Abstract
Somatic embryogenesis and plant regeneration are basic processes for the success of citrus somatic hybridization via protoplast fusion. In many cases, few embryos develop normally and only a small number of plants are recovered. The development of methodologies able to increase the recovery of plants after protoplast fusion experiments it is an important requirement to improve the efficiency of the procedure. Here, plants were regenerated at high efficiency using in vitro micrografting of shoots, roots, and embryos recovered after different somatic hybridizations. Hybridizations were performed using protoplasts isolated from Chios mandarin callus with protoplasts isolated from Clementine mandarin leaves and from Sanguinelli sweet orange callus. Recovered plants were analyzed with flow cytometry and nuclear simple sequence repeat (SSR), mitochondrial InDel, and chloroplast SSR markers to determine genomic structure. One tetraploid cybrid and numerous diploid cybrids were recovered, and these exhibited a range of mitochondrial and chloroplastic genome combinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant somatic hybridization by protoplast fusion is an important tool in citrus breeding programs (Grosser et al. 2010; Grosser and Gmitter 2011). Protoplast fusion facilitates the combination of somatic cells from different species or related genera to produce new genetic combinations. Citrus reproductive biology is complex, and sexual incompatibility, male or female sterility, and apomixis can all hamper sexual hybridization. Somatic hybridization can assist traditional breeding schemes by bypassing problems associated with sexual hybridization (Grosser and Gmitter 1990; Grosser et al. 2000). In citrus, the most important application of somatic hybridization is the production of allotetraploid somatic hybrids that can be used either as rootstocks or as tetraploid parents in interploid sexual hybridizations for the production of seedless triploid cultivars (Grosser and Gmitter 2005; Grosser et al. 2010). Somatic hybridization in citrus is also important for the recovery of cybrid plants, which contain the nuclear genome of one parent with the mitochondrial and/or chloroplast genomes of a second parent (Saito et al. 1993; Grosser et al. 1996; Cabasson et al. 2001; Guo and Deng 2001; Guo et al. 2004a, 2013; Cai et al. 2007). In the majority of higher plants there is maternal inheritance of cytoplasm organelles (Kumar and Cocking 1987). Nucellar embryony, which is the apomictic mechanism in citrus, hampers recovery of citrus hybrids when apomictic genotypes are used as female parents in sexual hybridization. In this way, somatic hybridization by protoplast fusion plays a very important role in the production of new genetic combinations with apomictic genotypes and also provides the possibility to create new nuclear cytoplasm combinations and novel genotypes to study the interactions between nuclear and cytoplasm genomes. Citrus cybrids can be frequently regenerated as a by-product from the application of standard somatic hybridization procedures (Guo et al. 2004a, b; Olivares-Fuster et al. 2005; Grosser et al. 2010; Grosser and Gmitter 2011; Guo et al. 2013) and has been used as an alternative to conventional breeding in attempts to transfer cytoplasmic male sterility with the objective to produce genotypes with no pollen viability (Melchers et al. 1992; Guo et al. 2004a).
Direct or indirect embryogenesis produced by fused cells and plant regeneration are both basic process for the success of citrus somatic hybridization (Olivares-Fuster 1988; Grosser et al. 2010). Onlya few embryos develop in a normal pathway (globular, heart-shape, torpedo, and cotyledonary) and a low number of embryos reach the cotyledonary stage and germinate normally to produce plants (Button and Kochba 1977; Ollitrault 1992). Germinating embryos that produce only shoots or roots are common, and in addition, various types of malformations and abnormal development have been observed in citrus somatic embryos (Button et al. 1974; Olivares-Fuster 1988; Niedz et al. 2002; Grosser et al. 2010), including cell proliferation in the shoot apical region, lack of protoderm continuity, abnormal elongation axis, multiple fasciated cotyledons, among others (Olivares-Fuster 1988; Tomaz et al. 2001). Also in many cases embryos proliferate without germination. All this abnormalities hamper embryo germination and consequently the recovery of somatic hybrids and cybrids.
Shoot-tip grafting (STG) in vitro is an important technique in the citrus industry (Navarro et al. 1975; Navarro and Juárez 2007). Currently, STG is mainly used to recover pathogen-free citrus plants; however, the technique is increasingly being used as a research tool for the regeneration of elite genotypes or for the production of plants that cannot be recovered by other means. STG facilitates genetic transformation, recovery of haploid and tetraploid plants, and generation of somaclonal variation (Navarro and Juárez 2007). Olivares-Fuster et al. (2005) successfully micrografted in vitro shoots that were produced by embryos in somatic hybridization experiments. Here, plants were recovered at high efficiency by micrografting in vitro shoots, roots, and embryos. Source embryos were produced by somatic hybridizations between protoplasts isolated from Chios mandarin callus, and protoplasts isolated from Clementine mandarin leaves and from Sanguinelli sweet orange callus. Flow cytometry analysis and analysis using nuclear, mitochondrial, and chloroplastic markers were used to assess the genetic configuration of recovered plants.
Materials and methods
Plant material
Fully expanded, but not completely hardened, leaves of Clementine mandarin (Citrus clementina Hort. ex Tan.) were used as the leaf donor parent. Embryogenic callus was obtained by ovule culture from Chios mandarin (C. deliciosa Ten.) and Sanguinelli sweet orange [C. sinensis(L.)Osb.] according to the methodology described by Pérez et al. (1998), and was used as a callus donor parent. The following protoplast fusions were performed: Chios plus Clementine (callus + leaf), and Chios plus Sanguinelli (callus + callus).
