Abstract
CMS (cytoplasmic male sterility) can be controlled by the mitochondrion genome in higher plants, including Satsuma mandarin. Somatic fusion experiments in citrus combining embryogenic callus protoplasts of one parent with leaf protoplasts of a second parent often produce cybrid plants of the leaf parent, a phenomenon occurring most often with interspecific fusion combinations. In an attempt to practically exploit this cybridization phenomenon, we conducted somatic fusion experiments combining embryogenic suspension-derived protoplasts of Satsuma mandarin, Citrus unshiu Marc. cv. Guoqing No. 1 (G1), a male-sterile cultivar, with leaf protoplasts of other seedy types—Hirado Buntan Pink pummelo (HBP) [Citrus grandis (L.) Osbeck], Sunburst mandarin (C. reticulata Blanco), Orie Lee hybrid (C. reticulata cv. Clementine × Murcott tangor), and Murcott tangor [C. reticulata × C. sinensis (L.) Osbeck], respectively—in an attempt to generate seedless cybrids by the targeted transfer of CMS. The genetic identities of regenerated plants from all four parental combinations were determined by flow cytometry, SSR, CAPS (or PCR-RFLP), RFLP, and chloroplast-SSR analyses. Regenerated plants from the first three parental combinations were diploids, and the cybrid nature of G1 + HBP with the mitochondrion genome from G1 and the chloroplast genome from HBP was confirmed, whereas the cybrid nature of the remaining two combinations was difficult to confirm because of the close phylogenetic relatedness of both fusion parents, as expected. Plants from G1 + Murcott were confirmed as tetraploid somatic hybrids. This is the first report of targeted citrus cybrid production by symmetric fusion with male-sterile Satsuma as the callus parent and other seedy cultivars as the leaf parents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytoplasmic male sterility (CMS), an important agronomic trait in crops, is controlled by the mitochondrial genome (Kumar and Cocking 1987). Protoplast fusion plays an impressive role in the transfer of CMS in higher plants, which otherwise requires at least several years to realize by sexual repetitive backcrossing. To date, numerous asymmetric somatic hybrids or cybrids have been produced as a means to transfer CMS into crop plants such as rice, rapeseed, potato, tobacco, etc. (Zelcer et al. 1978; Sakai and Imamura 1990; Creemers-Molenaar et al. 1992; Melchers et al. 1992; Akagi et al. 1995; Kirti et al. 1995; Liu et al. 1996; Cardi and Earle 1997; Sigareva and Earle 1997; Atanassov et al. 1998; Bhattacharjee et al. 1999; Akagi 2001; Motegi et al. 2003). There are two principal fusion methods to achieve CMS transfer. The first is by asymmetric protoplast fusion using irradiated protoplasts as a donor parent and chemically inactivated protoplasts as the recipient parent (Melchers et al. 1992; Sigareva and Earle 1997), and the second is by cytoplast-protoplast or microprotoplast fusion (Sakai and Imamura 1990; Spangenberg et al. 1991; Louzada et al. 2002). It is generally difficult to generate highly asymmetric hybrids or cybrids by asymmetric fusion, and the cytoplast-protoplast or microprotoplast fusion method is even more technically difficult (see above-mentioned references).
During the past two decades, great progress has been made in citrus somatic hybridization, and somatic hybrid plants from more than 200 different intergeneric and interspecific parental combinations have been produced (Grosser et al. 2000; Guo and Deng 2001). Somatic hybridization in citrus has circumvented some of the problems such as nucellar polyembryony, pollen/ovule sterility, and long juvenility encountered in sexual conventional breeding, and new hybrids are showing potential in citrus cultivar improvement (Grosser et al. 2000). For citrus somatic hybridization, the fusion model of “diploid embryogenic protoplasts + diploid mesophyll-derived protoplasts” has been used extensively. Normally, unfused mesophyll protoplasts do not divide and regenerate into plants. However, diploid plants morphologically resembling the leaf parent have been recovered unexpectedly from more than 40 symmetric fusion combinations (Grosser et al. 2000; Guo and Deng 2000; Deng et al. 2000a, 2000b). In all such cases examined, RFLP analysis showed that the regenerated diploid plants were not directly regenerated from unfused mesophyll protoplasts, but rather were cybrids with the nuclear DNA from the leaf parent and the mitochondria (mt)DNA from the corresponding embryogenic parent (Saito et al. 1993, 1994; Yamamoto and Kobayashi 1995; Grosser et al. 1996; Moriguchi et al. 1996, 1997; Moreira et al. 2000a; Cabasson et al. 2001). This indicates that the mitochondrial genome of the embryogenic parent plays an important role in mesophyll parent-type cybrid plant regeneration. Chloroplast (cp)DNA was randomly inherited in these plants. The same organelle inheritance pattern also occurs in all citrus allotetraploid somatic hybrids (Kobayashi et al. 1991; Moreira et al. 2000b; Guo and Deng 2001).
