantly induced by P. carotovorum (Table S4).
Differential signally pathways were induced by P. aroidearum and P. carotovorum
In addition to these common defense genes, 398 genes were downregulated and 707genes were upregulated by P. carotovorum, while 1338 upregulated genes and 304 downregulated genes were induced by P. aroidearum (Fig. 2C and Table S5). Plant hormones emerged as cellular signaling molecules with crucial functions in regulating immune responses to microbial pathogens. In our study, different hormone signaling was induced by P. aroidearum and P. carotovorum. Eight auxin-responsive proteins were upregulated significantly by P. carotovorum, indicating that auxin-mediated immunity plays an important role in defending against P. carotovorum. Additionally, five ethylene-responsive genes and four gibberellin-responsive genes were specifically upregulated by P. aroidearum (Fig. 4A and Table S5), and qRT-PCR was conducted to verified the expression (Fig. S4).
In addition to hormone signaling, many other specific immune signals were induced by P. carotovorum and P. aroidearum. A total of 12 GDSL esterase/lipase genes and three fasciclin-like arabinogalactan protein genes were upregulated by exposure to P. carotovorum, whereas 11 receptor-like kinases (RLKs), and eight disease resistance genes were upregulated only by P. aroidearum (Fig. 4B). This finding indicated that different degrees of disease occurred under the infection by two pathogens due to the expression of different genes.
Heatmap of genes involved in different pathway induced by P. aroidearum and P. carotovorum. (A) Heatmap of genes involved in different hormone signaling pathway. (B) Heatmap of key genes involved in other specific resistance signaling pathway. FPKM value was used for the heatmaps, and Z-score normalization method was applied
34 lectin genes differentially expressed in P. ternata induced by P. carotovorum and P. aroidearum
Lectin is an important ingredient in P. ternata, and it inhibits bacteria and kills insects. In our study, 34 lectin genes were differentially expressed in response to the invasion of pathogens. The structure of the 34 lectin proteins was analyzed, demonstrating that these lectin genes were primarily separated into three classes: class I, which consists of one B-lectin domain; class II, which consists of two similar B-lectin domains; class III, which consists of two different B-lectin domains (Fig. 5). We also found that most lectin genes were highly expressed upon P. aroidearum infection, which may be due to the participation of lectins in plant immunity against pathogen infection, while half of them were suppressed under infection by P. carotovorum (Fig. 5), resulting from the damage of plant tissues, causing a decrease in lectin content.
Lectin gene inhibited the infection of P. carotovorum
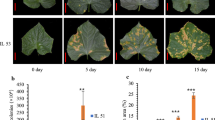
A lectin gene (part1transcript/39001) was chosen for functional verification, which is up-regulated by P. aroidearum and P. carotovorum (Fig. 6A). Agrobacterium‑mediated transient expression of the lectin gene was conducted in N. benthamiana leaves, with the empty vector (EV) expressing GFP protein used as a control. After 48 h of agro-infiltration, the lectin gene and empty vector were expressed in N. benthamiana leaves at 48 hpi (Fig. 6B). The leaves were detached and inoculated with P. carotovorum QJ-1 at a concentration of OD600 = 1. The disease lesion diameter was evaluated at 48 hpi. The findings showed that transient expression of the lectin gene significantly inhibited P. carotovorum colonization, reflected by smaller lesion diameters compared to the EV control (Fig. 6C and D). The above results demonstrated that the lectin gene positively regulates plant defense response against soft rot.
Transient expressing the lectin gene to verify their function in regulation of soft rot resistance. (A) qRT-PCR verified that the lectin gene was higher expressed with P. aroidearum and P. carotovorum induction. Gene expression levels were analyzed by the 2−△△CT method with 18S as the reference gene. One-way ANOVA, **, p < 0.01. Error bars represent mean ± SD of 3 biological replicates. (B) Lectin protein and GFP protein expressed in N. benthamiana leaves at 48 hpi, the leaf photos were taken under UV-light. (C) The infection with P. carotovorum QJ-1 on leaves transiently expressed the lectin gene and EV for 48 h, the leaf photos were taken at 48 hpi. (D) Disease lesion on N. benthamiana leaves was measured 2 d after P. carotovorum QJ-1 inoculation, one-way ANOVA, *, p < 0.05, 3 biological replicates with 20 leaves from 6–7 plants at least for each replicate. Error bars represent mean ± SD. Pa means P. aroidearum QS-1 and Pc means P. carotovorum QJ-1
Discussion
Soft rot is a critical disease in P. ternata production, which is primarily caused by P. carotovorum and P. aroidearum. However, different virulence is exerted by these two pathogens, which has been confirmed in our study (Fig. 1). Comparative genomics determined that P. carotovorum encodes more plant cell wall degrading enzymes and effectors than P. atrosepticum, including hrpK-like type III secretion system-dependent effector protein, resulting in the stronger pathogenicity of P. carotovorum [34]. The same phenomenon appears in potatoes, with more damage caused by P. carotovorum than by P. atrosepticum [35]. One of the most important pathogenic factors are exoenzymes, including pectate lyase (pel), polygalacturonase (PG), protease (Prt), and cellulase (cel), that adhere to and destroy plant cell walls by degrading pectin, resulting in the water stain presentation [36].