Protoplast isolation and electric fusion
Protoplasts were isolated from leaves and from embryogenic callus following the methodology described by Grosser and Gmitter (1990) and Grosser et al. (2010). Protoplast electric fusions were performed according to the methodology described by Dambier et al. (2011) with slight modifications. Leaf and callus protoplast densities were adjusted to 6 × 105 and 4.5 × 105 protoplasts/mL, respectively, in 0.8 M mannitol containing 0.25 mMCaCl2. Equal volumes of protoplast suspensions from the two parents were mixed, and 1 mL of the mixture was poured into 60 mm Petri dishes. Protoplast suspensions were subjected to an AC electric field for 30 s, and two pulses (35 μs) of 180 V (DC) were emitted to induce protoplast fusion. The electrofusion cycle was repeated once.
Plant regeneration
After protoplast fusion, 1 mL of protoplast suspension was mixed with 4.5 Ml of BH30.6M culture medium (Grosser et al. 2010) and plated on Murashige and Skoog (1962) culture medium (MS) supplemented with50 g/L sucrose, 500 mg/L malt extract, trace nutrients (1 g/L pyridoxine hydrochloride, 1 g/L thiamine hydrochloride, and 0.5 g/L nicotinic acid), and 2.3 g/L gelrite. Protoplasts were cultivated in the dark for 2 weeks at 24 ± 1 °C. Petri dishes were then transferred to a culture room with 16 h daily exposure to 40 μEm−2 s−1 illumination. After a further 1–2 months, globular and heart-shaped embryos (Fig. 1a) were transferred onto Petri dishes containing EME 1500 culture media (Grosser et al. 2010) for enlargement and germination.
Micrografting in vitro
A preliminary experiment was performed to test whether in vitro root grafting was a viable technique for plant regeneration in citrus. Seeds of Dweet tangor (C. tangerina × C. sinensis) were peeled by removing both seed coats. Seeds were then surface sterilized and sown in 25 × 150 mm culture tubes containing 25 mL of the plant cell culture MS medium described above, solidified with 1 % Bacto agar. Cultures were maintained at 24 ± 1 °C, 60 % humidity, and 16 h daily exposure to 40 µEm−2 s−1 illumination. When seedlings were 3–5 cm tall and had well-developed roots, 4–6 mm-long root segments were top-worked in vitro onto Carrizo citrange (C. sinensis × P. trifoliata) rootstock (Fig. 2). Seedlings of Carrizo citrange obtained by seed germination in vitro were used as rootstocks and were germinated in vitro following the methodology described above. Rootstock preparation was performed as described previously (Navarro et al. 1975; Navarro and Juárez 2007). Micrografted roots were cultured in a liquid culture media comprising MS plant cell culture salt solution supplemented with White’s vitamins and 75 g/L sucrose (Navarro et al. 1975).
Shoots and roots, over 3–8 mm in length, produced by abnormally germinated embryos recovered from protoplast fusion experiments (Fig. 1b, c) were micro-grafted in vitro as we indicated above according to the standard procedures described by Navarro et al. (1975) and Navarro and Juárez (2007) with slight modifications (Fig. 3). Abnormal embryos (Fig. 1d) were also micrografted in vitro. Each embryo was micrografted, according to its morphology, using a cut that allowed the largest possible area of contact between the embryo and the rootstock (Fig. 4).
In vitro micrografted shoots and roots from abnormally germinated embryos produced after protoplast fusion. a Freshly micrografted shoot. b Shoot development 4 weeks after in vitro micrografting ready to be transplanted to the greenhouse. c Micrografted root with adventitious buds 3 weeks after grafting. d Root micrografted plant 6 weeks after grafting ready to be transplanted to the greenhouse
In vitro micrograft to abnormal embryo onto Carrizo citrange rootstock. a Abnormal embryo 4 weeks after micrografting. b Growing shoot after micrografted embryo 2–3 months after grafting. c Abnormal embryo 4 weeks after micrografting, with no shoot development. d Adventitious buds and shoots produced on the surface of cut embryo 4 months after graftingready to be transplanted to the greenhouse
Transfer to soil
Recovered plants were transferred to pots containing steam-sterilized artificial soil mix appropriate for citrus (40 % black peat, 29 % coconut fiber, 24 % washed sand, and 7 % perlite). Composition was developed in our group to grow citrus in the greenhouse. Pots were enclosed in polyethylene bagsclosed with rubber bands, and placed in a shaded area in a temperature-controlled greenhouse set at 18–25 °C. After 8–10 days, the bags were opened, and, after another 8–10 days, the bags were removed and the plants were grown under greenhouse conditions (Navarro and Juárez 2007).
Ploidy level analysis
The ploidy level of regenerated plants was determined by flow cytometry following the methodology described by Aleza et al. (2009). Briefly, small leaf samples (~0.5 mm2) were collected from each regenerated plant and a diploid control plant. Samples were chopped together using a razor blade in the presence of a nuclei isolation solution (High Resolution DNA Kit Type P, solution A; Partec®, Münster, Germany). Nuclei were filtered through a 30 μm nylon filter and stained with 4,6-diamine-2-phenylindol (DAPI) (High Resolution DNA Kit Type P, solution B; Partec®). Following a 5 min incubation period, stained samples were run in a CyFlow® Ploidy Analyzer (Partec®) flow cytometer equipped with optical parameters for the detection of DAPI fluorescence. The DNA fluorochrome DAPI is excited by the UV-LED at 365 nm. Histograms were analyzed using CyView software (Partec®), which determined peak position, coefficient of variation (CV), arithmetic mean, and median of samples.
