Abstract
Current rheumatology guidelines recommend exercise as a key component in the management of people with RA, however, what is lacking is evidence on its impact on sleep. Objective is to assess the feasibility of a walking-based intervention on TST, sleep quality, and sleep disturbance and to generate potential effect size estimates for a main trial. Participants were recruited at weekly rheumatology clinics and through social media. Patients with RA were randomized to a walking-based intervention consisting of 28 sessions, spread over 8 weeks (2–5 times/week), with 1 per week being supervised by a physiotherapist, or to a control group who received verbal and written advice on the benefits of exercise. Primary outcomes were recruitment, retention, protocol adherence and participant experience. The study protocol was published and registered in ClinicalTrials.gov NCT03140995. One hundred and one (101) people were identified through clinics, 36 through social media. Of these, 24 met the eligibility criteria, with 20 randomized (18% recruitment; 100% female; mean age 57 (SD 7.3 years). Ten intervention participants (100%) and eight control participants (80%) completed final assessments, with both groups equivalent for all variables at baseline. Participants in the intervention group completed 87.5% of supervised sessions and 93% of unsupervised sessions. No serious adverse events were related to the intervention. Pittsburgh Sleep Quality Index global score showed a significant mean improvement between the exercise group-6.6 (SD 3.3) compared to the control group-0.25 (SD 1.1) (p = 0.012); Intervention was feasible, safe and highly acceptable to study participants, with those participants in the exercise group reporting improvements in sleep duration and sleep quality compared to the control group. Based on these findings, a fully powered randomized trial is recommended. Trial registration number: ClinicalTrials.gov Identifier: NCT03140995 (April 25th, 2017)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune condition that can lead to reduced activity levels in up to 60% of participants [1]. Poor sleep quality is prevalent in people with RA [2] with 40–64% of participants reporting reduced Total Sleep Time (TST) [3, 4].
Results from a recent systematic review could find no strong evidence for the effect of exercise on sleep in people with RA, partly due to the small number of studies (N = 5) available [5]. In addition, the lack of studies of the highest methodological quality complicates the interpretations of the findings, therefore, the most effective exercise prescription in terms of the Frequency, Intensity, Time and Type (FITT) principle, and the ideal approach to exercise delivery, requires further research.
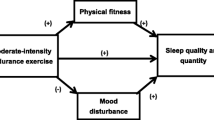
Evidence indicates that sleep is an essential aspect in maintaining the body’s circadian rhythm and maintaining health-related quality of life (HRQoL), therefore, sleep disturbances could have a detrimental impact on same in people with RA. Various sleep organisations support TST figures, with the American Academy of Sleep Medicine (AASM) and the Sleep Research Society (SRS) recommending adults sleep at least 7 h per night to promote optimal health and well-being [6], while the National Sleep Foundation (NSF) adults/older adults advocate a ‘sleep needs spectrum’ of 7–9 h sleep per night. This ‘spectrum’ is necessary, as sleep has a role to play in our immune system and is also important in the restoration and maintenance of homeostasis. Sleep disorders and reduced TST may lead to the development of autoimmune diseases like RA, due to the triggering of autoantibody production [7].
With sleep being identified as a major concern for people with RA, and disturbed sleep and fatigue known to affect up to 70% in this population [8], health professionals (HPs) should be concerned with the effect low TST and poor sleep quality has on HRQoL. Low TST and poor sleep quality, in addition to their effect on mental and physical health [9, 10], may lead to people with RA being less active [11]. Therefore, aiming to increase TST and improving sleep quality through exercise, may be a health promotion strategy that is feasible and safe for this population. As exercise prescription is a core skill for some HPs e.g. physiotherapists, they should play an important role in educating patients on the benefits of increasing their exercise levels [12], and its potential positive effect on sleep, however, based on research to date, interventions are required to show an effect [5].
A number of studies have investigated the effects of aerobic exercise on TST and sleep quality in other populations and while results suggest they are beneficial, it is unclear how large these benefits are [13, 14]. Cross-sectional self-report and objective studies in people with RA indicate a population with low TST, along with those who are more active indicating a longer TST, supports the need to further this area of research to test for effect [4, 12]. Exercise is recommended as a key component in the management of people with RA [15, 16], however, what is lacking is evidence of its impact on TST and sleep quality [5, 17].
It has been well established that being physically active and taking regular exercise are important for those who have been diagnosed with RA across the lifespan [18]. Research has shown that people with RA may benefit from several forms of exercise [19], which are safe and beneficial conferring benefits at low risk to people with RA [20]. However, adherence to exercise is often low or unrecorded [12], raising questions about the feasibility and acceptability of some forms of aerobic exercise in people with RA and, in particular, for improving TST and sleep quality.