To investigate the basic and variable immunity of P. ternata induced by P. carotovorum and P. aroidearum, RNA-seq was performed in this study. It found that genes involved in the regulation of cell walls, receptor-like kinase genes, and resistance-related genes were induced by both pathogens, forming the basic defense system of plants. Receptor-like kinase genes are elements of a critical signaling pathway for plant immunity (Table S1). For instance, BAK1, belonging to the LRR receptor-like protein kinase family, interacts with BRI1 to modulate brassinosteroid signaling [37]. FLS2 is an LRR receptor-like protein kinase that senses bacterial flagellin to activate plant immunity [38]. EDS1-PAD4-ADR1, a kind of LRR receptor kinase, mediates pattern-triggered immunity in Arabidopsis [39]. OsLRP, a leucine-rich repeat (eLRR) domain protein, has been introduced into Chinese cabbage, exhibiting enhanced disease resistance to bacterial soft rot [40]. Different classes of genes, such as WRKY transcription factors, receptor-like protein kinase, LRR domain proteins, and genes involved in the MAPK pathway, form the basis of plant immunity against soft rot. In this study, 219 R genes expressed in our study, and most of them have the same expression pattern (Table S1). 8 R genes were upregulated only by P. aroidearum (Table S5). And 64 WRKY genes were identified in RNAseq data (Table S1). WRKY40 and WRKY76 were induced by P. carotovorum. WRKY50, WRKY75, WRKY24-like were induced by P. aroidearum (Table S5).
Plant hormones have critical roles in the regulation of plant growth and development. They also have key functions in the regulation of immune responses to microbial pathogens [41]. Li et al. have demonstrated that MeJA could display improved performance in enhancing the resistance to disease in kiwifruit by regulating the phenylpropanoid and jasmonate pathways [42]. Jasmonate regulates plant resistance to P. brasiliense and D. dadantii by regulating indole glucosinolate biosynthesis [17, 43]. Abscisic acid deficiency causes rapid activation of tomato defense responses upon infection with Erwinia chrysanthemi [44]. N-3-Oxo-Octanoyl Homoserine Lactone primes plant resistance against the necrotrophic pathogen P. carotovorum by coordinating jasmonic acid and auxin-signaling pathways [45]. In our study, many genes responsive to plant hormones were substantially induced. Notably, some differences appeared in the hormone signaling induced by P. aroidearum and P. carotovorum. Elevated amounts of auxin-responsive protein genes were induced by P. carotovorum, and more ethylene and gibberellin-responsive protein genes were induced by P. aroidearum (Table S1). The conclusion that the auxin-signaling pathway figures prominently in plant resistance to P. carotovorum mirrors previous works [16, 46]. In addition, several studies suggest that ethylene and gibberellin pathways play a necessary role in plant immunity against other Pectobacterium species. Narváez-Barragán et al. have demonstrated that Expansin-like Exl1 from P. resilience and P. atrosepticum is a virulence factor, producing a plant immunity response in ROS, and jasmonate, ethylene, and salicylic acid signaling pathways in Arabidopsis thaliana [47]. ERF96 is a key player in the ERF network that positively regulates Arabidopsis resistance responses to necrotrophic pathogens [48]. GLIP1 functions independently of salicylic acid but requires ethylene signaling [49]. Potato gibberellin stimulated-like 2 (GSL2) gene in transgenic potatoes enhances resistance to blackleg disease produced by P. atrosepticum [50]. Meanwhile, many genes in JA and SA signalling pathways were identified, part1transcript/44,968 (23 kDa jasmonate-induced protein) was expressed higher under the infection of P. atrosepticum. But part2transcript/15,083, part2transcript/15,903 (jasmonic acid-amido synthetase JAR1-like), part2transcript/34,625 (jasmonate O-methyltransferase), part1transcript/44,416 (salicylic acid-binding protein 2-like) were identified without significantly difference.
GDSL esterase/lipase and asciclin-like arabinogalactan (FLA) genes, contributing to plant growth, were induced by P. carotovorum specifically to repair the severe damage to plants. Receptor-like kinases and disease-resistance genes, promoting plant immunity against pathogens, were induced by P. aroidearum specifically to avoid the further invasion of P. aroidearum, resulting in smaller spots on leaves (Fig. 1B). Many studies have confirmed that GDSL esterase/lipase (GELP) modulates plant immunity through lipid homeostasis by fatty acid degradation [51, 52]. Meanwhile, FLA genes, specifically situated in sclerenchyma cells [53], were required for stem development [54]. These genes were induced by P. carotovorum to defend against the pathogen infection by lipid homeostasis and stem development. RLKs play a central role in signaling during pathogen recognition, subsequent activation of plant defense mechanisms, and developmental control [55]. Disease-resistance protein genes are necessary for plants to avoid further infection by pathogens, playing a crucial role in plant immunity.