Molecular characterization
Regenerated plants were analyzed using twenty nuclear, three mitochondrial, and two chloroplast molecular markers. Nuclear simple sequence repeat (SSR) markers used are described in Table 1 and were distributed across the nine linkage groups (LGs) of the clementine genetic reference map of clementine (Ollitrault et al. 2012a). Mitochondrial InDel markers were rrn5/rrn18-1, nad2/4-3, and nad7/1-2 (Froelicher et al. 2011). Universal chloroplast SSR markers were NTCP9 (Cheng et al. 2005) and ccmp6 (Weising and Gardner 1999).
Genomic DNA extraction was performed according to Dellaporta and Hicks (1983), with some modifications. PCR amplification was performed using a ThermocyclerEP gradient S (Eppendorf®) in 10 µL final reaction volumes containing Taq DNA polymerase (Fermentas®) (0.8 U), template DNA (2 ng/µL), wellRED (Sigma®) dye-labelled forward primer (0.2 mM), unlabeled reverse primer (0.2 mM), dNTPs (0.2 mM each), 10 × PCR buffer, and MgCl2(1.5 mM). The PCR protocol was as follows: denaturation at 94 °C for 5 min; followed by 40 cycles of 30 s at 94 °C, 1 min at 50 or 55 °C (depending on the primer annealing temperature), and 45 s at 72 °C; and a final elongation step of 4 min at 72 °C.
Capillary electrophoresis was performed using a CEQ™ 8000 Genetic Analysis System (Beckman Coulter Inc.). GenomeLab™ GeXP v.10.0 genetic analysis software was used for data collection and analysis. PCR products were initially denatured at 90 °C for 2 min, injected at 2 kV for 30 s, and subsequently separated at 6 kV for 35 min. Alleles were sized usinga 400 bp DNA standard.
Results and discussion
Root grafting in vitro
Root grafting is a natural phenomenon that occurs frequently between roots of the same tree and between neighboring trees of the same species, and less frequently between trees belonging to different species (Goldschmidt 2014). Beddie (1942) described natural root grafting in at least 30 species of woody plants, including species belonging to Fuchsia, Myrtus, Podocarpus, and Schefflera. However, in vitro root grafting is used infrequently for plant regeneration. Here, 80 Dweet tangor seeds were germinated in vitro, and root segments of the seedlings were micrografted in vitro onto Carrizo citrange rootstock (Fig. 2a). All 80 micrografted roots survived. Adventitious buds were visible at the vascular ring after 2–3 weeks, and the buds grew vigorously and produced shoots (Fig. 2b). The resultant plants were transplanted to soil 4–6 weeks after micrografting. Although in vitro root grafting is rarely used in plants, our results indicate that this is an efficient strategy for the recovery of citrus plants.
Plant regeneration by micrografting shoots, roots, and embryos recovered after protoplast fusion
In total, 294 globular and heart-shaped embryos were obtained from the two somatic hybridizations (Chios + Clementine, and Chios + Sanguinelli; Fig. 1a). Two embryos germinated and produced plants directly. Eleven embryos produced only shoots, and 32 embryos produced only roots. Of these, 11 shoots and 30 roots were micrografted in vitro (Fig. 3), resulting in the regeneration of 11 and 29 plants, respectively (Table 2). Twenty embryos grew abnormally, exhibiting clusters of proliferating tissues, fasciated cotyledons, and abnormal elongation axes (Fig. 1d). Twenty micrografts were performed from these embryos (Fig. 4), and 18 plants were regenerated (Table 2). No differences were observed between embryos recovered from both somatic hybridizations.
Regeneration of plants using in vitro shoot and root micrografting was successful. Plants were regenerated with high frequency from abnormal germinating embryos that produced only shoots or roots. Apical meristems of micrografted shoots grew rapidly, and grafted plants were transplanted to pots after 3–4 weeks (Fig. 3a, b). In roots, adventitious buds were visible at the top of the micrografted root after 2–3 weeks, and the buds began to produce shoots after 2–3 additional weeks. Rootgraft-derived plants were then transplanted to pots and cultivated under greenhouse conditions (Fig. 3c, d).
Development of micrografted embryos differed from the development of shoot and root micrografts. Some embryos produced shoots directly from the micrografted embryo (Fig. 4a, b). By contrast, some embryos remained green but underwent no apparent development for several weeks after grafting (Fig. 4c). Such stalled embryos were cut, and 4–6 months after grafting, adventitious buds and shoots developed in the cut region (Fig. 4d).
All transplanted plants survived, development was normal, and plants were robust and vigorous. Plants generated by the methods described above exhibited no differences to plants regenerated by shoot-tip grafting or micro-grafting in vitro for production, propagation and regeneration of elite genotypes in several areas as regeneration of plants from irradiated shoots, regeneration of haploid plants, production of stable tetraploid plants of non apomictic genotypes, somaclonal variation and genetic transformation with close to 100 % of grafting success (Navarro and Juarez 2007).