The above-mentioned phenomenon provides a novel opportunity to transfer mitochondrion-controlled specific agronomic/horticultural traits simply by conducting symmetric fusion experiments. Seedlessness is a prerequisite for new fresh-market citrus cultivars, and seedlessness in diploid citrus generally relates to male/female sterility and/or self-incompatibility (Yamamoto et al. 1995). Since most citrus species exhibit some level of parthenocarpy, seedless fruits can develop normally. The seedless Satsuma mandarin is typically male-sterile, and its male sterility has been identified to be of a CMS type (Yamamoto et al. 1997). In the investigation reported here, we designed fusions between embryogenic callus protoplasts of Satsuma mandarin cv. Guoqing No. 1 (Citrus unshiu Marc.) and mesophyll protoplasts from four seedy cultivars in an effort to produce diploid seedless cybrids. Hirado Buntan pummelo [Citrus grandis (L.) Osbeck] produces a high-quality pink-fleshed fruit that is becoming increasingly popular in Florida. However, it is excessively seedy, averaging 91 seeds per fruit (Melinda Grosser, personal communication), a trait that inhibits its consumer acceptance. Murcott tangor [C. reticulata × Citrus sinensis (L.) Osbeck] and Sunburst mandarin hybrid (Clementine × Orlando tangelo) are the two most popular tangerine cultivars in Florida. However, their high seed content is allowing seedless Clementine fruit from Spain, Morocco, and California to displace them in the marketplace. The Orie Lee hybrid (Clementine × Murcott) is an unreleased selection with exceptional quality, but it is also seedy and susceptible to citrus scab disease. Seedless fruits of any of these cultivars should provide excellent marketing opportunities to Florida and other growers.
Materials and methods
Protoplast fusion and plant regeneration
A friable embryogenic callus line of Satsuma mandarin, Citrus unshiu Marc. cv. Guoqing No 1 (abbreviated as G1) was induced from unfertilized ovules by Huo et al. (1999) and maintained on MT (Murashige and Tucker 1969) basal medium. Suspension cultures of this line were established and maintained using a 2-week subculture cycle on agar-free H+H medium (Grosser and Gmitter 1990). A recently budded plant of Hirado Buntan pummelo [Citrus grandis (L.) Osbeck] (abbreviated as HBP) and nucellar seedlings of Sunburst [C. reticulata (L.) Osbeck], Orie Lee (C. reticulata cv. Clementine tangerine × Murcott tangor), and Murcott [C. reticulata × C. sinensis (L.) Osbeck] were maintained in a heavily shaded greenhouse (using double shadecloth), and fully expanded leaves were used for protoplast isolation. The procedures for protoplast isolation, PEG-mediated fusion, and culture were according to Grosser and Gmitter (1990). Regenerated embryos were also cultured over 0.22 μm acetate plus membrane filters, which were put on fresh EME solid medium (Grosser and Gmitter 1990) containing 0.15 M maltose instead of sucrose to normalize and enlarge the embryos (Niedz et al. 2002). The regenerated shoots were grafted in vitro or onto greenhouse rootstock seedlings to expedite growth.
Ploidy analysis
Ploidy analysis was carried out using a Partec flow cytometer (Partec, Münster, Germany) according to Miranda et al. (1997) with minor modifications. Approximately 1 cm2 of young leaf was chopped in a plastic petri dish containing 0.4 ml Partec HR-A buffer. After being filtered, the samples were stained with 0.8 ml of HR-B buffer and the relative fluorescence of total DNA measured. Each histogram was generated by the analysis of at least 3,000–5,000 nuclei.
Genomic DNA extraction
For SSR and CAPS analysis, we used a Sigma GenEluteTM plant genomic DNA miniprep kit (Sigma, St. Louis, Mo.). For RFLP analysis, DNA extraction was performed according to Cheng et al. (2003a): 2–5 g of fresh healthy leaves were harvested and ground in liquid nitrogen, mixed with SDS extraction buffer and incubated at 65°C. After removal of the RNA, the crude DNA solution was then extracted with water-saturated ether and NaCl and precipitated with isopropanol; the DNA was then dissolved in TE buffer.