Walking is an ideal form of aerobic exercise owing to its ease of accessibility and relatively low impact, with a low risk of musculoskeletal injury [21]. Low-to-moderate intensity walking has been shown to lead to improvements in aerobic capacity and body mass index [22]. Walking is, therefore, a low-cost and simple form of exercise, requiring little formal training and has been found to be feasible, acceptable and safe, for people with RA [23]. Previous studies have used walking as an exercise intervention for people with arthritis and have involved a non-randomized control (non-RCT) trial design, or have focused on community samples with self-reported diagnoses of arthritis [24]. A 2016 pilot RCT demonstrated that people with RA found their walking intervention feasible and acceptable as they participated in the required number of sessions per week [23]. Furthermore, the authors reported their intervention safe, as no adverse events (AEs) were reported and pain levels did not differ between the intervention and control groups. These findings concur with previous systematic reviews [25] which showed that AEs are rare for people with RA who participate in PA and exercise. Together, these findings should provide encouragement and reassurance to HPs, recommending walking as an aerobic exercise for people with RA. However, the potential role of walking as an exercise intervention in the management of RA, specifically to improve TST and sleep quality, has yet to be studied.
High-quality research is required to inform and implement evidence-based practice (EBP) [26], which requires an appropriate study design to answer the research question [27]. To investigate the effect of various types of interventions, like PA and exercise, randomized controlled trials (RCT) are recognized as the gold standard for study designs [28]. The successful development and implementation of an RCT can encounter many barriers from a design perspective and, therefore, it is prudent to undertake pilot work prior to fully engaging in a large study. The second phase of the Medical Research Council (MRC) framework recommends pilot studies to investigate the feasibility of the study design in the intended population and highlight any barriers to the success of a larger scale trial [29], thereby reducing research waste in exercise interventions. Therefore, the aim of this study is to assess the feasibility of a walking-based intervention to improve sleep (time, quality, and disturbance), in people with RA to inform an RCT.
Materials and methods
Study design
The design was a single blinded pilot RCT of a walking-based exercise intervention against information on the benefits of exercise for an 8-week period. All outcome measures were collected by a researcher blinded to the intervention (LC). The exercise intervention was delivered by the first author (SMcK) who is a chartered physiotherapist. Participant characteristics and outcome assessments were collected by the blinded assessor at baseline (T1) and 1-week post-intervention (T2). The study protocol was published [30] and registered in ClinicalTrials.gov NCT03140995.
Screening using the Pittsburgh Sleep Quality Index (PSQI) [31] was used to clarify whether participants fulfilled the criteria for poor sleep (PSQI global score > 5), and to ascertain their physical activity levels. Participants completed a consent form and were free to withdraw at any time. While no compensation was provided, participants were equipped with a ‘High Visibility Vest’, which is a piece of clothing that is highly luminescent in its natural matt property or a colour that is easily discernible from any background. This was provided for safety due to the intervention taking place in the late Autumn/early Winter.
Participant recruitment
A sample of convenience was used with participants meeting the inclusion and exclusion criteria (Table 1) being identified through weekly rheumatology clinics or contacted through social media.
Ethics
Approval was obtained from the Research Ethics Committee at a University Hospital (REC: 60/17) and procedures performed in the study involving human participants were in accordance with the ethical standards of the University and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Participants completed a consent form and were free to withdraw at any time. The reporting of results are recorded in accordance with the Consolidated Standard of Reporting trials (CONSORT) for pilot trials [32].
Intervention and control group
The exercise intervention is outlined in Table 2 and was a walking-based exercise intervention based on the American College of Sports Medicine (ACSM) aerobic exercise guidelines [33], which is similar to that as recommended by the World Health Organisation [34] and recent European League against Rheumatism (EULAR) PA guidelines [18], of being moderate intensity (50–80% of HRR) for at least 30 min on 5 or more days, for a total of 150 min per week. The supervised sessions were group based, while the unsupervised sessions were performed by the participant at a time and location of their choice.
As participants would not be meeting the relevant PA guidelines, the programme was devised using incremental targets for daily walks and participants were advised to monitor and progress their exercise intensity using the Borg Rating of Perceived Exertion (BORG) (RPE) scale (range 6–20) [35]. Participants were instructed to be moderately short of breath on exertion and were encouraged to maintain an RPE of 12–17 (equivalent to 50–80% of maximal exertion). This scale is a frequently used quantitative measure of perceived exertion during exercise [36] and has been found to be highly correlated with heart rate, lactate levels, %VO2max, and breathing [37]. Studies have supported the validity of the RPE scale in a wide range of populations, including inflammatory arthritis [38]. In addition, participants’ cardiorespiratory fitness was assessed using a sub-maximal treadmill test, i.e. modified Bruce protocol, which is walking based and involves walking at an increasing gradient, stopping at a HR/RPE threshold [39].