Abnormal embryos are often produced in protoplast fusion experiments (Olivares-Fuster 1988; Tomaz et al. 2001), and, as these embryos fail to produce viable plants, the recovery efficiency of somatic hybrids is adversely affected and potentially valuable genotypes can be lost. Here, only two plants were recovered by normal embryo germination from a total of 294 embryos (0.7 %). Shoot, root, and embryo micrografting allowed regeneration of a further 58 plants (20 %), representing an almost 30-fold increase in efficiency. These results demonstrated the utility of in vitro shoot, root, and embryo grafting for efficient plant recovery from protoplast fusions. De Pasquale et al. (1999) performed in vivo grafts using somatic embryos, shoots, and roots obtained from normal developed embryos derived from citrus embryogenic callus obtained from style and stigma in vitro culture. The rootstock used was Troyer citrange. Successful graft percentages were 29.4, 20.6, and 77.3 % for root, somatic embryo, and shoot grafts, respectively. Previously, Ollitrault (1992) performed in vivo grafts, with ~60 % success, using normally developed citrus somatic embryos recovered from embryogenic callus derived from in vitro ovule culture. This success rate was lower than the obtained in our study mainly because we have performed in vitro micrografting that is more efficient than in vivo grafting. In vitro micrografting has been a widely and very efficiently used in citrus as a tool in different research areas to regenerate elite genotypes or to produce plants that cannot be recover by other means (Navarro and Juárez 2007). Furthermore, while the previous studies used in vivo grafts from normally developed embryos, we regenerated plants from abnormally developed embryos with 96.7, 90, and 100 % success rates for root, embryo, and shoot micrografts, respectively. In addition, our in vitro procedure permitted the use of the smaller and weaker shoots and roots that are often produced by the abnormally developed embryos derived from protoplast fusion experiments. This micrografting procedure is thus highly effective for recovering somatic hybrid citrus plants and also has wider applications for other woody species in which plant regeneration by germination of embryos recovered from somatic embryogenesis is not well established. All the regenerated plants generated in this study were grafted in the field for further evaluation and potential variety selection.
Ploidy level and genetic analysis
Cells from 28 and 32 regenerated plants from the Chios + Clementine, and Chios + Sanguinelli, somatic hybridizations, respectively, were analyzed by flow cytometry. With the exception of a single tetraploid Chios + Clementine plant, all plants were diploid (Table 3).
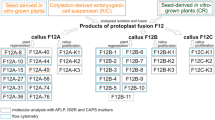
Parental genomes of Chios and Clementine mandarins and Sanguinelli sweet orange were analyzed using twenty nuclear SSR markers polymorphic between parents. Chios and Clementine always had one common allele (AA × AB or AB × BC), whereas Chios and Sanguinelli did not had common alleles (AA × BB, AB × CC, AB × CD) or had one common allele (AA × AB or AB × BC). Only two SSR markers, CIBE6126 and MEST123, noted the same alleles for Chios and Clementine and they were not used for the genetic analysis of plants recovered from Chios + Clementine somatic hybridization. Regenerated plant genotypes are shown in Table 3. Plants from Chios + Clementine somatic hybridization had the alleles of the leaf parent (Clementine) for all markers analyzed. Plants from Chios + Sanguinelli hybridization had Sanguinelli sweet orange alleles for all SSR markers. Mitochondrial and chloroplastic markers were not polymorphic for Chios and Clementine. Polymorphisms were observed between all markers for Chios and Sanguinelli. Four of the regenerated plants had identical mitochondrial and chloroplastic genotypes to the Sanguinelli parent, and 18 plants had different mitochondrial and chloroplastic genome combinations (Fig. 5). Three plants were recovered with Chios mitochondrial and chloroplastic genomes, one plant was recovered with Chios mitochondrial and Sanguinelli chloroplastic genomes and 11 plants were recovered with Sanguinelli mitochondrial and Chios chloroplastic genomes. Finally, three plants were recovered that exhibited a Chios chloroplastic genome alongside a recombined mitochondrial genome (Chios/Sanguinelli).