Lectins are fundamental to plant life and have necessary roles in cell-to-cell communication, constituting versatile recognition systems at the cell surface and contributing to the detection of symbionts and pathogens [56]. In this study, a lectin gene was chosen from common DEGs to validate its defensive function with P. carotovorum treatment. RNA-seq and qRT-PCR demonstrated that the lectin gene is highly expressed upon treatment with P. aroidearum and P. carotovorum (Fig. 6A). Functional verification indicated that the transient expression of the lectin gene in N. benthamiana decreases the lesion damage by P. carotovorum, demonstrating that the lectin gene elevates the immune response against P. carotovorum (Fig. 6C and D). Furthermore, a down regulated lectin gene (part1transcript25085) was also selected to verify the function, and the same result was obtained (Fig. S5). Expression of the Pinellia pedatisecta lectin gene in transgenic wheat enhances resistance to wheat aphids [57]. Rice, wheat, and barley plants overexpressing OsJAC1, a member of Jacalin-like lectins, are resistant to several fungal pathogens [58]. These results demonstrate that the lectin genes benefit plant immunity against P. aroidearum and P. carotovorum, providing guidance and promising practices to unravel the molecular foundation of plant immunity.
Conclusion
Soft rot is a devastating disease in P. ternata and other plants, with a great impact on their yield and quality. The soft rot pathogens in P. ternata included P. aroidearum and P. carotovorum, causing varying degrees of disease. RNA-seq analysis of P. ternata following exposure to each pathogen showed that they could cause the differential expression of a large number of cell wall membrane-related genes, transport-related genes, MAPK pathway-related genes, and hormone response genes, and these regulatory networks formed the broad-spectrum immune mechanism of P. ternata in response to bacterial diseases. However, many functional genes are specifically regulated by P. aroidearum and P. carotovorum. For instance, more auxin-responsive genes and growth-related genes are induced by P. carotovorum, while more ethylene-responsive genes, gibberellin-responsive genes, and disease-resistance-related genes are regulated by P. aroidearum, which may be responsible for the variable degrees of soft rot in P. ternata. Lectins are a class of important proteins in P. ternata. The transient expression of the lectin gene indicated that the lectin protein could enhance plant resistance to soft rot. In this study, the role of lectins in plant resistance to bacterial diseases was investigated for the first time. Future studies will focus on the molecular mechanisms involved in how lectin gene regulate host immunity to promote the green prevention and control of P. ternata production.
Materials and methods
Plant and pathogen material
P. ternata “Yingshan” specimens were collected on April 25, 2022, at Hubei University of Chinese Medicine and authenticated by Prof. Liu Dahui (voucher No. Yingshan202204), and stored in the medical plants garden of Hubei University of Chinese Medicine. Tissue culture seedlings were acclimated for 15 days in floating dishes, then transferred to pots and grown under controlled conditions (24 °C; 12 h of light/12 hours of dark; 60% humidity) for 30 days.
P. carotovorum QJ-1 and P. aroidearum QS-1 were previously isolated and identified [9], and stored at -80 ℃.
The N. benthamiana seeds used in this work were preserved in our laboratory in Hubei University of Chinese Medicine. The seeds of Nicotiana benthamiana were grown under standard conditions (24 °C; 12 h light/12 h dark photoperiod; and 60% relative humidity) in a chamber for approximately 4 weeks for transient expression and pathogen inoculation.
The extraction of RNA and inverse transcription
Total RNA was extracted using TRIzol reagent and quality-assessed using an RNA Nano 6000 Assay Kit (Bioanalyzer 2100, Agilent Technologies, CA, USA). Reverse transcription was performed using Moloney Murine Leukemia Virus Reverse Transcriptase (MMLV RT; Promega, USA).
Library preparation and transcriptome sequencing
mRNA was purified from total RNA using poly-T oligo-attached magnetic beads and fragmented using divalent cations under elevated temperatures in First Strand Synthesis Reaction Buffer (5×). cDNA fragments 370 to 420 bp in length were selected using the AMPure XP approach (Beckman Coulter, Beverly, USA). Libraries were PCR-amplified, purified using AMPure XP beads, pooled according to their concentration, and sequenced on an Illumina NovaSeq 6000 (150-bp paired-end reads).
Raw reads in fastq format were quality-checked using FastQC and trimmed using Trimmomatic [59]. Clean reads were obtained by discarding reads containing adapters, N bases, and low-quality reads. Simultaneously, Q20, Q30, and GC content of the clean data were calculated. All downstream analyses were based on clean, high-quality data.
Differentially expressed gene (DEG) analysis and enrichment analysis
Paired-end clean reads were mapped to the full-length transcript (PRJNA893095) from our previous work using bowtie2 with a 1% mismatch [60]. Calculations and differentialy expression analysis (FDR < 0.05 and the |log2 (fold change)| > 1) were performed with rsem [61]. The GO and KEGG analysis were conducted using the OmicShare tools, a free online platform for data analysis (http://www.omicshare.com/ tools).