Participants were advised to seek medical assistance if there was adverse reaction during the intervention e.g. flare-up, fall, or if the participant feels unwell.
Randomization and blinding
Randomization was performed by computer generated random numbers with a 1:1 allocation ratio. Allocations were stored in a locked cabinet and an envelope was handed to participants after completion of their baseline assessments. Each envelope contained a code number, which participants used on all outcome assessments in place of their names.
Primary outcomes
Recruitment
As this is a pilot study, sample size calculations were not required [40]. The target recruitment was 40 participants, which was considered to be a realistic target for the timeframe available (3 months) and was a similar sample size to other pilot RCTs in people with RA [2, 23, 41]
Retention
Conservative rate of 80%, which is established a priori as acceptable for this type of study [17]
Protocol adherence
Attendance at weekly supervised sessions; completion of a weekly exercise log for the unsupervised part and completion of a weekly National Sleep Foundation’s (NSF) sleep diary. Accepted levels of adherence were based on the nature and frequency of reported attendance, with data being reported for those who completed and did not complete the intervention with a priori level of 80%.
Participant experience
According to the protocol [30], a qualitative evaluation was conducted using semi-structured face-to-face interviews. These will be reported separately.
Safety
Primary safety outcomes included the type and frequency of adverse events (AEs) [42].
Secondary outcomes
Physical activity profile
This was quantified using an activPAL3 accelerometer continuously worn on the right thigh by the participants for 8 days, beginning week 1 before start of intervention and for 8 days 1 week post-intervention [43], with the first 24 h were not included in the analysis to minimise the effects reactivity. Though an 8-day wear-time was employed, the first 24 h were not included in the analysis to minimise the effects reactivity to wearing the device. A minimum recording duration of 3 days from the last 7, including at least 1 weekend day was required for data processing; samples of lower than 3 days were not included. Recordings were processed for daily minutes of moderate to vigorous physical activity (MVPA). The activPAL activity monitor, classifies an individual’s free-living activity into periods spent in sedentary, standing, and walking behaviours through the use of proprietary algorithms. It has been found to be a reliable and valid measure of sedentary and physical activity behaviours and transition and step counts in other populations [44, 45], including adults who are healthy and adults who are overweight, in addition to walking behaviours in people with RA [46].
Sleep
Measured using the Pittsburgh Sleep Quality Index (PSQI) [31], with sleeping pattern measured by the National Sleep Foundation’s (NSF) Sleep Diary. The PSQI measures TST, sleep quality, sleep latency, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction over the last month.
Pain (RA)
Measured with a 10 cm visual analogue scale (VAS), which has good reliability and validity, and is sensitive to detecting changes in pain in inflammatory conditions [47].
Mood
Which includes depression and anxiety, measured by the Profile of Mood States questionnaire (POMS) [48], which has an internal consistency of 0.63–0.96 Cronbach alpha rating; Quick Inventory of Depressive Symptomatology (QIDS-SR16) [49], which has an internal consistency of 0.86 Cronbach alpha and has high concurrent validity and the State Trait Anxiety Inventory (STAI) [50], which has an internal consistency ranging from 0.86 to 0.95.
Functional limitation
Measured by the Health Assessment questionnaire disability index (HAQ-DI), which is the most commonly used measure of functional disability in RA and has good-to-excellent reliability and validity [51].
Disease activity: evaluated using the Clinical Disease Activity Index (CDAI) which has been implemented and validated for RA using several clinical trial datasets [52].
Health-related Quality of life: measured by EuroQoL, which measures health-related quality of life and contains five dimension, with each dimensions having five levels from ‘no problems’ to ‘extreme problems’ [53].
Fatigue
Measured by the Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF-NS) [54]. This measures the impact of fatigue for people with RA and disease specific and has acceptable to good convergent validity.
Barriers to exercise
Evaluated using the Exercise Benefits and Barriers Scale (EBBS) [55].Validation studies show internal consistencies between 0.80 and 0.94.
Data analysis
Scoring of the standardized questionnaires was carried out according to the guidelines from the instrument developers with participant code numbers ensuring blinding of data analysis. Data were double entered Microsoft Office Excel (2013), which was used for descriptive analysis of demographic questions. Categorical data were described using counts and percentages. As recommended, continuous data presented using medians and interquartile ranges (IQR) whether they are normal or not [56]. A 5% level of significance was used for all statistical tests. Data analysis was undertaken in SPSS version 22 (IBM corporation, New York, USA) with activPAL version 7.2.32 being used for physical activity profile.