Electropherograms corresponding to mitochondrial (Nad2 and Nad7), chloroplastic (ccmp6 and NTCP9) and MEST015 nuclear SSR markers for genetic analysis of Chios, Sanguinelli, and diploid cybrid 1.5.11 from Sanguinelli + Chios somatic hybridization (S + C). a Sanguinelli, b Chios, and c 1.5.11 diploid cybrid of S + C amplified alleles for Nad2 and Nad7 mitochondrial InDel markers. For the Nad2 mitochondrial marker, the diploid cybrid displays the 260 nt Sanguinelli allele. For the Nad7 mitochondrial marker, the diploid cybrid displays the 152 nt Chios mandarin allele. d Sanguinelli, e Chios, and f 1.5.11 diploid cybrid of S + C amplified alleles for ccmp6 and NTCP9 chloroplastic SSR markers. The diploid cybrid displays the Chios mandarin alleles for both the ccmp6 (128 nt) and NTCP9 (259 nt) markers. g Sanguinelli, h Chios, and i 1.5.11 diploid cybrid of S + C amplified alleles for MEST015 SSR marker. The diploid cybrid displays the Sanguinelli sweet orange alleles
Wu et al. (2014) confirmed that clementines arose from hybridization of Common mandarin (female parent) and Sweet orange (male parent) (Carbonell-Caballero et al. 2015), as previously proposed by Deng et al. (1996), Nicolosi et al. (2000), Ollitrault et al.(2012b), and Garcia-Lor et al. (2012). Froelicher et al. (2011) observed seven different mitotypes in Citrus and related genera. One mitotype permitted the differentiation of an acidic group of mandarins from other mandarins, but it was not possible to distinguish between Common mandarin and Clementines. As Chios mandarin is a type of Common mandarin, it is not possible to distinguish Clementine from Chios mandarin with mitochondrial markers. Diploid and tetraploid plants regenerated from the Chios + Clementine somatic hybridization had Clementine nuclear genomes. This strongly suggests that the plants produced in this combination are cybrids because in citrus it has not been possible to regenerate plants from leaf protoplasts and only protoplast isolated from embryogenic callus or leaf protoplasts that incorporate the mitochondrial genome from callus protoplasts have the capacity to produce embryos and subsequently plants (Kobayashi et al. 1991; Grosser and Gmitter 2005; Guo et al. 2006). The recovery of citrus cybrid plants as a by-product of symmetric somatic hybridization was reported previously, with both diploid and (less frequently) tetraploid cybrids obtained (Grosser et al. 1996, 2000; Ollitrault et al. 2001; Guo et al. 2004a, b, 2006; Dambier et al. 2011; Xiao et al. 2014). Tetraploid cybrids were proposed to arise from protoplast fusion between one protoplast from the callus parent and two diploid protoplasts from the leaf parent, followed by failed nuclear fusion and the subsequent loss of the nucleus from the callus parent and the incorporation of mitochondria released from ruptured embryogenic cells into the fused leaf protoplasts (Grosser et al. 1996; Guo et al. 2006). Clementines are non-apomictic plants (Navarro et al. 2005), and the tetraploid cybrid recovered from Chios + Clementine somatic hybridization may therefore prove useful as male and female breeding parents in interploid hybridizations for the generation of triploid hybrids.
Guo et al. (2004a, b) suggested that the phenomenon of cybridization by symmetric fusion was dependent on the genotype of the embryogenic parent and the combination of parents. Of the 22 diploid plants regenerated from somatic hybridization between Chios callus protoplasts and Sanguinelli callus protoplasts, 18 were cybrids containing the nuclear genome of sweet orange and different combinations of mitochondrial and chloroplastic genomes. Dambier et al. (2011) performed somatic hybridizations with Chios mandarin callus and leaf protoplasts from three intergeneric hybrids: Citrange, Citrumelo (C. paradisi × P. trifoliata), and Citrandarin (C. reticulata × P. trifoliata). The recovered plants were all diploid cybrids that had nuclear and chloroplastic genomes from the intergeneric hybrid parent and the mitochondrial genome from Chios mandarin. Chios mandarin callus therefore has substantial potential as a producer of diploid cybrids via protoplast fusion.
Further genetic analysis of the cybrids produced in this study will allow the influence of mitochondrial and chloroplastic genomes on cybrid plants to be assessed. Previous studies examined the effect of cybridization on citrus phenotype, including changes to aroma (Fanciullino et al. 2005), fruit organic content (Bassene et al. 2008) and fruit organoleptic qualities (Satpute et al. 2015), resistance to ‘mal secco’ citrus disease caused by Phoma tracheiphila (Tusa et al. 2000), alteration in photosynthesis and stress resistance (Wang et al. 2010), reduced petal and retarded stamen primordia developments, and modifications of carbohydrate metabolism pathway and mitochondrial proteins in a male sterile cybrid of pummelo and satsuma mandarin (Zheng et al. 2012, 2014). Bassene et al. (2011) performed large-scale transcriptional profiling in a Willow leaf mandarin + Eureka lemon cybrid and found that mitochondrial replacement affected the expression of different nuclear genes, including some genes predicted to be involved in mitochondrial retrograde signaling.
The mitochondrial genome of the callus parent was prevalent in recovered cybrids and somatic hybrids in previous callus + leaf protoplast fusion experiments (Kobayashi et al. 1991; Saito et al. 1993; Yamamoto and Kobayashi 1995; Moriguchi et al. 1997; Moreira et al. 2000; Cabasson et al. 2001; Ollitrault et al. 2001; Guo et al. 2002; Xiao et al. 2014); however, rearrangements of the parental mitochondrial genomes were observed in some cases (Vardi et al. 1987; Moriguchi et al. 1997; Cheng et al. 2003; Dambier et al. 2011). The chloroplast genome was inherited from either the callus or leaf parent. In callus + callus protoplast fusions, although one of the mitochondrial genomes appeared to be prevalent (15/22 Chios + Sanguinelli plants had the Sanguinelli mitochondrial genome), the mitochondrial genome of the second parent (4/22) and recombination between both parents (3/22) was also observed. The chloroplast genome was inherited from either of the two callus parents. Callus + callus protoplast fusions appear to produce more variable mitochondrial and chloroplast genome combinations than callus + leaf hybridizations. Callus + callus fusions may therefore be useful for the production of new genetic combinations for citrus breeding schemes that cannot be obtained by traditional methods.