SSR and cp-SSR analysis
SSR analysis of nuclear genomes using three primer pairs (Table 1) and cp-SSR analysis by one pre-screened primer pair (NTCP9, primer 1: 5′-CTT CCA AGC TAA CGA TGC-3′; primer 2: 5′-CTG TCC TAT CCA TTA GAC AAT G-3′) (Cheng et al. 2003b) from the cp-SSR primer pairs list described by Bryan et al. (1999) were conducted according to the procedure of Kijas et al. (1997) with minor modifications. Approximately 20 ng of genomic DNA, 0.2 mM dNTPs, 1.5 mM MgCl2, 0.5 U Taq DNA polymerase (Promega, Madison, Wis.) and corresponding 1× reaction buffer and 0.2 μM of each primer pair were mixed in a 25-μl reaction volume. PCR cycles were programmed as follows: one initial denaturing cycle at 94ºC for 3 min; 32 cycles of 1 min at 94ºC (denaturing), 40 s at 55ºC (annealing), 2 min at 72ºC (elongation); a final cycle of 10 min at 72ºC. The products were analyzed on 3.0% (w/v) Metaphore agarose (BMA, Rockland, Me.) gels and visualized under UV light.
CAPS (or PCR-RFLP) analysis
Organelle DNA was amplified using three chloroplast and three mitochondrial universal primer pairs (Table 2). The PCR reactions were conducted in a PTC-100 thermocycler. The reaction mixture (50 μl) consisted of 0.2 mM dNTPs, 1.5 mM MgCl2, 2 U Taq DNA polymerase (Promega) and corresponding 1× reaction buffer, 0.2 μM of each primer, and 100 ng of sample DNA. PCR conditions of the CAPS analysis were the same as that of the SSR analysis. The PCR products were digested with 5 U of restriction enzymes (NEB, Beverly, Mass.), including DraI, EcoRI, FspI, HaeIII, HindIII, HinfI, PstI, SacII, or TaqI, and electrophoresed on 3.0% Metaphore agarose gels.
RFLP analysis
Approximately 10 μg of genomic DNA was digested with EcoRI, HindIII, BamHI, DraI, and PstI. The digested DNA was electrophoresed on a 0.8% agarose gel and blotted onto a Hybond-N+ membrane (Amersham, UK) using an alkali-downward capillary blotting procedure according to the manufacturer’s recommendation. Probe labeling, hybridization, and stringency washing were conducted using the procedure described by Feinberg and Vogelstein (1983). The mtDNA probes—aptA, cob, cox I, cox II, and cox III—were labeled with d(CTP)-[32P]. Hybridization was performed in tubes at 65ºC overnight, and membranes were washed in high-stringency solution (0.1× SSC, 0.1% SDS) at 65°C for 4 h. The membranes were exposed to X-ray film at –80ºC overnight to 1 week before the film was developed.
Results and discussion
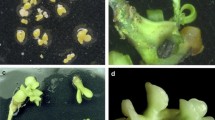
Fusions were conducted combining embryogenic suspension protoplasts of G1 with mesophyll protoplasts from HBP, Sunburst, Orie Lee, and Murcott, respectively. The G1 callus line had been in culture for several years and had lost its capacity for direct protoplast regeneration. Embryos were recovered from all four fusion combinations and transferred to 0.22 μm acetate plus membrane filters placed directly on fresh EME solid medium containing 0.15 M maltose instead of sucrose (Perez et al. 1998) to expedite growth and development (Niedz et al. 2002). Great differences in embryo and plantlet recovery were found from the four fusion combinations. Only one embryo was germinated from the G1 + HBP fusion combination, and approximately 20, 5, and 2 shoots were produced from the G1 + Sunburst, G1 + Orie Lee, and G1 + Murcott combinations, respectively (Table 3). The regenerated shoots were then grafted in vitro or grafted directly onto greenhouse rootstocks of a sour orange + Carrizo citrange somatic hybrid to expedite their growth.