Results
Patient characteristics
Twenty-four (24) participants were recruited, with 4 withdrawing pre-assessment resulting in 20 randomised to the intervention (N = 10) or control (N = 10) (Fig. 1). Baseline demographic and clinical characteristics are reported in Table 3.
All participants were female, with a mean age of 57 (SD 7.5); mean RA diagnosis of 10.7 (SD 6.4) years; moderate-to-severe disability (HAQ-DI: 1.4 (SD 0.63). Participants were predominately married (85%), in employment (55%), and educated to third level (50%). Biological Disease-modifying antirheumatic drugs (DMARDS) were taken by 75%, with 50% taking sleep medications, either ‘prescribed or over the counter’.
The DAS28 score was noted but not recorded for this study, however, it should be for any future trial.
Primary outcome measures
Recruitment
137 participants were invited to join the study, of whom 58 (42%) expressed interest and were screened for eligibility. Thirty-four (34) were excluded at assessment with the remaining 24 meeting all eligibility criteria and were recruited, with 20 being randomised. The final recruitment rate was 18% and all were female.
Retention
Participant retention exceeded the a priori level of 80% at baseline (100% intervention and control) and primary time point (100% for intervention; 80% for control).
Protocol adherence
Supervised sessions
For the supervised sessions, participant attendance was 87.5%, with a mean of 9 (SD 2) sessions across the group.
Unsupervised sessions
For the unsupervised part, the participants’ exercise log indicated 93% completed, with a mean of 18.5 (SD 4) sessions.
Sleep diary
Poor compliance with the NSF’s sleep diary, with many missing values in the data, made it difficult to analyse.
Adverse events
Participants reported several adverse events (AEs) during the 8-week intervention period; however, none were serious. The most common AE was increased musculoskeletal pain, which was generally mild, short-term and located in the lower body (Table 4).
Power and sample size calculations
Based on the results from the PSQI with different SDs in each treatment group, a sample size of 18 in each group will have a 90% power to detect a difference in means of 3.000, assuming that the Group 1 SD is 3.500 and the Group 2 SD is 1.200 (ratio of Group 2 to Group 1 standard deviation is 0.343), using a two group Satterthwaite t test with a 0.050 two-sided significance level [57].
A sample size of 18 in each group will have a 90% power to detect a difference in means of 3.000, assuming that the common standard deviation is 2.620 using a two-group t test with a 0.050 two-sided significance level. This is the same result if we assume equal SDs in the groups [57].
These are both estimating the number needed to detect a difference of at least 3 (PSQI) between the groups. Assuming a drop-out rate of 30% provides a figure of 26 per group, however, if researchers think the dropout will be higher than this, then they should recruit more e.g. 30 per group.
Secondary outcome measures
Descriptive statistics for secondary outcomes are reported in Table 5.
Physical activity profile
Those in the Intervention group were meeting the ACSM activity guidelines pre-intervention (moderate intensity, for at least 30 min on 5 or more days, for a total of 150 min per week) of 152 min [IQR 93, 211]; there was no difference compared to the control group at baseline (p = 0.22). Post-intervention, participants in the exercise group showed a significant improvement in their weekly MVPA (p < 0.003). With regards to their fitness, both groups were comparable at baseline (p = 0.27), with exercise participants significantly improving post intervention (p < 0.001).
Sleep
Baseline scores were similar for both groups, indicating a sample with poor sleep (PSQI: Intervention 13.4 [SD 2.6], Control 12.8 [SD 2.9]); reporting low TST (Intervention 6.1 [SD 0.6], Control 5.45 [SD 1.1]).
PSQI global scores showed a mean improvement for the intervention group of 6.6 (SD 3.3) (6 h 36 min) and control 0.25 (SD 1.1) (15 min), while TST showed a mean improvement for the intervention group of 1.65 (SD 0.39) (1 h 39 min) hours and control 0.56 (SD 0.46) (34 min) (Table 6).
PSQI subcomponent for ‘Sleep Latency’ indicates an improvement for the Intervention group of 16 min (SD 17.2), compared to 3 min (SD 22.0) for Control, while PSQI subcomponent ‘Sleep efficiency’ improved 12.5% (SD 8.3) for the Intervention group compared to 5% (SD 7.1) for Control.
PSQI subcomponent ‘Sleep quality’ indicated those in the intervention improved their sleep quality from very bad/fairly bad to fairly good/very good, while those in control reported no change at very bad/fairly bad.