References
Ahmad R, Struss D, Southwick SM (2003) Development and characterization of microsatellite markers in Citrus. J Am Soc Hortic Sci 128:584–590
Aleza P, Juárez J, Ollitrault P, Navarro L (2009) Production of tetraploid plants of non apomictic citrus genotypes. Plant Cell Rep 28:1837–1846
Aleza P, Froelicher Y, Schwarz S, Agustí M et al (2011) Tetraploidization events by chromosome doubling of nucellar cells are frequent in apomictic citrus and are dependent on genotype and environment. Ann Bot 108(1):37–50
Bassene JB, Berti L, CarcouetE Dhuique-Mayer C, Fanciullino AL, Bouffin J, Ollitrault P, Froelicher Y (2008) Influence of mitochondria origin on fruit quality in a Citrus cybrid. J Agric Food Chem 56:8635–8640
Bassene JB, Froelicher Y, Navarro L, Ollitrault P, Ancillo G (2011) Influence of mitochondria on gene expression in a citrus cybrid. Plant Cell Rep 30:1077–1085
Beddie AD (1942) Natural root grafts in New Zealand trees. Transact Proc R Soc New Zeal 71:199–203
Button J, Kochba J (1977) Tissue culture in the citrus industry. In: Reinert J, Bajaj YPS (eds) Applied and fundamental aspects of plant cell, tissue and organ culture. Springer, Berlin, pp 70–92
Button J, Kochba J, Bornman CH (1974) Fine structure of and embryoid development from embryogenic ovular callus of ‘Shamouti’ orange (Citrus sinensis Osb.). J Exp Bot 25:446–457
Cabasson C, Luro F, Ollitrault P, Grosser JW (2001) Non random inheritance of mitochondrial genomes in Citrus hybrids produced by protoplast fusion. Plant Cell Rep 20:604–609
Cai XD, Fu J, Deng XX, Guo WW (2007) Production and molecular characterization of potential seedless cybrid plants between pollen sterile Satsuma and two seedy Citrus cultivars. Plant Cell Tiss Organ Cult 90:275–283
Carbonell-Caballero J, Alonso R, Ibañez V, Terol J, Talon M, Dopazo J (2015) A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus Citrus. Mol Biol Evol. doi:10.1093/molbev/msv082
Cheng YJ, Guo WW, Deng XX (2003) Molecular characterization of cytoplasmic and nuclear genomes in phenotypically abnormal Valencia orange (Citrus sinensis) + Meiwa kumquat (Fortunella crassifolia) intergeneric somatic hybrids. Plant Cell Rep 21:445–451
Cheng Y, de Vicente MC, Meng H, Guo W, Tao N, Deng XA (2005) Set of primers for analyzing chloroplast DNA diversity in Citrus and related genera. Tree Physiol 25(6):661–672
Cuenca J, Froelicher Y, Aleza P, Juarez J et al (2011) Multilocus half-tetrad analysis and centromere map-ping in citrus: evidence of SDR mechanism for 2n megagametophyte production and partial chiasma interference in mandarin cv ‘Fortune’. Heredity 107:462–470
Dambier D, Benyahia H, Pensabene-Bellavia G, Aka Kacar Y, Froelicher Y, Belfalah Z, Beniken L, Handaji N, Printz B, Morillon R, Yesiloglu T, Navarro L, Ollitrault P (2011) Somatic hybridization for citrus rootstock breeding: an effective tool to solve important issues of the Mediterranean citrus industry. Plant Cell Rep 30:883–900
De Pasquale F, Giuffrida S, Carimi F (1999) Minigrafting of shoots, roots, inverted roots, and somatic embryos for rescue of in vitro Citrus regenerants. J Am Soc Hortic Sci 124(2):152–157
Dellaporta J, Hicks JB (1983) A plant DNA mini preparation: version II. Plant Mol Biol Rep 1:19–21
Deng Z, Gentile A, Nicolosi E, Continella G, Tribulato E (1996) Parentage determination of some citrus hybrids by molecular markers. Proc Int Soc Citric 2:849–854
Fanciullino AL, Gancel AL, Froelicher Y, Luro F, Ollitrault P, Brillouet JM (2005) Effects of nucleo–cytoplasmic interactions on leaf volatile compounds from citrus somatic diploid hybrids. J Agric Food Chem 53:4517–4523
Froelicher Y, Dambier D, Bassene JB, Costantino G, Lotfy S, Didout C, Beaumont V, Brottier P, Risterucci AM, Luro F, Ollitrault P (2008) Characterization of microsatellite markers in mandarin orange (Citrus reticulata Blanco). Mol Ecol Resour 8(1):119–122
Froelicher Y, Mouhaya W, Bassene JB, Costantino G, Kamiri M, Luro F, Morillon R, Ollitrault P (2011) New universal mitochondrial PCR markers reveal new information on maternal citrus phylogeny. Tree Genet Genomes 7(1):49–61
Garcia-Lor A, Luro F, Navarro L, Ollitrault P (2012) Comparative use of InDel and SSR markers in deciphering the interspecific structure of cultivated citrus genetic diversity: a perspective for genetic association studies. Mol Genet Genomics 287(1):77–94
Goldschmidt EE (2014) Plant grafting: new mechanisms, evolutionary implications. Front Plant Sci 5:727. doi:10.3389/fpls.2014.00727
Grosser JW, Gmitter FG Jr (1990) Protoplast fusion and citrus improvement. Plant Breed Rev 8:339–374