PCR and qRT‑PCR
Standard PCR was performed using 2 x Es Taq MasterMix (CWBIO, China). For qRT-PCR, cDNA was synthesized using reverse transcriptase M-MLV (RNase H-) (Code No.: 2641 A) from TaKaRa with 2 µg of total RNA in the 20 µL reaction for reverse transcription. qRT-PCR was conducted using Gloria Nova HS 2X Master Mix (RK20717; ABclonal) on a QuantStudio 12 K Flex Real-Time PCR system. A total of 10 µL of the mixture was used for qRT-PCR, encompassing 5 µL of 2 × ChamQ SYBR qPCR Master Mix, 1 µL of cDNA, 0.5 µL of forward primer, 0.5 µL of reverse primer, and 3 µL of H2O. The annealing temperature was 60 °C with a total of 35 amplification cycles in triplicate for each sample. The expression level of each gene was computed using the 2−ΔΔCt approach using 18S as an internal reference gene [62], Primers for this analysis (Table S6) were designed using NCBI primer designing tools.
Plasmid constructs for transient expression
The full-length CDS of the lectin gene from P. ternata “Yingshan” cDNA was cloned using specific primers with recombinant adaptors (Table S6) according to sequences deposited in NCBI. An overexpression plasmid, pH7Lic-GFP, driven by the 35S promoter (Fig. S6), underwent digestion with StuI, and the full-length CDS with adaptors was inserted into the vector with SE recombinase. The constructs were verified by sequencing. The empty vector with GFP was used as a negative control.
Agrobacterium-mediated transient gene expression assays
Constructs were transformed into the Agrobacterium strain GV3101. A sample of 1 µg of plasmid mixture was added to 50 µL GV3101, mixed and placed on ice for 10 min, before being flash-frozen in liquid nitrogen for 5 min. The mixtures were placed in a water bath at 37 ℃ for 5 min and placed on ice again for 5 min. This mixture was then added to 500 µL of liquid LB medium and cultured at 28 ℃ for 2 to 3 h. The liquid was evenly applied to a solid LB agar medium. The plates were cultured on inverted plates at 28 ℃ for 2 to 3 days. Colonies were selected from plates and inoculated in liquid YEB overnight at 28 °C while being shaken. Agrobacterium cultures were centrifuged at 4000 rpm for 10 min, and the pellet was resuspended in 10 mM MES and 10 mM MgCl2 buffer. The OD600 was adjusted to 0.2 for agroinfiltration, with acetosyringone being added at a concentration of 200 mM. N. benthamiana leaves were infiltrated using a 1 mL syringe following wounding with a needle. The agroinfiltration approach followed the procedure outlined by Luo et al. [63].
Pathogen inoculation
P. carotovorum QJ-1 and P. aroidearum QS-1 were utilized for inoculation to infect Pinellia ternata “Yingshan” plants, detached leaves, and N. benthamiana leaves. Pathogens were cultured on LB solid medium, then grown in LB liquid medium, centrifuged at 4000 rpm for 10 min, and resuspended with ddH2O to OD600 = 1.0. “Yingshan” plants were sprayed with the P. carotovorum and P. aroidearum solutions for 0 h and 48 h. The detached leaves of P. ternata were inoculated with the P. carotovorum and P. aroidearum solutions using 10 µL droplets pipetted onto the surfaces of detached leaves, which were maintained in sealed boxes with moist tissue paper. Boxes were kept in the dark for the first 24 h before being transferred to normal light conditions. Lesion diameters were assessed every 24 h. When used for infection after transient expression, P. carotovorum was inoculated 48 h after infiltration with Agrobacterium suspension to transiently express selected genes. Each group underwent triplicate experimentation, with leaves flash-frozen in liquid nitrogen for total RNA extraction.
Statistical analysis
One-way ANOVA was employed to analyze statistical significance using GraphPad Prism 8.0 software (GraphPad Prism Software Inc.). The lesion-size calculation was represented by the mean ± SD from three independent experiments, while the qRT-PCR results are shown as the mean ± SD from three biological replicates.
Data availability
The datasets supporting the conclusions of this article are included within the article (and its supplemental files). The Illumina sequence data generated during the current study are accessible through BioProject accession number PRJNA932221. The sequence of the lectin gene (part1transcript/39001) is accessible through Genebank (OR394647).
References
Kong X, Yang M, Abbas HMK, Wu J, Li M, Dong W. Antimicrobial genes from Allium sativum and Pinellia ternata revealed by a Bacillus subtilis expression system. Sci Rep. 2018;8(1):14514. https://doi.org/10.1038/s41598-018-32852-x.
Zhang ZH, Zhao YY, Cheng XL, Lin RC, Dai Z, Zhou C. Metabonomic study of biochemical changes in the rat urine induced by Pinellia ternata (Thunb.) Berit. J Pharm Biomed Anal. 2013;85:186–93. https://doi.org/10.1016/j.jpba.2013.07.026.