Pain (RA)
Mean reduction (VAS 0/10) for intervention of -1.9 (SD 1.2) compared to 0.4 (SD 0.8) for control.
Mood
Moderate depression (QIDS-SR: Intervention 11.7 [SD 3.9], Control 12.2 [SD 3.3]), and moderate to high for both state anxiety (STAI-State: Intervention 47.3 [SD 2.2], Control 45.4 [SD 3.9]) and trait anxiety (STAI-Trait: Intervention 42.6 [SD 3.0], Control 45.5 [SD 3.9]).
Functional limitation
HAQ scores mean difference − 0.60 (SD 0.42) for intervention and 0.14 (SD 0.28) for control.
Fatigue
Reduced levels of fatigue for intervention − 11 (IQR − 16, − 7) compared to control 1 (IQR − 1, 3).
Barriers to exercise
The EBBS statement number 26, ‘Exercise helps me sleep better at night’, asks participants to indicate the degree to which they agree or disagree with the above statement. Pre-intervention 9/10 Intervention participants and 9/10 Control Disagreed/Strongly Disagreed; Post-intervention 10/10 Intervention participants Strongly Agreed/Agreed, and 8/10 Control Disagreed/Strongly Disagreed with the above statement.
Discussion
The aim of this pilot RCT was to determine the feasibility of walking as an exercise intervention in RA management for improving sleep (time, quality and disturbance), and to examine if a larger adequately powered trial would be indicated. The data from this study indicate that the walking-based intervention was both feasible and safe for people with RA who have poor sleep, moderate-to-severe disability and moderate disease activity. This study provides preliminary evidence that this approach to exercise could be a beneficial option in improving TST and sleep quality, thus a larger study powered to test for effect is warranted. Benefits of such a programme could extend beyond sleep to include increased self-efficacy, improved pain, stiffness, and physical function.
Identifying participants at two rheumatology clinics resulted in 60% (N = 24) of the targeted sample size (N = 40) being achieved within a three-month recruitment period, indicating an interest among people with RA trialling walking for the management of poor sleep. A commonly reported issue with the conduct of RCTs is that recruitment is often slower or more difficult than expected [58]. There are promising strategies for increasing recruitment to trials, most notably telephone reminders, open-trial designs with focus groups to investigate methods to improve the recruitment of males would be recommended. There is also evidence to indicate that recruitment in September, is more beneficial with potential increased interest from participants [59].
An excellent retention of participants from both groups was achieved, indicating satisfaction and acceptability. High PSQI scores from the participants were consistent with other studies in patients with chronic pain [60], therefore, the PSQI would be a useful screening tool for future trials. Further refinement of the inclusion criteria using a cut-off point from the PSQI of ≥ 5 would ensure a greater homogeneity of the sample and only recruit those with significant RA-related poor sleep quality. Sleep efficiency (SE) ranged from 66.5% pre-intervention to 79% post-intervention, which is considered below normative limits ≥ 85%. It is acknowledged that self-reported sleep may have conflicting reports from participants’ recall of their sleep quality, which highlights the need to collect objective data in a future RCT. Although polysomnography (PSG) is the gold standard sleep assessment method, it is expensive and time-consuming, therefore, actigraphy might be a more appropriate method. Advances in the availability of smartphone apps and wearables for health monitoring is staring to provide a previously unobtainable mechanism to collect regular self-reported symptoms and objective sleep data, while embedding data collection into participants’ everyday lives e.g. a triaxial accelerometer MotionWatch8 [61]. However, it is important to note that quiet wakefulness is categorized as sleep by some actigraphy methods, thus highlighting the need to continue to use both subjective and objective outcome measures in a future RCT.
From the NSF sleep diary, it was possible to obtain TST, SOL, and SE for each participant on a nightly basis during the intervention. However, due to issues with compliance in completing the diary, there were many missing values in these data and as a result the data could not be analysed. Therefore, the poor compliance with the sleep diary over a 7-night period, as recommended by the NSF and the American Academy of Sleep Medicine (AASM) [6] needs to be addressed. Recent developments in phone and tablet technology that can integrate an electronic diary application, with automatic prompts for those not responding or forgetting, present alternatives to improve the rate of completion of sleep diaries.
Although there is a growing consensus that exercise will benefit sleep for those experiencing a chronic health disorder, research is still inadequate for those with a rheumatic condition [5, 14, 62]. Because of the multifactorial nature of RA, that is, how it affects a person both physically and psychosocially, engaging in exercise may not only improve sleep quality but also mitigate some of its symptoms [8, 17]. As exercise prescription is a core skill for some health professionals (HPs) [16], they should, therefore, play an important role in educating people with RA on the benefit of increasing their exercise levels in improving their TST and sleep quality. Given that a recent systematic review has provided further evidence that being physically active is an important contributor to symptom management in people with RA [63], it is essential that any negative beliefs regarding exercise’s impact on sleep are challenged by HPs when seeking to promote their exercise levels.