Grosser JW, Gmitter FG Jr (2005) 2004 SIVB congress symposium proceedings ‘Thinking outside the cell’: applications of somatic hybridization and cybridization in crop improvement, with citrus as a model. In Vitro Cell Dev Biol Plant 41:220–225
Grosser JW, Gmitter FG Jr (2011) Protoplast fusion for production of tetraploids and triploids: applications for scion and rootstock breeding in citrus. Plant Cell Tiss Organ Cult 104:343–357
Grosser JW, Gmitter FG Jr, Tusa N, Reforgiato Recupero G, Cucinotta P (1996) Further evidence of a cybridization requirements for plant regeneration from citrus leaf protoplasts following somatic fusion. Plant Cell Rep 15:672–676
Grosser JW, Ollitrault P, Olivares-Fuster O (2000) Somatic hybridization in citrus: an effective tool to facilitate variety improvement. In vitro Cell Biol Plant 36:434–449
Grosser JW, Calovic M, Louzada ES (2010) Technology—somatic hybridization and cybridization. In: Davey MR, Anthony P (eds) Plant cell culture. Essential methods. Wiley, New York, pp 175–198. doi:10.1002/9780470686522
Guo WW, Deng XX (2001) Wide somatic hybrids of Citrus with its related genera and their potential in genetic improvement. Euphytica 118:175–183
Guo WW, Cheng YJ, Deng XX (2002) Regeneration and molecular characterization of intergeneric somatic hybrids between Citrus reticulata and Poncirus trifoliata. Plant Cell Rep 20:829–834
Guo WW, Cai XD, Grosser JW (2004a) Somatic cell cybrids and hybrids in plant improvement. In: Daniell H, Chase CD (eds) Molecular biology and biotechnology of plant organelles. Kluwer Academic Publisher, Dordrecht, pp 635–659
Guo WW, Prasad D, Cheng YJ, Serrano P, Deng XX, Grosser JW (2004b) Targeted cybridization in Citrus: transfer of Satsuma cytoplasm to seedy cultivars for potential seedlessness. Plant Cell Rep 22:752–758
Guo WW, Cheng YJ, Chen CL, Deng XX (2006) Molecular analysis revealed autotetraploid, diploid and tetraploid cybrid plants regenerated from an interspecific somatic fusion in Citrus. Sci Hortic 108:162–166
Guo WW, Xiao SX, Deng XX (2013) Somatic cybrid production via protoplast fusion for citrus improvement. Sci Hortric 163:20–26
Kijas JMH, Thomas MR, Fowler JCS, Roose ML (1997) Integration of trinucleotide microsatellites into a linkage map of Citrus. Theor Appl Genet 94(5):701–706
Kobayashi S, Ohgawara T, Fujiwara K, Oiyama I (1991) Analysis of cytoplasmic genomes in somatic hybrids between navel orange (Citrus sinensis Osb.) and ‘‘Murcott’’ tangor. Theor Appl Genet 82:6–10
Kumar A, Cocking EC (1987) Protoplast fusion—a novel-approach to organelle genetics in higher plants. Am J Bot 74:1289–1303
Melchers G, Mohri Y, Watanabe K, Wakayabashi S, Harada K (1992) One-step generation of cytoplasmic male sterility by fusion of mitochondrial-inactivated tomato protoplasts withnuclear-inactivated Solanum protoplasts. Proc Natl Acad Sci USA 89:6832–6836
Moreira CD, Chase CD, Gmitter FG, Grosser JW (2000) Inheritance of organelle genomes in citrus somatic cybrids. Mol Breed 6:401–405
Moriguchi T, Motomura T, Hidaka T, Akihama T, Omura M (1997) Analysis of mitochondrial genomes among Citrus plants produced by the interspecific somatic fusion of ‘Seminole’ tangelo with rough lemon. Plant Cell Rep 16:397–400
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Navarro L, Juárez J (2007) Shoot-tip grafting in vitro. In: Khan IA (ed) Citrus genetics. Breeding and biotechnology. CABI Head Office, Wallingford, pp 353–364
Navarro L, Roistacher CN, Murashige T (1975) Improvement ofshoot-tip grafting in vitro for virus-free citrus. J Am Soc Hortic Sci 100:471–479
Navarro L, Juárez J, Aleza P, Pina JA, Olivares-Fuster O, Cuenca J, Julve JM (2005) Programa de obtención de híbridos triploides de mandarino en España. Phytoma 170:36–41
Nicolosi E, Deng ZN, Gentile A, Malfa S, Continella G, Tribulato E (2000) Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor Appl Genet 100:1155–1166
Niedz RP, Hyndman SE, Wynn ET, Bauscher MG (2002) Normalizing sweet orange (C. sinensis (l.) Osbeck) somatic embryogenesis with semi-permeable membranes. In Vitro Cell Dev Biol Plant 38:552–557
Olivares-Fuster O (1988) Fusión de protoplastos de cítricos. PhD thesis, Universidad Politécnica de Valencia, Valencia, Spain
Olivares-Fuster O, Duran-Vila N, Navarro L (2005) Electrochemical protoplast fusion in citrus. Plant Cell Rep 24:112–119
Ollitrault P (1992) Somatic embryo grafting: a promising technique for citrus breeding and propagation. Fruit 47:213–218
Ollitrault P, Dambier D, Froelicher Y, Luro F, Cottin R (2001) La diversite´ des agrumes: structuration et exploitation par hybridation somatique. Comptes-rendus de l’Academied’agriculture 86:197–221