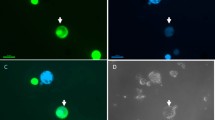
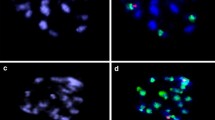
Flow cytometry (Fig. 1) analysis confirmed that the regenerated plants of the G1 + HBP, G1 + Sunburst, and G1 + Orie Lee fusion combinations were all diploids, whereas the two plants from the G1 + Murcott combination were tetraploid. In addition, the regenerated plant from G1 + HBP was morphologically identical to HBP, which suggested its putative cybrid nature. The diploid plants from the other two fusion combinations also exhibited a leaf morphology similar to that of their respective leaf parents.
To determine the origin of the nuclear genotype, we conducted SSR analysis. The results showed that the regenerated diploids from the three different fusion combinations had a banding pattern identical to that of their corresponding leaf parent, indicating that the regenerated diploid plants were putative cybrids with the nuclear genome from their corresponding leaf parent (Figs. 2, 3). The regenerated tetraploid from G1 + Murcott had complementary specific bands from both parents, thus confirming its somatic hybrid nature (Fig. 4A). This hybrid should have excellent potential to serve as a breeding parent for use in interploid crosses to produce seedless triploids (Grosser et al. 2000).
SSR analysis of G1 + Sunburst and G1 + Orie Lee hybrid diploid regenerated plants. Lanes: 1, 4, 8, 11 G1; 2, 5 G1 + Sunburst diploid plant; 3, 6 Sunburst; 7100-bp ladder; 9, 12 G1 + Orie Lee hybrid diploid plant; 10, 13 Orie Lee hybrid. Primer pairs: lanes 1–3, 8–10 TAA15, lanes 4–6, 11–13 TAA41. Arrows indicate specific bands
SSR (A) and mt-DNA (B) analysis of G1 + Murcott tetraploid somatic hybrid. Lanes: 1, 8 100-bp ladder; 2, 5, 9, 12 G1, 3, 6, 10, 13 G1 + Murcott tetraploid plant; 4, 7, 11, 14 Murcott. SSR primer pairs: lanes 2–4 TAA15, lanes 5–7 TAA41. Mt-DNA primer pair/enzyme combinations: lanes 9–11 nad4Exon1-nad4Exon2/TaqI, lane 12–14 18S rRNA-5S rRNA/TaqI. Arrows indicate specific bands
To further confirm the cybrid nature of the diploid regenerates, we carried out CAPS, RFLP, and cp-SSR analysis. CAPS analysis results showed that mt-DNA primer pair/enzyme combinations of nad4exon1-nad4exon2/TaqI, nad4exon1-nad4exon2/HaeIII, nad4exon1-nad4exon2/HinfI, and 18S rRNA-5S rRNA/TaqI were able to distinguish both parents of G1 and HBP and that the mtDNA of the G1 + HBP diploid plant was always from G1 (Fig. 5, lanes 1–6). RFLP analysis of the mtDNA further confirmed the results of CAPS analysis (Fig. 6A). For cpDNA, the primer pair/enzyme combinations of rbcL-psaI/TaqI and trnD-trnT/TaqI were effective in distinguishing both parents, and the cpDNA of G1 + HBP diploid plant was verified to be from HBP (Fig. 5, lanes 8–13); cp-SSR analysis further confirmed this inheritance pattern (Fig. 6B). With respect to the inheritance of the mitochondrial genome of the G1 + Murcott tetraploid somatic hybrid, the mtDNA primer pair/enzyme combinations of nad4exon1-nad4exon2/TaqI and 18S rRNA-5S rRNA/TaqI were able to distinguish both parents; the mtDNA of the hybrid was always from G1 (Fig. 4B). We could not distinguish the cpDNA of this fusion combination by either CAPS or cp-SSR analysis. In addition, CAPS analysis with the primer pair/enzyme combinations used could not identify the origin of the cytoplasmic genomes in the G1 + Sunburst and G1 + Orie Lee diploid regenerates due to the close phylogenetic relatedness of both fusion parents, although they were thought to be cybrid plants. CAPS analysis has only recently been applied to identify the cytoplasmic inheritance of somatic hybrids in higher plants and has proven to be very efficient (Bastia et al. 2001; Guo et al. 2002; Liu et al. 2002; Cheng et al. 2003a; Lotfy et al. 2003). Chloroplast microsatellite (cp-SSR) analysis is a new tool for plant ecology and evolution studies (Provan et al. 2001) and has only recently been introduced into citrus phylogenetic study (Cheng et al. 2003b). Compared with CAPS analysis, it is even more convenient and efficient since enzyme cutting following PCR reaction is not needed.