Lee MY, Shin IS, Jeon WY, Lim HS, Kim JH, Ha H. Pinellia ternata Breitenbach attenuates ovalbumin-induced allergic airway inflammation and mucus secretion in a murine model of asthma. Immunopharmacol Immunotoxicol. 2013;35(3):410–8. https://doi.org/10.3109/08923973.2013.770522.
Mao R, He Z. Pinellia ternata (Thunb.) Breit: a review of its germplasm resources, genetic diversity and active components. J Ethnopharmacol. 2020;263:113252. https://doi.org/10.1016/j.jep.2020.113252.
Hu XF, Ying FX, He YB, et al. Characterization of Pectobacterium carotovorum subsp. carotovorum causing soft-rot disease on Pinellia ternata in China. Eur J Plant Pathol. 2008;120:305–10. https://doi.org/10.1007/s10658-007-9219-4.
Wang F, Tang T, Mao T, Guo J, Guo X, Duan Y, You J. Occurrence of Dickeya Fangzhongdai Causing Soft Rot of Banxia (Pinellia ternata) in China. Plant Dis. 2021. https://doi.org/10.1094/PDIS-01-21-0030-PDN.
Ying FX, Hu XF, Chen JS. First Report of Soft Rot caused by Pectobacterium carotovorum on Pinellia ternata in China. Plant Dis. 2007;91(10):1359. https://doi.org/10.1094/PDIS-91-10-1359C.
Agyemang PA, Kabir MN, Kersey CM, Dumenyo CK. The bacterial soft rot pathogens, Pectobacterium carotovorum and P. Atrosepticum, respond to different classes of virulence-inducing host chemical signals. Horticulturae. 2020;6(1):13. https://doi.org/10.3390/horticulturae6010013.
Xu JW, Luo M, Xu R, Wang MX, Ma CJ, Miao YH, Dahui Liu. Isolation, identification and comparative study of soft rot pathogens of Pinellia ternata in China. Southwest China J Agricultural Sci. 2023;36:1950–61. https://doi.org/10.16213/j.cnki.scjas.2023.9.015.
Jones JD, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–9. https://doi.org/10.1038/nature05286.
Zhang J, Zhou JM. Plant immunity triggered by microbial molecular signatures. Mol Plant. 2010;3(5):783–93. https://doi.org/10.1093/mp/ssq035.
Nicaise V, Roux M, Zipfel C. Recent advances in PAMP-triggered immunity against bacteria: pattern recognition receptors watch over and raise the alarm. 2009;150(4):1638–47. https://doi.org/10.1104/pp.109.139709
Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol. 2011;14(1):54–61. https://doi.org/10.1016/j.mib.2010.12.005.
Luderer R, Joosten MH. Avirulence proteins of plant pathogens: determinants of victory and defeat. Mol Plant Pathol. 2001;2(6):355–64. https://doi.org/10.1046/j.1464-6722.2001.00086.x.
Davidsson PR, Kariola T, Niemi O, Palva ET. Pathogenicity of and plant immunity to soft rot pectobacteria. Front Plant Sci. 2013;4:191. https://doi.org/10.3389/fpls.2013.00191.
Liu M, Wu F, Wang S, Lu Y, Chen X, Wang Y, Gu A, Zhao J, Shen S. Comparative transcriptome analysis reveals defense responses against soft rot in Chinese cabbage. Hortic Res. 2019;6:68. https://doi.org/10.1038/s41438-019-0149-z.
Yi SY, Lee M, Park SK, Lu L, Lee G, Kim SG, Kang SY, Lim YP. Jasmonate regulates plant resistance to Pectobacterium brasiliense by inducing indole glucosinolate biosynthesis. Front Plant Sci. 2022;13:964092. https://doi.org/10.3389/fpls.2022.964092.
Augustine L, Varghese L, Kappachery S, Ramaswami VM, Surendrababu SP, Sakuntala M, Thomas G. Comparative analyses reveal a phenylalanine ammonia lyase dependent and salicylic acid mediated host resistance in Zingiber zerumbet against the necrotrophic soft rot pathogen Pythium myriotylum. Plant Sci. 2024;340:111972. https://doi.org/10.1016/j.plantsci.2023.111972.
Hong CY, Zheng JL, Chen TY, Chao HR, Lin YH. PFLP-Intensified Disease Resistance Against Bacterial Soft rot through the MAPK pathway in PAMP-Triggered immunity. Phytopathology. 2018;108(12):1467–74. https://doi.org/10.1094/PHYTO-03-18-0100-R.
Liau CH, Lu JC, Prasad V, Hsiao HH, You SJ, Lee JT, Yang NS, Huang HE, Feng TY, Chen WH, Chan MT. The sweet pepper ferredoxin-like protein (pflp) conferred resistance against soft rot disease in Oncidium orchid. Transgenic Res. 2003;12(3):329–36. https://doi.org/10.1023/a:1023343620729.