Ollitrault F, Terol J, Pina JA, Navarro L, Talon M, Ollitrault P (2010) Development of SSRmarkers from Citrus clementina (Rutaceae) BAC end sequences and interspecific transferability in Citrus. Am J Bot 97(11):e124–e129
Ollitrault P, Terol J, Chen C, Federici C, Lotfy S et al (2012a) A reference genetic map of C. clementine Hort. ex Tan.; citrus evolution inferences from comparative mapping. BMC Genomics 13:593
Ollitrault P, Terol J, Garcia-Lor A, Berard A, Chauveau A, Froelicher Y, Belzile C, Morillon R, Navarro L, Brunel D, Talon M (2012b) SNP mining in C. clementina BAC end sequences; transferability in the Citrus genus (Rutaceae), phylogenetic inferences and perspectives for genetic mapping. BMC Genomics 13:13
Pérez RM, Galiana AM, Navarro L, Duran-Vila N (1998) Embryogenesis in vitro of several Citrus species and cultivars. J Hortic Sci Biotechnol 73:796–802
Saito W, Ohgawara T, Shimizu J, Ishii S, Kobayashi S (1993) Citrus cybrid regeneration following cell fusion between nucellar cells and mesophyll cells. Plant Sci 88:195–201
Satpute AD, Chen C, Gmitter FG Jr, Ling P, Yu Q, Grosser MR, Chase CD, Grosser JW (2015) Cybridization of grapefruit with ‘Dancy’ mandarin leads to improved fruit characteristics. J Am Soc Hortic Sci 140(5):427–435
Tomaz ML, Mendes BMJ, Mourao FA, Demetrio CGB, Jansakul N, Martinelli-Rodriguez AP (2001) Somatic embryogenesis in citrus spp.: carbohydrate stimulation and histo differentiation. In Vitro Cell Dev Biol Plant 37:446–452
Tusa N, Bosco SFD, Nigro F, Ippolito A (2000) Response of cybrids and a somatic hybrid of lemon to Phoma tracheiphila infections. HortScience 35:125–127
Vardi A, Breiman A, Galun E (1987) Citrus cybrids: production by donor-recipient protoplast fusion and verification by mitochondrial-DNA restriction profiles. Theor Appl Genet 75:51–58
Wang L, Pan Z-Y, Guo WW (2010) Proteomic analysis of leaves from a diploid cybrid produced by protoplast fusion between Satsuma mandarin and pummelo. Plant Cell Tissue Organ Cult 103:165–174
Weising K, Gardner RC (1999) A set of conserved PCR primers for the analysis of simple sequence repeat polymorphisms in chloroplast genomes of dicotyledonous angiosperms. Genome 42:9–19
Wu AG, Prochnik S, Jenkins J, Salse J, Hellsten U, Murat F, Perrier X, Ruiz M, Scalabrin S, Terol J, Takita MA, Labadie K, Poulain J, Couloux A, Jabbari K, Cattonaro F, Del Fabbro C, Pinosio S, Zuccolo A, Chapman J, Grimwood J, Tadeo FR, Estornell LH, Muñoz-Sanz JV, Ibañez V, Herrero-Ortega A, Aleza P, PérezPérez J, Ramón D, Brunel D, Luro F, Chen C, Farmerie WG, Desany B, Kodira C, Mohiuddin M, Harkins T, Fredrikson K, Burns P, Lomsadze Borodovsky M, Reforgiato G, Freitas-Astúa J, Quetier F, Navarro L, Roose M, Wincker P, Schmutz J, Morgante M, Machado MA, Talon M, Jaillon O, Ollitrault P, Gmitter F, Rokhsar D (2014) Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat Biotechnol. doi:10.1038/nbt.2906
Xiao SX, Biswas MK, Li MY, Deng XX, Xu Q, Guo WW (2014) Production and molecular characterization of diploid and tetraploid somatic cybrid plants between male sterile Satsuma mandarin and seedy sweet orange cultivars. Plant Cell Tiss Organ Cult 116:81–88
Yamamoto M, Kobayashi S (1995) A cybrid plant produced by electrofusion between Citrus unshiu (satsuma mandarin) and C. sinensis (sweet orange). Plant Tissue Culture Lett 12:131–137
Zheng BB, Wu XM, Ge XX, Deng XX, Grosser JW, Guo WW (2012) Comparative transcript profiling of a male sterile cybrid pummelo and its fertile type revealed altered gene expression related to floral organ development. PLoS One 7(8):e43758
Zheng BB, Fang YN, Pan ZY, Sun L, Deng XX, Grosser JW, Guo WW (2014) iTRAQ based quantitative proteomics analysis revealed alterations of carbohydrate metabolism pathways and mitochondrial proteins in a male sterile cybrid pummelo. J Proteome Res 13:2998–3015
Acknowledgments
This work was supported by a grant [AGL2011-26490] from the Ministry of Economía y Competividad–Fondo Europeo de Desarrollo Regional (FEDER).
Authors’ contribution
P. Aleza and L. Navarro conceived and designed the experiments. P. Aleza performed protoplast fusions. J. Jua´rez analyzed flow cytometry data. A. Garcia-Lor performed the genetic analysis. P. Aleza and L. Navarro wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aleza, P., Garcia-Lor, A., Juárez, J. et al. Recovery of citrus cybrid plants with diverse mitochondrial and chloroplastic genome combinations by protoplast fusion followed by in vitro shoot, root, or embryo micrografting. Plant Cell Tiss Organ Cult 126, 205–217 (2016). https://doi.org/10.1007/s11240-016-0991-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-0991-8