Mt- and cp-DNA analysis of G1 + HBP diploid cybrid plant by CAPS markers. Lanes: 1, 4, 8, 11 G1; 2, 5, 9, 12 G1 + HBP diploid cybrid plant; 3, 6, 10, 13 HBP; 7, 14100-bp ladder. Primer pair/enzyme combinations: lanes 1–3 nad4Exon1-nad4Exon2/HaeIII, lanes 4–6 nad4Exon1-nad4Exon2/HinfI, lanes 8–10 rbcL-psaI/TaqI, lanes 11–13 trnD-trnT/TaqI. Arrows indicate specific bands
The molecular analysis confirmed that we had produced not only a cybrid plant between G1 and HBP with mtDNA from G1 but also many putative cybrid plants between G1 and Sunburst tangerine and G1 and the Orie Lee hybrid. Additional plants from G1 + Sunburst and G1 + Orie Lee were tested by flow cytometry and SSR analysis. The results also showed that they were all diploids and that their nuclear genome was always from their corresponding leaf parent. There is also strong circumstantial evidence that the plants regenerated from these latter two combinations are also cybrids. In the more than 25 cases of citrus callus/suspension + leaf protoplast fusion experiments examined where diploid plants of leaf-parent morphology were recovered, all were confirmed as cybrids containing the nuclear DNA of the leaf parent but only the mtDNA of the callus/suspension parent (Saito et al. 1993; Yamamoto and Kobayashi 1995; Grosser et al. 1996; Moriguchi et al. 1996; Guo and Deng 2000; Moreira et al. 2000a; Cabasson et al. 2001; Y.J. Cheng, unpublished data; M.T. Scarano, personal communication). The same phenomenon has been shown for allotetraploid somatic hybrids, as all of the regenerated plants examined always contain only the mtDNA of the callus/suspension parent (Kobayashi et al. 1991; Ohgawara et al. 1994; Moreira et al. 2000b). The current research demonstrates that it is possible to take advantage of this phenomenon to transfer CMS from Satsuma mandarin to other seedy cultivars simply by symmetric fusion. This method is easier and more efficient than cytoplast-protoplast fusion or asymmetric fusion, which are technically complicated with difficult cybrid plant recovery.
Several possible mechanisms leading to the regeneration of cybrid plants by symmetric fusion have been proposed by various researchers (Gleba and Sytnik 1984; Grosser et al. 1996; Moriguchi et al. 1996; Guo and Deng 2000; Moreira et al. 2000a), but none are conclusive. Moreira et al. (2000a) proposed that quantitative differences in the mitochondria content of callus/suspension cells versus leaf cells could be responsible. Leaf cells and corresponding protoplasts contain much fewer mitochondria per cell than callus/suspension cells and may not be capable of producing the energy required for direct regeneration. Cytoplasmic fusion with callus/suspension cells may provide the necessary mitochondria and energy to achieve regeneration. In such cases, it is not known what happens to the callus/suspension donor cell nuclei. Our experiences with Citrus indicate that the phenomenon of cybrid production by symmetric fusion is dependent on the genotype of the embryogenic parent and the parental combination and that the possibility of obtaining cybrid plants is higher when higher ratios of mesophyll protoplasts to callus/suspension cells are used in the fusion.
Several seedless sexual hybrid cultivars have been obtained and released with Satsuma mandarin as the seed parent in China and Japan. One cybrid plant between Juman Satsuma and Washington navel orange has also been produced (Yamamoto and Kobayashi 1995; Yamamoto et al. 2001). Since Washington navel orange is a typical seedless cultivar just as Satsuma, it is difficult to determine if the seedlessness of the cybrid plant is due to sterile cytoplasm from Satsuma. In our investigation reported here, we were able to produce cybrid plants between G1 Satsuma and other seedy cultivars within a year; a task that would require years by conventional repetitive backcrossing—if it is possible at all. In this sense, the transfer of CMS by cell fusion in citrus may be even more efficient than in other annual crops. These cybrid plants have been top-worked to mature trees to expedite flowering and fruiting, which is necessary to determine the practical value of this approach. It must still be determined if the substitution of Satsuma mtDNA in the present combinations can result in new functional mitochondria-nuclear interactions that could result in making these cultivars seedless without otherwise altering their cultivar integrity. Any resulting seedless fruit with expected cultivar integrity would have instant marketability. If successful, this strategy could be applied to eliminate seed production from many superior diploid citrus cultivars. In addition, even if the cybrid HBP containing the Satsuma CMS cytoplasm turns out to be seedy, it will still have value as a mono-embryonic female parent that could be used in crosses with mandarins of the appropriate nuclear background to produce seedless diploid tangelos.