Yip MK, Huang HE, Ger MJ, Chiu SH, Tsai YC, Lin CI, Feng TY. Production of soft rot resistant calla lily by expressing a ferredoxin-like protein gene (pflp) in transgenic plants. Plant Cell Rep. 2007;26(4):449–57. https://doi.org/10.1007/s00299-006-0246-y.
Yan J, Yu X, Ma W, Sun X, Ge Y, Yue X, Han J, Zhao J, Lu Y, Liu M. Genome-wide identification and expression analysis of WRKY family genes under soft rot in Chinese cabbage. Front Genet. 2022;13:958769. https://doi.org/10.3389/fgene.2022.958769.
Kim HS, Park YH, Nam H, Lee YM, Song K, Choi C, Ahn I, Park SR, Lee YH, Hwang DJ. Overexpression of the Brassica rapa transcription factor WRKY12 results in reduced soft rot symptoms caused by Pectobacterium carotovorum in Arabidopsis and Chinese Cabbage. Plant Biol (Stuttg). 2014;16(5):973–81. https://doi.org/10.1111/plb.12149.
Wang X, Basnayake BM, Zhang H, Li G, Li W, Virk N, Mengiste T, Song F. The Arabidopsis ATAF1, a NAC transcription factor, is a negative regulator of defense responses against necrotrophic fungal and bacterial pathogens. Mol Plant Microbe Interact. 2009;22(10):1227–38. https://doi.org/10.1094/MPMI-22-10-1227.
Bonnardel F, Perez S, Lisacek F, Imberty A. Structural database for lectins and the UniLectin web platform. Methods Mol Biol. 2020;2132:1–14. https://doi.org/10.1007/978-1-0716-0430-4_1.
Fonseca VJA, Braga AL, Filho JR, Teixeira CS, da Hora GCA, Morais-Braga MFB. A review on the antimicrobial properties of lectins. Int J Biol Macromol. 2022;195:163–78. https://doi.org/10.1016/j.ijbiomac.2021.11.209.
Sarkar A, Paul S, Singh C, Chowdhury N, Nag P, Das S, Kumar S, Sharma A, Das DK, Dutta D, Thakur KG, Bagchi A, Shriti S, Das KP, Ringe RP, Das S. A novel plant lectin, NTL-125, interferes with SARS-CoV-2 interaction with hACE2. Virus Res. 2022;315:198768. https://doi.org/10.1016/j.virusres.2022.198768.
Zhang XW, Yang CH, Zhang HQ, Pan XT, Jin ZY, Zhang HW, Xia XH. A C-type lectin with antibacterial activity in weather loach, Misgurnus anguillicaudatus. J Fish Dis. 2020;43(12):1531–9. https://doi.org/10.1111/jfd.13255.
Pinheiro AQ, Melo DF, Macedo LM, Freire MG, Rocha MF, Sidrim JJ, Brilhante RS, Teixeira EH, Campello CC, Pinheiro DC, Lima MG. Antifungal and marker effects of Talisia esculenta lectin on Microsporum canis in vitro. J Appl Microbiol. 2009;107(6):2063–9. https://doi.org/10.1111/j.1365-2672.2009.04387.x.
Carneiro RF, Torres RC, Chaves RP, de Vasconcelos MA, de Sousa BL, Goveia AC, Arruda FV, Matos MN, Matthews-Cascon H, Freire VN, Teixeira EH, Nagano CS, Sampaio AH, Purification. Biochemical characterization, and amino acid sequence of a novel type of lectin from Aplysia dactylomela Eggs with Antibacterial/Antibiofilm potential. Mar Biotechnol (NY). 2017;19(1):49–64. https://doi.org/10.1007/s10126-017-9728-x.
Weidenbach D, Esch L, Möller C, Hensel G, Kumlehn J, Höfle C, Hückelhoven R, Schaffrath U. Polarized defense against fungal pathogens is mediated by the Jacalin-related lectin domain of Modular Poaceae-Specific proteins. Mol Plant. 2016;9(4):514–27. https://doi.org/10.1016/j.molp.2015.12.009.
Van Damme EJ, Goossens K, Smeets K, Van Leuven F, Verhaert P, Peumans WJ. The major tuber storage protein of araceae species is a lectin. Characterization and molecular cloning of the lectin from Arum maculatum L. Plant Physiol. 1995;107(4):1147–58. https://doi.org/10.1104/pp.107.4.1147.
Barre A, Bourne Y, Van Damme EJ, Peumans WJ, Rougé P. Mannose-binding plant lectins: different structural scaffolds for a common sugar-recognition process. Biochimie. 2001;83(7):645–51. https://doi.org/10.1016/s0300-9084(01)01315-3.
Glasner JD, Marquez-Villavicencio M, Kim HS, Jahn CE, Ma B, Biehl BS, Rissman AI, Mole B, Yi X, Yang CH, Dangl JL, Grant SR, Perna NT, Charkowski AO. Niche-specificity and the variable fraction of the Pectobacterium pan-genome. Mol Plant Microbe Interact. 2008;21(12):1549–60. https://doi.org/10.1094/MPMI-21-12-1549.