Comprehensive reporting of adverse events (AEs) indicated that the current study was low risk for individuals with RA, with no serious AEs attributed to the study. The most common AE associated with the intervention was delayed onset of musculoskeletal soreness (DOMS), lasting 24–48 h after class, due to previous inactivity. The risk of falling was not part of this study and as the adverse events were low the intervention is not deemed to increase the risk of falling. However, the risk of falling is an important area of research that still has to be fully explored in people with RA. In any future, fully powered RCT to assessing participants for their risk of falls should be considered.
Sleep has an essential role to play in our immune system and is necessary in the restoration and maintenance of homeostasis [7]. Sleep disorders may trigger immune system abnormalities inducing autoantibody production, which may lead to the development of autoimmune disease such as RA [7]. People with RA have varied sleep patterns and from our study have reduced TST, in addition to having lower physical activity and exercise profiles. The results presented from our pilot RCT will contribute data to the field of exercise and sleep which is currently lacking [64].
Study strengths and limitations
This study presents preliminary evidence that a walking-based exercise intervention has a positive impact on sleep in people with RA. However, exercise, is not, by itself, enough evidence that it is the primary impact, therefore, as sleep is a complex issue it may require several lifestyle changes to improve same.
This was a rigorous and controlled, single-blinded intervention conducted at a University research centre, with no dropouts from the Intervention group and all data analysed. The study indicates that the intervention is feasible, and that participant compliance with the exercise intervention and recording measures was high. However, as this study was a pilot RCT, it was not adequately powered to detect significant differences between the intervention and control group and may be of insufficient length to determine any impact on clinical outcomes. Results of statistical analysis should be interpreted with caution due to small sample size; however, preliminary results are encouraging. In addition, data on comorbidities and specific steroid medications were not collected in this study, but are recommended in any future RCT.
A further limitation relates to the sample size of the study. Recruitment took place over the summer period due to the timeline of the study. A summer recruitment period was less than ideal and reduced the availability of potential participants due to the holiday season. Future research should consider recruitment in the Autumn.
Participants in this study were independently mobile and able to be active and, therefore, may not be representative of those with greater mobility limitations and with a variety of activity levels.
Involvement of public and patients in research is associated with improved outcomes and translation into practice and is advocated by the European League against Rheumatism (EULAR) recommendations through the inclusion of two patient representatives in scientific projects [65], which this study has facilitated. Aiming to improving TST and sleep quality through increasing exercise may be a health promotion strategy that is feasible and safe for this population. Therefore, the implications of this study provide a framework for larger intervention studies and based on these findings, a fully powered trial of walking as an exercise intervention is recommended, preceded by focus groups to investigate methods to improve the recruitment of males.
Conclusion
This pilot RCT explored the potential of a walking-based exercise intervention to improve sleep in people with RA, to inform the development of a fully powered trial. This intervention was found to be feasible and safe to study participants, with those participants in the exercise group reporting improvements in TST and sleep quality compared to the control group.
The consistent positive improvements in sleep outcomes observed provide preliminary evidence of the effect of a physiotherapist led walking intervention on sleep in a sample of people with RA. Participants expressed positive comments in relation to the intervention, including overall enjoyment of the exercise programme. These findings should inform the design for a future larger trial RCT of a walking-based exercise intervention for people with RA, to improve TST, sleep quality, and sleep disturbances. The poor compliance with the sleep diary and the lack of males being recruited are limitations that warrant further investigation, however.