Abbreviations
- CAPS::
-
Cleaved amplified polymorphic sequence
- CMS::
-
Cytoplasmic male sterility
- cp-SSR::
-
Chloroplast simple sequence repeat
- PEG::
-
Polyethylene glycol
- SSR::
-
Simple sequence repeat
- RFLP::
-
Restriction fragment length polymorphism
References
Akagi H (2001) Cybridization in Oryza sativa L. (rice). In: Nagata T, Bajaj YPS (eds) Somatic hybridization in crop improvement II, vol 49. Biotechnology in agriculture and forestry. Springer, Berlin Heidelberg New York, pp 17–36
Akagi H, Taguchi T, Fujimura T (1995) Stable inheritance and expression of the CMS traits introduced by asymmetric protoplast fusion. Theor Appl Genet 91:563–567
Al-Janabi SM, McClelland M, Petersen C, Sobral BWS (1994) Phylogenetic analysis of organellar DNA sequences in the Andropogoneae: Saccharinea. Theor Appl Genet 88:933–944
Atanassov II, Atanassova SA, Dragoeva AI, Atanassov AI (1998) A new CMS source in Nicotiana developed via somatic cybridization between N. tabacum and N. alata. Theor Appl Genet 97:982–985
Bastia T, Scotti N, Cardi T (2001) Organelle DNA analysis of Solanum and Brassica somatic hybrids by PCR with ‘universal primers’. Theor Appl Genet 102:1265–1272
Bhattacharjee B, Sane AP, Gupta HS (1999) Transfer of wild abortive cytoplasmic male sterility through protoplast fusion in rice. Mol Breed 5:319–327
Bryan GJ, McNicoll J, Ramsay G, Meyer RC, De Jong WS (1999) Polymorphic simple sequence repeat markers in chloroplast genomes of Solanaceous plants. Theor Appl Genet 99:859–867
Cabasson CM, Luro F, Ollitrault P, Grosser JW (2001) Non-random inheritance of mitochondrial genomes in Citrus hybrids produced by protoplast fusion. Plant Cell Rep 20:604–609
Cardi T, Earle ED (1997) Production of new CMS Brassica oleracea by transfer of ‘Anand’ cytoplasm from B. rapa through protoplast fusion. Theor Appl Genet 94:204–212
Cheng YJ, Guo WW, Deng XX (2003a) Molecular characterization of cytoplasmic and nuclear genomes in phenotypically abnormal Valencia orange (Citrus sinensis) + Meiwa kumquat (Fortunella crassifolia) intergeneric somatic hybrids. Plant Cell Rep 21:445–451
Cheng YJ, Guo WW, Deng XX (2003b) cpSSR: a new tool to analyze chloroplast genome of citrus somatic hybrids. Acta Bot Sin 45:906–909
Creemers-Molenaar J, Hall RD, Krens FA (1992) Asymmetric protoplast fusion aimed at intraspecific transfer of cytoplasmic male-sterility (CMS) in Lolium perenne L. Theor Appl Genet 84:763–770
Demesure B, Sodzi N, Petit RJ (1995) A set of universal primers for amplification of polymorphic non-coding regions of mitochondrial and chloroplast DNA in plants. Mol Ecol 4:129–131
Deng XX, Guo WW, Yu GH (2000a) Citrus somatic hybrids regenerated from protoplast electrofusion. Acta Hortic 535:163–168
Deng XX, Guo WW, Yu GH (2000b) Diploid mesophyll parent type plants regenerated from nine symmetrical protoplast fusion combinations in Citrus (in Chinese). Acta Hortic Sin 27:1–5
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Gleba YY, Sytnik KM (1984) Protoplast fusion. In: Shoeman R (ed) Genetic engineering in higher plants. Springer, Berlin Heidelberg, New York, pp 63–114
Grosser JW, Gmitter FG Jr (1990) Protoplast fusion and citrus improvement. Plant Breed Rev 8:339–374
Grosser JW, Gmitter FG Jr, Tusa N, Reforgiato Recupero G, Cucinotta P (1996) Further evidence of a cybridization requirements for plant regeneration from citrus leaf protoplasts following somatic fusion. Plant Cell Rep 15:672–676
Grosser JW, Ollitrault P, Olivares-Fuster O (2000) Somatic hybridization in citrus: an effective tool to facilitate variety improvement. In Vitro Cell Dev Biol Plant 36:434–449
Guo WW, Deng XX (2000) Citrus cybrids and their cytoplasmic genetic recombination (in Chinese). Acta Hortic Sin 27:487–491