Marquez- Villavicencio MDP, Groves RL, Charkowski AO. Soft rot Disease Severity is affected by Potato Physiology and Pectobacterium taxa. Plant Dis. 2011;95(3):232–41. https://doi.org/10.1094/PDIS-07-10-0526.
Barras F, Gijsegem FV, Chatterjee AK. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu Rev Phytopathol. 1994;32:201–34. https://doi.org/10.1146/annurev.py.32.090194.001221.
Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110(2):213–22. https://doi.org/10.1016/s0092-8674(02)00812-7.
Gómez-Gómez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5(6):1003–11. https://doi.org/10.1016/s1097-2765(00)80265-8.
Pruitt RN, Locci F, Wanke F, Zhang L, Saile SC, Joe A, Karelina D, Hua C, Fröhlich K, Wan WL, Hu M, Rao S, Stolze SC, Harzen A, Gust AA, Harter K, Joosten MHAJ, Thomma BPHJ, Zhou JM, Dangl JL, Weigel D, Nakagami H, Oecking C, Kasmi FE, Parker JE, Nürnberger T. The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature. 2021;598(7881):495–9. https://doi.org/10.1038/s41586-021-03829-0.
Park YH, Choi C, Park EM, Kim HS, Park HJ, Bae SC, Ahn I, Kim MG, Park SR, Hwang DJ. Over-expression of rice leucine-rich repeat protein results in activation of defense response, thereby enhancing resistance to bacterial soft rot in Chinese cabbage. Plant Cell Rep. 2012;31(10):1845–50. https://doi.org/10.1007/s00299-012-1298-9.
Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. https://doi.org/10.1146/annurev-cellbio-092910-154055.
Li S, Xiao L, Chen M, Cao Q, Luo Z, Kang N, Jia M, Chen J, Xiang M. The involvement of the phenylpropanoid and jasmonate pathways in methyl jasmonate-induced soft rot resistance in kiwifruit (Actinidia chinensis). Front Plant Sci. 2022;13:1097733. https://doi.org/10.3389/fpls.2022.1097733.
Taurino M, Abelenda JA, Río-Alvarez I, Navarro C, Vicedo B, Farmaki T, Jiménez P, García-Agustín P, López-Solanilla E, Prat S, Rojo E, Sánchez-Serrano JJ, Sanmartín M. Jasmonate-dependent modifications of the pectin matrix during potato development function as a defense mechanism targeted by Dickeya dadantii virulence factors. Plant J. 2014;77(3):418–29. https://doi.org/10.1111/tpj.12393.
Asselbergh B, Achuo AE, Höfte M, Van Gijsegem F. Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi. Mol Plant Pathol. 2008;9(1):11–24. https://doi.org/10.1111/j.1364-3703.2007.00437.x.
Liu F, Zhao Q, Jia Z, Zhang S, Wang J, Song S, Jia Y. N-3-oxo-octanoyl Homoserine Lactone Primes Plant Resistance against Necrotrophic Pathogen Pectobacterium carotovorum by coordinating Jasmonic Acid and Auxin-Signaling pathways. Front Plant Sci. 2022;13:886268. https://doi.org/10.3389/fpls.2022.886268.
Alvarez A, Montesano M, Schmelz E, Ponce de León I. Activation of Shikimate, Phenylpropanoid, Oxylipins, and Auxin pathways in Pectobacterium carotovorum Elicitors-treated Moss. Front Plant Sci. 2016;7:328. https://doi.org/10.3389/fpls.2016.00328.
Narváez-Barragán DA, Tovar-Herrera OE, Torres M, Rodríguez M, Humphris S, Toth IK, Segovia L, Serrano M, Martínez-Anaya C. Expansin-like Exl1 from Pectobacterium is a virulence factor required for host infection, and induces a defence plant response involving ROS, and jasmonate, ethylene and salicylic acid signalling pathways in Arabidopsis thaliana. Sci Rep. 2020;10(1):7747. https://doi.org/10.1038/s41598-020-64529-9.
Catinot J, Huang JB, Huang PY, Tseng MY, Chen YL, Gu SY, Lo WS, Wang LC, Chen YR, Zimmerli L. ETHYLENE RESPONSE FACTOR 96 positively regulates Arabidopsis resistance to necrotrophic pathogens by direct binding to GCC elements of jasmonate - and ethylene-responsive defence genes. Plant Cell Environ. 2015;38(12):2721–34. https://doi.org/10.1111/pce.12583.
Kwon SJ, Jin HC, Lee S, Nam MH, Chung JH, Kwon SI, Ryu CM, Park OK. GDSL lipase-like 1 regulates systemic resistance associated with ethylene signaling in Arabidopsis. Plant J. 2009;58(2):235–45. https://doi.org/10.1111/j.1365-313X.2008.03772.x.