References
Firestein GS (2005) Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. JCR J Clin Rheumatol 11(3):S39–S44
Løppenthin K et al (2014) Effect of intermittent aerobic exercise on sleep quality and sleep disturbances in patients with rheumatoid arthritis–design of a randomized controlled trial. BMC Musculoskelet Disord 15(1):49
Mckenna S et al (2018) Sleep and physical activity: a survey of people with inflammatory arthritis and their engagement by health professionals in rheumatology in Ireland. Disabil Rehabil 40(19):2260–2266
Mckenna S et al (2018) Sleep and physical activity: a cross-sectional objective profile of people with rheumatoid arthritis. Rheumatol Int 38(5):845–853
McKenna S et al (2017) Does exercise impact on sleep for people who have rheumatoid arthritis? A systematic review. Rheumatol Int 37(6):963–974
Consensus Conference P et al (2015) Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep 38(8):1161–1183
Sangle SR, Tench CM, D’Cruz DP (2015) Autoimmune rheumatic disease and sleep: a review. Curr Opin Pulm Med 21(6):553–556
Løppenthin K et al (2015) Physical activity and the association with fatigue and sleep in Danish patients with rheumatoid arthritis. Rheumatol Int 35(10):1655–1664
Roehrs T et al (2013) Nocturnal sleep, daytime sleepiness and fatigue in fibromyalgia patients compared to rheumatoid arthritis patients and healthy controls: a preliminary study. Sleep Med 14(1):109–115
Mckenna S et al (2018) OP0275-HPR The effects of exercise on depressive and anxiety symptoms in rheumatoid arthritis: a systematic review and meta-analysis. BMJ Publishing Group Ltd., London
Tierney M, Fraser A, Norelee K (2012) Physical activity in rheumatoid arthritis: a systematic review. J Phys Activity Health 9:1036
Kennedy NM et al (2018) A survey across four European countries to determine rheumatology health professionals’ awareness of physical activity measures in people with inflammatory joint diseases. BMJ open 8(5):e020809
Kredlow MA et al (2015) The effects of physical activity on sleep: a meta-analytic review. J Behav Med 38(3):427–449
Youngstedt SD (2005) Effects of exercise on sleep. Clin Sports Med 24(2):355–365
Swärdh E, Brodin N (2016) Effects of aerobic and muscle strengthening exercise in adults with rheumatoid arthritis: a narrative review summarising a chapter in Physical activity in the prevention and treatment of disease (FYSS 2016). Br J Sports Med 50(6):362–367
McKenna S, Kelly G, Kennedy N (2019) A survey of physiotherapists’ current management and the promotion of physical activity, in people with rheumatoid arthritis. Disabil Rehabil 41(18):2183–2191
Durcan L, Wilson F, Cunnane G (2014) The effect of exercise on sleep and fatigue in rheumatoid arthritis: a randomized controlled study. J Rheumatol 41(10):1966–1973
Osthoff A-KR et al (2018) 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 77(9):1251–1260
van den Berg MH et al (2007) Are patients with rheumatoid arthritis less physically active than the general population? JCR J Clin Rheumatol 13(4):181–186
Stenström CH, Minor MA (2003) Evidence for the benefit of aerobic and strengthening exercise in rheumatoid arthritis. Arthr Care Res 49(3):428–434
Hootman JM et al (2001) Association among physical activity level, cardiorespiratory fitness, and risk of musculoskeletal injury. Am J Epidemiol 154(3):251–258
Eijsvogels T et al (2010) Effect of prolonged walking on cardiac troponin levels. Am J Cardiol 105(2):267–272
Baxter SV et al (2016) Walking is a feasible physical activity for people with rheumatoid arthritis: a feasibility randomized controlled trial. Musculoskelet care 14(1):47–56
Nyrop KA, Cleveland R, Callahan LF (2014) Achievement of exercise objectives and satisfaction with the Walk With Ease program—group and self-directed participants. Am J Health Promot 28(4):228–230
Baillet A et al (2010) Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta-analysis of randomized controlled trials. Arthr Care Res 62(7):984–992
Rousseau DM, Gunia BC (2016) Evidence-based practice: the psychology of EBP implementation. Annu Rev Psychol 67:667–692
Thompson D, Oldham J, Woby S (2016) Does adding cognitive-behavioural physiotherapy to exercise improve outcome in patients with chronic neck pain? A randomised controlled trial. Physiotherapy 102(2):170–177
Frieden TR (2017) Evidence for health decision making—beyond randomized, controlled trials. N Engl J Med 377(5):465–475
Moore GF et al (2015) Process evaluation of complex interventions: Medical Research Council guidance. BMJ 350:h1258
McKenna SG et al (2018) The impact of exercise on sleep (time, quality, and disturbance) in patients with rheumatoid arthritis: a study protocol for a pilot randomised controlled trial. Rheumatol Int 2018:1–8
Buysse DJ et al (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
Eldridge SM et al (2016) CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibil Stud 2(1):64
Nelson ME et al (2007) Physical activity and public health in older adults. Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116:1094