Guo WW, Deng XX (2001) Wide somatic hybrids of Citrus with its related genera and their potential in genetic improvement. Euphytica 118:175–183
Guo WW, Cheng YJ, Deng XX (2002) Regeneration and molecular characterization of intergeneric somatic hybrids between Citrus reticulata and Poncirus trifoliata. Plant Cell Rep 20:829–834
Huo HQ, Hao YJ, Deng XX (1999) Induction of embryogenic callus of loose skin mandarins (in Chinese). Acta Biol Exp Sin 32:289–295
Kijas JMH, Thomas MR, Fowler JCS, Roose ML (1997) Integration of trinucleotide microsatellite into a linkage map of Citrus. Theor Appl Genet 94:701–708
Kirti PB, Banga SS, Prakash S, Chopra VL (1995) Transfer of Ogu cytoplasmic male-sterility to Brassica juncea and improvement of the male-sterile line through somatic-cell fusion. Theor Appl Genet 91:517–521
Kobayashi S, Ohgawara T, Fujiwara K, Oiyama I (1991) Analysis of cytoplasmic genomes in somatic hybrids between navel orange (Citrus sinensis Osb) and ‘Murcott’ tangor. Theor Appl Genet 82:6–10
Kumar A, Cocking EC (1987) Protoplast fusion—a novel-approach to organelle genetics in higher plants. Am J Bot 74:1289–1303
Liu JH, Landgren M, Glimelius K (1996) Transfer of the Brassica tournefortii cytoplasm to B. napus for the production of cytoplasmic male sterile B. napus. Physiol Plant 96:123–129
Liu JH, Pang XM, Cheng YJ, Meng HJ, Deng XX (2002) Molecular characterization of the nuclear and cytoplasmic genomes of intergeneric diploid plants from cell fusion between Microcitrus papuana and Rough lemon. Plant Cell Rep 21:327–332
Lotfy S, Luro F, Carreel F, Froelicher Y, Rist D, Ollitrault P (2003) Application of cleaved amplified polymorphic sequence method for analysis of cytoplasmic genome among Aurantioideae intergeneric somatic hybrids. J Am Soc Hortic Sci 128:225–230
Louzada ES, del Rio HS, Xia D, Moran-Mirabal JM (2002) Preparation and fusion of citrus sp. microprotoplasts. J Am Soc Hortic Sci 127:484–488
Melchers G, Mohri Y, Watanabe K, Wakabayashi S, Harada K (1992) One-step generation of cytoplasmic male-sterility by fusion of mitochondrial-inactivated tomato protoplasts with nuclear-inactivated Solanum protoplasts. Proc Natl Acad Sci USA 89:6832–6836
Miranda M, Motomura T, Ikeda F, Ohgawara T, Saito W, Endo T, Omura M, Moriguchi T (1997) Somatic hybrids obtained by fusion between Poncirus trifoliata (2x) and Fortunella hindsii (4x) protoplasts. Plant Cell Rep 16:401–405
Moreira CD, Chase CD, Gmitter FG Jr, Grosser JW (2000a) Inheritance of organelle genomes in citrus somatic cybrids. Mol Breed 6:401–405
Moreira CD, Chase CD, Gmitter FG Jr, Grosser JW (2000b) Transmission of organelle genomes in citrus somatic hybrids. Plant Cell Tissue Organ Cult 61:165–168
Moriguchi T, Hidaka T, Omura M, Motomura T, Akihama T (1996) Genotypes and parental combination influence efficiency of cybrid induction in Citrus by electrofusion. HortScience 31:275–278
Moriguchi T, Motomura T, Hidaka T, Akihama T, Omura M (1997) Analysis of mitochondrial genomes among Citrus plants produced by the interspecific somatic hybrids of ‘Seminole’ tangelo with rough lemon. Plant Cell Rep 16:397-400
Morton BR, Clegg MT (1993) A chloroplast DNA mutational hotspot and gene conversion in a noncoding region near rbcL in the grass family (Poaceae). Curr Genet 24:357–365
Motegi T, Nou IS, Zhou J, Kanno A, Kameya T, Hirata Y (2003) Obtaining an Ogura-type CMS line from asymmetrical protoplast fusion between cabbage (fertile) and radish (fertile). Euphytica 129:319–323
Murashige T, Tucker DPH (1969) Growth factor requirements of citrus tissue culture. In: Chapman HD (ed) Proc 1st Citrus Symp, vol 3. University of California Press, Riverside, pp 1155–1161