Mohan S, Meiyalaghan S, Latimer JM, Gatehouse ML, Monaghan KS, Vanga BR, Pitman AR, Jones EE, Conner AJ, Jacobs JM. GSL2 over-expression confers resistance to Pectobacterium atrosepticum in potato. Theor Appl Genet. 2014;127(3):677–89. https://doi.org/10.1007/s00122-013-2250-2.
Gao M, Yin X, Yang W, Lam SM, Tong X, Liu J, Wang X, Li Q, Shui G, He Z. GDSL lipases modulate immunity through lipid homeostasis in rice. PLoS Pathog. 2017;13(11):e1006724. https://doi.org/10.1371/journal.ppat.1006724.
Huang LM, Lai CP, Chen LO, Chan MT, Shaw JF. Arabidopsis SFAR4 is a novel GDSL-type esterase involved in fatty acid degradation and glucose tolerance. Bot Stud. 2015;56(1):33. https://doi.org/10.1186/s40529-015-0114-6.
Ito S, Suzuki Y, Miyamoto K, Ueda J, Yamaguchi I. AtFLA11, a fasciclin-like arabinogalactan-protein, specifically localized in sclerenchyma cells. Biosci Biotechnol Biochem. 2005;69(10):1963–9. https://doi.org/10.1271/bbb.69.1963.
Liu E, MacMillan CP, Shafee T, Ma Y, Ratcliffe J, van de Meene A, Bacic A, Humphries J, Johnson KL. Fasciclin-like Arabinogalactan-Protein 16 (FLA16) is required for Stem Development in Arabidopsis. Front Plant Sci. 2020;11:615392. https://doi.org/10.3389/fpls.2020.615392.
Afzal AJ, Wood AJ, Lightfoot DA. Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Mol Plant Microbe Interact. 2008;21(5):507–17. https://doi.org/10.1094/MPMI-21-5-0507.
Bellande K, Bono JJ, Savelli B, Jamet E, Canut H. Plant lectins and Lectin Receptor-like Kinases: how do they sense the outside? Int J Mol Sci. 2017;18(6):1164. https://doi.org/10.3390/ijms18061164.
Duan X, Hou Q, Liu G, Pang X, Niu Z, Wang X, Zhang Y, Li B, Liang R. Expression of Pinellia pedatisecta lectin gene in Transgenic Wheat Enhances Resistance to wheat aphids. Molecules. 2018;23(4):748. https://doi.org/10.3390/molecules23040748.
Esch L, Schaffrath U. An update on Jacalin-Like Lectins and their role in Plant Defense. Int J Mol Sci. 2017;18(7):1592. https://doi.org/10.3390/ijms18071592.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. https://doi.org/10.1093/bioinformatics/btu170.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. 2011;12:323. https://doi.org/10.1186/1471-2105-12-323
Xue T, Zhang H, Zhang Y, Wei S, Chao Q, Zhu Y, Teng J, Zhang A, Sheng W, Duan Y, Xue J. Full-length transcriptome analysis of shade-induced promotion of tuber production in Pinellia ternata. BMC Plant Biol. 2019;19(1):565. https://doi.org/10.1186/s12870-019-2197-9.
Sun M, Qi X, Zhou Y, Wu J, Tian X. Phytophthora infestans RXLR effector Pi04089 perturbs diverse defense-related genes to suppress host immunity. BMC Plant Biol. 2021;21(1):582. https://doi.org/10.1186/s12870-021-03364-0.
Acknowledgements
We thank Prof. Zhendong Tian from Huazhong Agricultural University for providing the pH7LIC vector.
Funding
This research was joint supported by Hubei Provincial Natural Science Foundation and Traditional Chinese Medicine Innovation and Development-of China (2024AFD294); the National Key R&D Program “Modernization of Traditional Chinese Medicine” Key Special Project, China (2023YFC3503804); the Key Special Fund for Traditional Chinese Medicine of Hubei University of Chinese Medicine (2022ZZXZ001); the Major science and technology research project of Hubei University of Chinese Medicine (2023ZDXM008); the key projects at the central government level: The Ability Establishment of Sustainable Use for Valuable Chinese Medicine Resources (2060302-2202-18).
Author information
Authors and Affiliations
Contributions
Conceptualization, D.L.; methodology, Y.M.; software, M.L.; validation, M.W. and M.L.; formal analysis, M.L. and J.X.; investigation, M.L. and K.Q.; resources, M.W. and J.X.; data curation, Y.M.; writing—original draft preparation, M.L.; writing—review and editing, Y.M.; visualization, M.L.; supervision, D.L.; project administration, D.L.; funding acquisition, D.L. and M.L. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval and informed consent
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, M., Wang, M., Xu, J. et al. Comparative transcriptome analysis reveals defense responses against soft rot induced by Pectobacterium aroidearum and Pectobacterium carotovorum in Pinellia ternata. BMC Genomics 25, 831 (2024). https://doi.org/10.1186/s12864-024-10746-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10746-9