World Health Organization (2018) Summary report of the update of systematic reviews of the evidence to inform the WHO guidelines on physical activity, sedentary behaviour and sleep in children under 5 years of age. World Health Organization, Geneva
Borg E, Kaijser L (2006) A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sports 16(1):57–69
Dawes HN et al (2005) Borg’s rating of perceived exertion scales: do the verbal anchors mean the same for different clinical groups? Arch Phys Med Rehabil 86(5):912–916
Borg G, Hassmén P, Lagerström M (1987) Perceived exertion related to heart rate and blood lactate during arm and leg exercise. Eur J Appl Physiol 56(6):679–685
Chen MJ, Fan X, Moe ST (2002) Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci 20(11):873–899
Goldman L et al (1981) Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation 64(6):1227–1234
Senn B et al (2013) Developing and evaluating complex interventions: the new Medical Research Council guidance. Studies 59:587–592
Ward L et al (2014) Yoga for pain and sleep quality in rheumatoid arthritis: a pilot randomized controlled trial. J Altern Complement Med 20(5):A87–A87
Ioannidis JP (2005) Why most published research findings are false. PLoS Med 2(8):e124
Powell C et al (2016) Simultaneous validation of five activity monitors for use in adult populations. Scand J Med Sci Sport 27:1881
Bassett DR Jr et al (2014) Detection of lying down, sitting, standing, and stepping using two activPAL monitors. Med Sci Sports Exerc 46(10):2025–2029
Grant PM et al (2006) The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br J Sports Med 40(12):992–997
Larkin L et al (2016) Criterion validity of the activ PAL activity monitor for sedentary and physical activity patterns in people who have rheumatoid arthritis. Phys Ther 96(7):1093–1101
Hawker GA et al (2011) Measures of adult pain: Visual analog scale for pain (vas pain), numeric rating scale for pain (nrs pain), mcgill pain questionnaire (mpq), short-form mcgill pain questionnaire (sf-mpq), chronic pain grade scale (cpgs), short form-36 bodily pain scale (sf-36 bps), and measure of intermittent and constant osteoarthritis pain (icoap). Arthr Care Res 63(S11):S240–S252
McNair DM (1971) Profile of mood states instrument. Manual for the profile of mood states, pp 3–29
Rush AJ et al (2003) The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiat 54(5):573–583
Spielberger, C.D., State‐Trait anxiety inventory. The Corsini encyclopedia of psychology, 2010: p. 1–1.
Bruce B, Fries JF (2005) The health assessment questionnaire (HAQ). Clin Exp Rheumatol 23(5):S14
Smolen J et al (2003) A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 42(2):244–257
Group, T.E. (1990) EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 16(3):199–208
Nicklin J et al (2010) Measuring fatigue in rheumatoid arthritis: a cross-sectional study to evaluate the bristol rheumatoid arthritis fatigue multi-dimensional questionnaire, visual analog scales, and numerical rating scales. Arthr Care Res 62(11):1559–1568
Sechrist KR, Walker SN, Pender NJ (1987) Development and psychometric evaluation of the exercise benefits/barriers scale. Res Nurs Health 10(6):357–365
Leech NL, Barrett KC, Morgan GA (2005) SPSS for intermediate statistics: use and interpretation. Psychology Press
O’Hagan A, Stevens JW, Campbell MJ (2005) Assurance in clinical trial design. Pharm Stat J Appl Stat Pharm Ind 4(3):187–201
Treweek S et al (2013) Methods to improve recruitment to randomised controlled trials: cochrane systematic review and meta-analysis. BMJ open 3(2):e002360
Warner ET et al (2013) Recruitment and retention of participants in a pragmatic randomized intervention trial at three community health clinics: results and lessons learned. BMC Public Health 13(1):192
Marin R, Cyhan T, Miklos W (2006) Sleep disturbance in patients with chronic low back pain. Am J Phys Med Rehabil 85(5):430–435
Buysse DJ (2014) Sleep health: can we define it? Does it matter? Sleep 37(1):9–17
Dolezal BA et al (2017) Interrelationship between sleep and exercise: a systematic review. Adv Prev Med 2017:1364387
Van Zanten JJCSV et al (2015) Perceived barriers, facilitators and benefits for regular physical activity and exercise in patients with rheumatoid arthritis: a review of the literature. Sports Med 45(10):1401–1412
Cramp F, Hewlett S, Almeida C, Kirwan JR, Choy EH, Chalder T, Pollock J, Christensen R (2013) Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev (8):CD008322. https://doi.org/10.1002/14651858.CD008322.pub2
de Wit MPT et al (2011) European League Against Rheumatism recommendations for the inclusion of patient representatives in scientific projects. Ann Rheum Dis 70:722
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McKenna, S.G., Donnelly, A., Esbensen, B.A. et al. The feasibility of an exercise intervention to improve sleep (time, quality and disturbance) in people with rheumatoid arthritis: a pilot RCT. Rheumatol Int 41, 297–310 (2021). https://doi.org/10.1007/s00296-020-04760-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04760-9