Nicolosi E, Deng ZN, Gentile A, La Malfa S (2000) Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor Appl Genet 100:1155–1166
Niedz RP, Hyndman SE, Wynn ET, Bausher MG (2002) Normalizing sweet orange [C. sinensis (L.) Osbeck] somatic embryogenesis with semi-permeable membranes. In Vitro Cell Dev Biol-Plant 38:552–557
Ohgawara T, Uchimiya H, Ishii S, Kobayashi S (1994) Somatic hybridization between Citrus sinensis and Poncirus trifoliata. In: Bajaj YPS (ed) Somatic hybridization in crop improvement I, vol 27. Biotechnology in agriculture and forestry. Springer, Berlin Heidelberg New York, pp 439–454
Perez RM, Galiana AM, Navarro L, Duran-Vila N (1998) Embryogenesis in vitro of several Citrus species and cultivars. J Hortic Sci Biotechnol 73:796–802
Provan J, Powell W, Hollingsworth PM (2001) Chloroplast microsatellites: new tools for studies in plant ecology and evolution. Trends Ecol Evol 16:142–147
Saito W, Ohgawara T, Shimizu J, Kobayashi S (1993) Citrus cybrid regeneration following cell fusion between nucellar cells and mesophyll cells. Plant Sci 88:195–201
Saito W, Ohgawara T, Shimizu J, Kobayashi S (1994) Somatic hybridization in Citrus using embryogenic cybrid callus. Plant Sci 99:89–95
Sakai T, Imamura J (1990) Intergeneric transfer of cytoplasmic male-sterility between Raphanus sativus (CMS line) and Brassica napus through cytoplast-protoplast fusion. Theor Appl Genet 80:421–427
Sigareva MA, Earle ED (1997) Direct transfer of a cold-tolerant Ogura male sterile cytoplasm into cabbage (Brassica oleracea ssp capitata) via protoplast fusion. Theor Appl Genet 94:213–220
Spangenberg G, Freydl E, Osusky M, Nagel J, Potrykus I (1991) Organelle transfer by microfusion of defined protoplast-cytoplast pairs. Theor Appl Genet 81:477–486
Yamamoto M, Kobayashi SA (1995) Cybrid plant produced by electrofusion between Citrus unshiu (Satsuma mandarin) and C. sinensis (sweet orange). Plant Tissue Cult Lett 12:131–137
Yamamoto M, Matsumoto R, Yamada Y (1995) Relationship between sterility and seedlessness in Citrus. J Jpn Soc Hortic Sci 64:23-29
Yamamoto M, Matsumoto R, Okudai N, Yamada Y (1997) Aborted anthers of Citrus result from gene-cytoplasmic male sterility. Sci Hortic 70:9–14
Yamamoto M, Kobayashi S, Yoshioka T, Matsumoto R (2001) Cybridization in Citrus unshiu Marc. (Satsuma mandarin) and C. sinensis (L.) Osbeck (Sweet orange). In: Nagata T, Bajaj YPS (eds) Somatic hybridization in crop improvement II, vol 49. Biotechnology in agriculture and forestry. Springer, Berlin Heidelberg New York, pp 124–138
Zelcer A, Aviv D, Galun E (1978) Interspecific transfer of cytoplasmic male-sterility by fusion between protoplasts of normal Nicotiana sylvestris and x-ray-irradiated protoplasts of male-sterile N. tabacum. Z Pflanzenphysiol 90:397–407
Acknowledgements
This research was financially supported by the Florida Agricultural Experiment Station and approved for publication as Journal Series No. R-09483. The authors also thank the Florida Citrus Production Research Advisory Council, the 863 High-Tech Project of China, the National Natural Science Foundation of China (NSFC), the International Foundation for Science (IFS) in Stockholm, Sweden, and the Chengguang Youth Project of Wuhan City of China for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.C. Phillips
Rights and permissions
About this article
Cite this article
Guo, W.W., Prasad, D., Cheng, Y.J. et al. Targeted cybridization in citrus: transfer of Satsuma cytoplasm to seedy cultivars for potential seedlessness. Plant Cell Rep 22, 752–758 (2004). https://doi.org/10.1007/s00299-003-0747-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0747-x